Optimization of Electrolyte Composition for Sodium Metal Anodes
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Battery Electrolyte Development Background and Objectives
The development of sodium-ion batteries has gained significant momentum in recent years as a promising alternative to lithium-ion batteries. This surge in interest stems primarily from concerns about the long-term sustainability and cost of lithium resources. Sodium, being the sixth most abundant element in the Earth's crust, offers a more economically viable and geographically distributed resource base compared to lithium.
The evolution of sodium battery technology can be traced back to the 1970s and 1980s, when initial research was conducted alongside lithium-ion battery development. However, the superior energy density of lithium-ion batteries led to their commercial dominance, causing sodium battery research to be temporarily sidelined. The resurgence of interest in sodium-ion batteries began around 2010, driven by increasing concerns about lithium supply chain vulnerabilities and cost escalations.
A critical challenge in sodium battery development has been the electrolyte composition, particularly for systems utilizing sodium metal anodes. Unlike lithium, sodium metal is more reactive and forms less stable solid electrolyte interphase (SEI) layers, leading to poor cycling performance and safety concerns. The optimization of electrolyte formulations is therefore paramount to enabling practical sodium metal batteries.
Current electrolyte development for sodium batteries has focused on several approaches: conventional organic liquid electrolytes with carefully selected salts and solvents, solid-state electrolytes to physically contain sodium dendrite growth, and hybrid systems combining aspects of both. Each approach presents unique advantages and challenges in terms of ionic conductivity, electrochemical stability, and compatibility with electrode materials.
The technical objectives for sodium battery electrolyte optimization include achieving high ionic conductivity (>1 mS/cm at room temperature), wide electrochemical stability windows (>4V), improved interfacial stability with sodium metal anodes, suppression of dendrite formation, and enhanced safety characteristics. Additionally, the electrolyte must be economically viable for large-scale production and environmentally benign.
Recent breakthroughs in fluorinated solvents, ether-based electrolytes, and concentrated electrolyte systems have shown promising results in stabilizing the sodium metal interface. These developments suggest that properly engineered electrolyte compositions could overcome the historical limitations of sodium metal anodes.
The ultimate goal of this research direction is to develop sodium battery systems that can achieve energy densities approaching those of lithium-ion batteries (>250 Wh/kg) while maintaining significantly lower costs (<$100/kWh) and improved sustainability profiles. Such advancements would position sodium batteries as viable alternatives for grid-scale energy storage applications and potentially certain electric vehicle segments where cost considerations outweigh the need for maximum energy density.
The evolution of sodium battery technology can be traced back to the 1970s and 1980s, when initial research was conducted alongside lithium-ion battery development. However, the superior energy density of lithium-ion batteries led to their commercial dominance, causing sodium battery research to be temporarily sidelined. The resurgence of interest in sodium-ion batteries began around 2010, driven by increasing concerns about lithium supply chain vulnerabilities and cost escalations.
A critical challenge in sodium battery development has been the electrolyte composition, particularly for systems utilizing sodium metal anodes. Unlike lithium, sodium metal is more reactive and forms less stable solid electrolyte interphase (SEI) layers, leading to poor cycling performance and safety concerns. The optimization of electrolyte formulations is therefore paramount to enabling practical sodium metal batteries.
Current electrolyte development for sodium batteries has focused on several approaches: conventional organic liquid electrolytes with carefully selected salts and solvents, solid-state electrolytes to physically contain sodium dendrite growth, and hybrid systems combining aspects of both. Each approach presents unique advantages and challenges in terms of ionic conductivity, electrochemical stability, