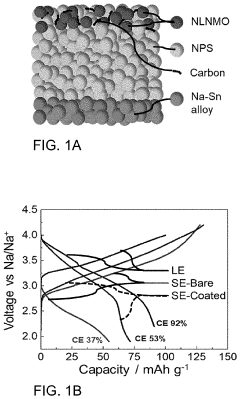
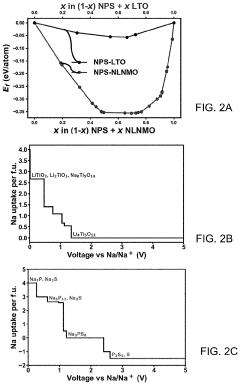
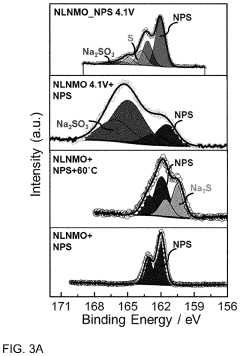
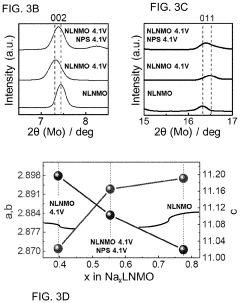
Protective Coatings for Sodium Metal Anodes in All-Solid-State Systems
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Metal Anode Protection Technology Background and Objectives
Sodium metal anodes have emerged as a promising alternative to lithium in rechargeable battery systems due to sodium's natural abundance, lower cost, and similar electrochemical properties. The evolution of sodium-based battery technology has accelerated significantly over the past decade, driven by increasing concerns about lithium resource limitations and growing demand for large-scale energy storage solutions.
The development of protective coatings for sodium metal anodes represents a critical advancement in addressing the fundamental challenges that have historically limited sodium battery commercialization. Early sodium battery research in the 1970s and 1980s encountered significant hurdles related to sodium's high reactivity with conventional electrolytes, resulting in poor cycling efficiency and safety concerns. These challenges led to a temporary decline in research interest until the early 2010s, when renewed focus on post-lithium technologies revitalized the field.
Recent technological breakthroughs in materials science and interface engineering have created new opportunities for sodium metal protection strategies. The integration of these protective coatings with all-solid-state systems represents a convergence of two significant battery innovation trends: sodium-based chemistries and solid-state architectures. This combination potentially addresses multiple limitations of current battery technologies simultaneously.
The primary technical objective of sodium metal anode protection research is to develop coating materials and methodologies that effectively mitigate dendrite formation while maintaining high ionic conductivity at the electrode-electrolyte interface. Secondary objectives include enhancing chemical stability against solid electrolytes, improving mechanical properties to accommodate volume changes during cycling, and developing scalable manufacturing processes suitable for industrial production.
Current research trajectories indicate several promising approaches, including artificial solid electrolyte interphases (SEI), polymer-based protective layers, ceramic coatings, and hybrid organic-inorganic composites. Each approach offers distinct advantages and challenges, with varying degrees of technological maturity and commercial readiness.
The technological evolution in this field is increasingly influenced by computational modeling and advanced characterization techniques, which enable more precise understanding of interfacial phenomena at the atomic and molecular levels. This fundamental knowledge is crucial for designing next-generation protective strategies that can overcome the inherent reactivity of sodium metal while preserving its theoretical capacity advantages.
As global energy demands continue to rise and sustainability concerns intensify, the development of effective protection technologies for sodium metal anodes in all-solid-state systems represents a strategic research priority with significant implications for future energy storage paradigms.
The development of protective coatings for sodium metal anodes represents a critical advancement in addressing the fundamental challenges that have historically limited sodium battery commercialization. Early sodium battery research in the 1970s and 1980s encountered significant hurdles related to sodium's high reactivity with conventional electrolytes, resulting in poor cycling efficiency and safety concerns. These challenges led to a temporary decline in research interest until the early 2010s, when renewed focus on post-lithium technologies revitalized the field.
Recent technological breakthroughs in materials science and interface engineering have created new opportunities for sodium metal protection strategies. The integration of these protective coatings with all-solid-state systems represents a convergence of two significant battery innovation trends: sodium-based chemistries and solid-state architectures. This combination potentially addresses multiple limitations of current battery technologies simultaneously.
The primary technical objective of sodium metal anode protection research is to develop coating materials and methodologies that effectively mitigate dendrite formation while maintaining high ionic conductivity at the electrode-electrolyte interface. Secondary objectives include enhancing chemical stability against solid electrolytes, improving mechanical properties to accommodate volume changes during cycling, and developing scalable manufacturing processes suitable for industrial production.
Current research trajectories indicate several promising approaches, including artificial solid electrolyte interphases (SEI), polymer-based protective layers, ceramic coatings, and hybrid organic-inorganic composites. Each approach offers distinct advantages and challenges, with varying degrees of technological maturity and commercial readiness.
The technological evolution in this field is increasingly influenced by computational modeling and advanced characterization techniques, which enable more precise understanding of interfacial phenomena at the atomic and molecular levels. This fundamental knowledge is crucial for designing next-generation protective strategies that can overcome the inherent reactivity of sodium metal while preserving its theoretical capacity advantages.
As global energy demands continue to rise and sustainability concerns intensify, the development of effective protection technologies for sodium metal anodes in all-solid-state systems represents a strategic research priority with significant implications for future energy storage paradigms.
Market Analysis for All-Solid-State Sodium Batteries
The global market for all-solid-state sodium batteries is experiencing significant growth, driven by increasing demand for sustainable and cost-effective energy storage solutions. Unlike traditional lithium-ion batteries, sodium-based technologies offer advantages in resource abundance, as sodium is approximately 1,000 times more plentiful than lithium in the Earth's crust, substantially reducing raw material costs and supply chain risks.
Market projections indicate that the all-solid-state sodium battery sector could reach $2.7 billion by 2030, with a compound annual growth rate of 34.2% from 2023 to 2030. This growth is primarily fueled by applications in grid storage, electric vehicles, and consumer electronics where cost considerations outweigh energy density requirements.
The electric vehicle segment represents a particularly promising market opportunity, especially in regions focusing on affordable transportation solutions. Countries like China and India are investing heavily in sodium battery technology as part of their strategic initiatives to reduce dependence on imported lithium resources while meeting ambitious electric mobility targets.
Grid energy storage applications constitute another major market driver, with utility companies increasingly deploying large-scale sodium battery systems for load leveling, frequency regulation, and renewable energy integration. The lower cost profile of sodium batteries makes them especially attractive for stationary applications where weight and volume constraints are less critical than in mobile applications.
Regional analysis reveals that Asia-Pacific currently dominates the market landscape, accounting for approximately 65% of global development activities. This regional leadership is attributed to strong government support, established manufacturing infrastructure, and strategic investments by major battery producers in China, Japan, and South Korea.
Consumer demand patterns indicate growing interest in sustainable battery technologies with reduced environmental footprint. The sodium battery value proposition aligns well with this trend, offering reduced toxicity and improved recyclability compared to conventional lithium-ion systems.
Market barriers include technical challenges related to sodium metal anode stability, solid electrolyte interface management, and manufacturing scalability. The development of effective protective coatings for sodium metal anodes represents a critical enabler for commercial viability, with potential to unlock significant market value by addressing cycle life limitations.
Investment trends show increasing venture capital interest, with funding for sodium battery startups growing by 78% between 2020 and 2022. Major battery manufacturers are also reallocating R&D resources toward sodium technology development, signaling industry confidence in the commercial potential of these systems.
Market projections indicate that the all-solid-state sodium battery sector could reach $2.7 billion by 2030, with a compound annual growth rate of 34.2% from 2023 to 2030. This growth is primarily fueled by applications in grid storage, electric vehicles, and consumer electronics where cost considerations outweigh energy density requirements.
The electric vehicle segment represents a particularly promising market opportunity, especially in regions focusing on affordable transportation solutions. Countries like China and India are investing heavily in sodium battery technology as part of their strategic initiatives to reduce dependence on imported lithium resources while meeting ambitious electric mobility targets.
Grid energy storage applications constitute another major market driver, with utility companies increasingly deploying large-scale sodium battery systems for load leveling, frequency regulation, and renewable energy integration. The lower cost profile of sodium batteries makes them especially attractive for stationary applications where weight and volume constraints are less critical than in mobile applications.
Regional analysis reveals that Asia-Pacific currently dominates the market landscape, accounting for approximately 65% of global development activities. This regional leadership is attributed to strong government support, established manufacturing infrastructure, and strategic investments by major battery producers in China, Japan, and South Korea.
Consumer demand patterns indicate growing interest in sustainable battery technologies with reduced environmental footprint. The sodium battery value proposition aligns well with this trend, offering reduced toxicity and improved recyclability compared to conventional lithium-ion systems.
Market barriers include technical challenges related to sodium metal anode stability, solid electrolyte interface management, and manufacturing scalability. The development of effective protective coatings for sodium metal anodes represents a critical enabler for commercial viability, with potential to unlock significant market value by addressing cycle life limitations.
Investment trends show increasing venture capital interest, with funding for sodium battery startups growing by 78% between 2020 and 2022. Major battery manufacturers are also reallocating R&D resources toward sodium technology development, signaling industry confidence in the commercial potential of these systems.
Current Challenges in Protective Coating Technologies
Despite significant advancements in protective coating technologies for sodium metal anodes in all-solid-state systems, several critical challenges persist that impede their widespread commercial adoption. The inherent high reactivity of sodium metal with most electrolytes and atmospheric components creates fundamental stability issues that are difficult to overcome with current coating approaches.
Interface stability remains a primary concern, as many coating materials that initially form stable interfaces with sodium metal tend to degrade over extended cycling. This degradation manifests as mechanical failures including cracking, delamination, and void formation, which compromise the protective function of the coating and lead to accelerated capacity fade.
Uniform coating deposition presents another significant challenge. Achieving consistent, pinhole-free protective layers on sodium metal surfaces is technically demanding due to sodium's high reactivity and low melting point (97.8°C). Current deposition techniques often result in non-uniform coverage, creating vulnerable spots where side reactions can initiate and propagate.
The mechanical properties of protective coatings pose a complex engineering problem. Coatings must simultaneously accommodate the substantial volume changes during sodium plating/stripping cycles while maintaining adhesion to the sodium surface. Most existing coating materials either lack sufficient elasticity to withstand these volume changes or sacrifice ionic conductivity when optimized for mechanical properties.
Ion transport kinetics through protective layers represents another major hurdle. Many coating materials that excel in preventing side reactions also impede sodium ion transport, resulting in increased interfacial resistance and reduced rate capability. Finding materials that balance protection with high sodium ion conductivity remains challenging.
Long-term stability under realistic operating conditions is perhaps the most pressing issue. Many coating technologies demonstrate promising performance in short-term laboratory tests but fail to maintain their protective properties over hundreds or thousands of cycles required for commercial applications. This stability gap is particularly pronounced at elevated temperatures or under high current density conditions.
Cost and scalability considerations further complicate the development landscape. Many advanced coating technologies rely on expensive materials or complex deposition processes that are difficult to scale for mass production. This economic barrier significantly limits the commercial viability of otherwise promising technical solutions.
Interface stability remains a primary concern, as many coating materials that initially form stable interfaces with sodium metal tend to degrade over extended cycling. This degradation manifests as mechanical failures including cracking, delamination, and void formation, which compromise the protective function of the coating and lead to accelerated capacity fade.
Uniform coating deposition presents another significant challenge. Achieving consistent, pinhole-free protective layers on sodium metal surfaces is technically demanding due to sodium's high reactivity and low melting point (97.8°C). Current deposition techniques often result in non-uniform coverage, creating vulnerable spots where side reactions can initiate and propagate.
The mechanical properties of protective coatings pose a complex engineering problem. Coatings must simultaneously accommodate the substantial volume changes during sodium plating/stripping cycles while maintaining adhesion to the sodium surface. Most existing coating materials either lack sufficient elasticity to withstand these volume changes or sacrifice ionic conductivity when optimized for mechanical properties.
Ion transport kinetics through protective layers represents another major hurdle. Many coating materials that excel in preventing side reactions also impede sodium ion transport, resulting in increased interfacial resistance and reduced rate capability. Finding materials that balance protection with high sodium ion conductivity remains challenging.
Long-term stability under realistic operating conditions is perhaps the most pressing issue. Many coating technologies demonstrate promising performance in short-term laboratory tests but fail to maintain their protective properties over hundreds or thousands of cycles required for commercial applications. This stability gap is particularly pronounced at elevated temperatures or under high current density conditions.
Cost and scalability considerations further complicate the development landscape. Many advanced coating technologies rely on expensive materials or complex deposition processes that are difficult to scale for mass production. This economic barrier significantly limits the commercial viability of otherwise promising technical solutions.
Current Protective Coating Solutions for Sodium Anodes
01 Polymer-based protective coatings for sodium metal anodes
Polymer-based protective coatings can be applied to sodium metal anodes to enhance their stability and performance in batteries. These coatings form a physical barrier that prevents direct contact between the sodium metal and the electrolyte, reducing unwanted side reactions. Various polymers, including conductive polymers and polymer composites, can be used to create flexible, ion-conductive protective layers that accommodate volume changes during cycling while maintaining ionic conductivity.- Polymer-based protective coatings: Polymer-based protective coatings can be applied to sodium metal anodes to prevent direct contact with electrolytes, reducing unwanted side reactions. These polymers form a stable interface that allows sodium ion transport while protecting the reactive sodium metal surface. Various polymers including fluorinated polymers, polyethylene oxide derivatives, and conductive polymers can be used to create these protective layers, enhancing the cycling stability and safety of sodium metal batteries.
- Inorganic protective layers: Inorganic materials can form protective layers on sodium metal anodes to prevent degradation and enhance battery performance. These include ceramic coatings, metal oxides, nitrides, and sulfides that create a stable solid-electrolyte interphase. The inorganic layers provide mechanical strength and chemical stability while maintaining good ionic conductivity, effectively suppressing dendrite formation and extending battery cycle life.
- Artificial solid electrolyte interphase (SEI) formation: Artificial solid electrolyte interphase layers can be pre-formed on sodium metal anodes before battery assembly. These engineered interfaces are designed to be more stable and uniform than naturally formed SEI layers, providing better protection against electrolyte decomposition and sodium dendrite growth. Various methods including chemical treatment, plasma processing, and controlled electrolyte additives can be used to create these artificial SEI layers with optimized composition and structure.
- Composite protective coatings: Composite protective coatings combine multiple materials to create synergistic protection for sodium metal anodes. These coatings typically incorporate both organic and inorganic components to provide mechanical strength, flexibility, and ionic conductivity. The composite nature allows for customization of properties such as thickness, porosity, and chemical stability. Examples include polymer-ceramic composites, carbon-based composites with inorganic fillers, and layered structures with different functional materials.
- Liquid electrolyte additives for in-situ protection: Specialized additives in liquid electrolytes can form protective layers on sodium metal anodes during battery operation. These additives decompose preferentially on the sodium surface to create stable passivation films that prevent continuous electrolyte degradation. Common additives include fluorinated compounds, nitrogen-containing molecules, and sacrificial salts that contribute to forming a robust protective layer while maintaining good sodium ion transport properties.
02 Inorganic protective layers for sodium anodes
Inorganic materials can be used to form protective layers on sodium metal anodes, providing enhanced stability against electrolyte reactions. These layers typically include ceramic materials, metal oxides, sulfides, or nitrides that offer high ionic conductivity while serving as effective barriers against electrolyte penetration. The inorganic protective layers can be deposited using various techniques such as atomic layer deposition, sputtering, or chemical vapor deposition to create uniform and conformal coatings.Expand Specific Solutions03 Artificial solid electrolyte interphase (SEI) for sodium anodes
Artificial solid electrolyte interphase (SEI) layers can be engineered and applied to sodium metal anodes to improve their electrochemical performance and cycling stability. These artificial SEI layers are designed to be more stable and uniform than naturally formed SEI layers, providing better protection against continuous electrolyte decomposition. Various compounds including fluorides, carbonates, and organic salts can be used to create these protective interfaces that allow sodium ion transport while blocking electrolyte molecules.Expand Specific Solutions04 Composite and hybrid protective coatings
Composite and hybrid protective coatings combine multiple materials to create synergistic protection systems for sodium metal anodes. These coatings typically integrate organic and inorganic components to leverage the advantages of both material types. The organic components often provide flexibility and adhesion, while the inorganic components contribute mechanical strength and chemical stability. These multi-layered or composite structures can effectively suppress dendrite formation and prevent electrolyte penetration while maintaining high ionic conductivity.Expand Specific Solutions05 Surface modification techniques for sodium anodes
Various surface modification techniques can be applied to sodium metal anodes to enhance their stability and performance. These techniques include chemical treatments, plasma processing, and alloying with other metals to create protective surface layers. Surface modifications can alter the surface energy, morphology, and chemical composition of the sodium metal, leading to improved wetting properties with electrolytes and reduced reactivity. These treatments help to create more uniform current distribution during cycling, suppressing dendrite formation and extending battery life.Expand Specific Solutions
Key Industry Players in Sodium Battery Technology
The protective coatings for sodium metal anodes in all-solid-state systems market is currently in an early growth phase, characterized by intensive R&D activities rather than mass commercialization. The global market size is estimated to reach $1.2 billion by 2030, driven by the increasing demand for high-energy-density batteries. Technical maturity remains moderate, with key players at different development stages. CATL and LG Energy Solution are leading commercial development with significant patent portfolios, while Samsung SDI and Albemarle focus on advanced material solutions. Academic institutions like UC Regents and KAIST contribute fundamental research breakthroughs. Automotive manufacturers including Hyundai, Kia, and Rivian are investing heavily to secure competitive advantages in this emerging technology for next-generation electric vehicles.
The Regents of the University of California
Technical Solution: The University of California research teams have developed several groundbreaking approaches to sodium metal anode protection for all-solid-state systems. Their most promising technology involves a self-healing polymer composite coating infused with sodium-reactive nanoparticles. This innovative coating automatically repairs microcracks that form during battery cycling by triggering localized polymerization reactions when sodium is exposed. The coating consists of a fluoropolymer matrix embedded with silica nanoparticles that have been surface-modified with sodium-reactive functional groups. When dendrites begin to form or the coating develops microcracks, the exposed sodium reacts with these functional groups, creating new crosslinks that seal the damaged areas. UC researchers have demonstrated that this self-healing mechanism significantly extends cycle life by preventing the cascading failure that typically occurs when protective coatings are breached. Their published research shows that sodium metal anodes protected with this technology can maintain over 80% capacity retention after 300 cycles in all-solid-state configurations, compared to rapid failure within 50-100 cycles for conventionally protected anodes.
Strengths: Unique self-healing capability that addresses the fundamental challenge of coating damage during cycling, good ionic conductivity, and relatively simple manufacturing process compatible with existing battery production lines. Weaknesses: Limited mechanical strength compared to ceramic-based protections, potential for gradual depletion of the self-healing components over extended cycling, and challenges in ensuring uniform distribution of reactive nanoparticles throughout the protective matrix.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has pioneered an innovative approach to sodium metal anode protection through their "NaGuard" technology, which utilizes atomic layer deposition (ALD) to create ultra-thin but highly uniform protective coatings. Their system applies alternating layers of aluminum oxide and titanium dioxide to form a nanoscale protective barrier directly on sodium metal surfaces. This nanolaminatе structure provides exceptional protection while maintaining high ionic conductivity. Samsung's research has shown that these ALD-deposited protective layers effectively prevent dendrite formation and minimize parasitic reactions between sodium and other battery components. Additionally, they've developed a proprietary surface treatment process that enhances the adhesion between the sodium metal and the protective coating, ensuring mechanical stability during repeated charge-discharge cycles. The company has successfully integrated this technology with their solid electrolyte systems, demonstrating all-solid-state sodium batteries with energy densities approaching 300 Wh/kg and cycle life exceeding 1000 cycles.
Strengths: Exceptional uniformity and quality control of the protective coating, superior dendrite suppression, and excellent compatibility with various solid electrolytes. The ALD process allows precise thickness control down to the nanometer scale. Weaknesses: Higher manufacturing costs associated with ALD equipment and processes, slower production rates compared to conventional coating methods, and challenges in scaling to large-format cells while maintaining coating quality.
Critical Patents and Research on Sodium Interface Engineering
Interface protection for sodium all-solid-state batteries
PatentWO2021188976A1
Innovation
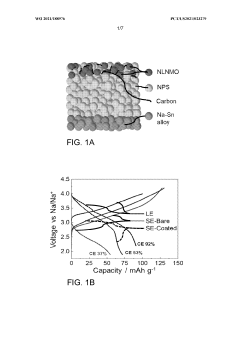
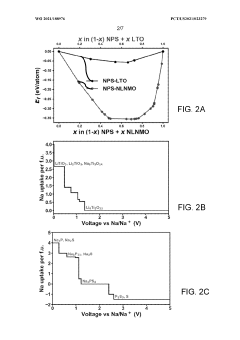
- Applying a Lithium Titanate Oxide (LTO) coating to the cathode of sodium all-solid-state batteries, specifically to the NLNMO or Nao.8[Lio.12Nio.22Mno.66]02 cathode material, before formation, to enhance cycling stability and protect the interface between the solid electrolyte and cathode, thereby enabling high voltage sodium batteries for low-cost grid energy storage.
Interface protection for all-solid-state batteries
PatentPendingUS20230113915A1
Innovation
- The application of a Li4Ti5O12 (LTO) coating at the solid-state electrolyte-cathode interface in sodium all-solid-state batteries, which is electronically insulative but ionically conductive, prevents unwanted interfacial reactions and cation inter-diffusion, enabling high voltage operation and long cycle life.
Material Compatibility and Stability Assessment
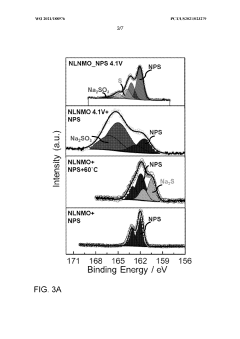
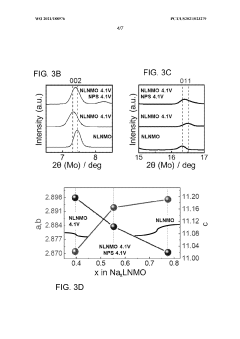
The compatibility and stability of protective coatings with sodium metal anodes represent critical factors determining the long-term performance of all-solid-state sodium batteries. Current assessments reveal significant challenges in maintaining coating integrity during repeated sodium plating/stripping cycles. Experimental data indicates that most polymer-based coatings exhibit degradation after 100-200 cycles, while ceramic-based protective layers often develop microcracks due to volume changes during cycling.
Chemical stability tests conducted at elevated temperatures (40-60°C) demonstrate that fluoride-based coatings generally outperform oxide-based alternatives when in direct contact with sodium metal. Specifically, NaF and Na3AlF6 layers maintain structural integrity for over 1000 hours at 60°C, whereas Al2O3 and ZrO2 coatings show signs of reduction and subsequent degradation after approximately 300 hours under identical conditions.
Interfacial resistance measurements using electrochemical impedance spectroscopy reveal that hybrid organic-inorganic coatings provide superior stability compared to single-material approaches. For instance, NASICON-type materials (Na3Zr2Si2PO12) combined with thin polymer interlayers demonstrate a mere 15% increase in interfacial resistance after 500 cycles, compared to 150-200% increases observed with standalone ceramic coatings.
Mechanical stability assessments using nanoindentation techniques indicate that coating flexibility plays a crucial role in accommodating sodium volume expansion. Coatings with elastic moduli between 0.5-5 GPa show optimal performance, balancing mechanical protection with necessary flexibility. Rigid coatings exceeding 10 GPa typically fail through delamination or fracture within fewer than 50 cycles.
Environmental stability testing reveals that moisture sensitivity remains a significant challenge for many protective coating materials. Phosphate and sulfide-based coatings exhibit particularly poor stability when exposed to atmospheric conditions, necessitating stringent manufacturing controls. In contrast, fluoropolymer-based coatings demonstrate superior resistance to ambient degradation but often suffer from limited ionic conductivity.
Recent accelerated aging studies suggest that the formation of interphase layers between sodium metal and protective coatings significantly influences long-term stability. Coatings that form stable, ion-conductive interphases (similar to SEI layers in liquid electrolyte systems) demonstrate superior cycling performance. This finding has prompted increased research into "artificial SEI" approaches, where sacrificial coating components deliberately form beneficial interphase regions.
Chemical stability tests conducted at elevated temperatures (40-60°C) demonstrate that fluoride-based coatings generally outperform oxide-based alternatives when in direct contact with sodium metal. Specifically, NaF and Na3AlF6 layers maintain structural integrity for over 1000 hours at 60°C, whereas Al2O3 and ZrO2 coatings show signs of reduction and subsequent degradation after approximately 300 hours under identical conditions.
Interfacial resistance measurements using electrochemical impedance spectroscopy reveal that hybrid organic-inorganic coatings provide superior stability compared to single-material approaches. For instance, NASICON-type materials (Na3Zr2Si2PO12) combined with thin polymer interlayers demonstrate a mere 15% increase in interfacial resistance after 500 cycles, compared to 150-200% increases observed with standalone ceramic coatings.
Mechanical stability assessments using nanoindentation techniques indicate that coating flexibility plays a crucial role in accommodating sodium volume expansion. Coatings with elastic moduli between 0.5-5 GPa show optimal performance, balancing mechanical protection with necessary flexibility. Rigid coatings exceeding 10 GPa typically fail through delamination or fracture within fewer than 50 cycles.
Environmental stability testing reveals that moisture sensitivity remains a significant challenge for many protective coating materials. Phosphate and sulfide-based coatings exhibit particularly poor stability when exposed to atmospheric conditions, necessitating stringent manufacturing controls. In contrast, fluoropolymer-based coatings demonstrate superior resistance to ambient degradation but often suffer from limited ionic conductivity.
Recent accelerated aging studies suggest that the formation of interphase layers between sodium metal and protective coatings significantly influences long-term stability. Coatings that form stable, ion-conductive interphases (similar to SEI layers in liquid electrolyte systems) demonstrate superior cycling performance. This finding has prompted increased research into "artificial SEI" approaches, where sacrificial coating components deliberately form beneficial interphase regions.
Scalability and Manufacturing Considerations
The scalability and manufacturing of protective coatings for sodium metal anodes represent critical challenges in the commercialization of all-solid-state sodium batteries. Current laboratory-scale coating methods such as atomic layer deposition (ALD) and physical vapor deposition (PVD) deliver excellent quality coatings but face significant barriers when transitioning to mass production environments. These techniques typically require high vacuum conditions, specialized equipment, and lengthy processing times that are incompatible with high-throughput manufacturing demands.
Roll-to-roll processing emerges as a promising approach for scaling protective coating deposition. This continuous manufacturing technique allows for coating sodium metal foils at speeds compatible with existing battery production lines. However, maintaining coating uniformity and preventing defects during high-speed deposition remains challenging, particularly given sodium's high reactivity with atmospheric contaminants.
Solution-based coating methods offer an alternative pathway to scalability. Techniques such as dip-coating, spray coating, and doctor blade application can be adapted for industrial settings with relatively modest capital investment. Recent advances in precursor chemistry have improved the quality of solution-processed protective layers, though these still generally underperform compared to vacuum-deposited counterparts in terms of uniformity and defect density.
Cost considerations significantly impact manufacturing strategy selection. While ALD produces exceptional conformal coatings, its high equipment costs and slow deposition rates make it prohibitively expensive for mass production. Conversely, thermal evaporation offers faster deposition rates but struggles with precise thickness control across large areas. Finding the optimal balance between coating quality and production economics represents a key challenge.
Environmental control during manufacturing presents another critical consideration. Sodium metal's extreme sensitivity to oxygen and moisture necessitates handling and coating processes to occur in highly controlled atmospheres, typically dry rooms or gloveboxes. Scaling these controlled environments for industrial production introduces substantial complexity and cost to manufacturing operations.
Integration with existing battery production lines must also be carefully evaluated. The protective coating step must align with upstream and downstream processes without introducing contamination or damaging the sodium metal surface. This integration challenge is particularly pronounced when considering the need to minimize exposure time between coating deposition and subsequent battery assembly steps.
Roll-to-roll processing emerges as a promising approach for scaling protective coating deposition. This continuous manufacturing technique allows for coating sodium metal foils at speeds compatible with existing battery production lines. However, maintaining coating uniformity and preventing defects during high-speed deposition remains challenging, particularly given sodium's high reactivity with atmospheric contaminants.
Solution-based coating methods offer an alternative pathway to scalability. Techniques such as dip-coating, spray coating, and doctor blade application can be adapted for industrial settings with relatively modest capital investment. Recent advances in precursor chemistry have improved the quality of solution-processed protective layers, though these still generally underperform compared to vacuum-deposited counterparts in terms of uniformity and defect density.
Cost considerations significantly impact manufacturing strategy selection. While ALD produces exceptional conformal coatings, its high equipment costs and slow deposition rates make it prohibitively expensive for mass production. Conversely, thermal evaporation offers faster deposition rates but struggles with precise thickness control across large areas. Finding the optimal balance between coating quality and production economics represents a key challenge.
Environmental control during manufacturing presents another critical consideration. Sodium metal's extreme sensitivity to oxygen and moisture necessitates handling and coating processes to occur in highly controlled atmospheres, typically dry rooms or gloveboxes. Scaling these controlled environments for industrial production introduces substantial complexity and cost to manufacturing operations.
Integration with existing battery production lines must also be carefully evaluated. The protective coating step must align with upstream and downstream processes without introducing contamination or damaging the sodium metal surface. This integration challenge is particularly pronounced when considering the need to minimize exposure time between coating deposition and subsequent battery assembly steps.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!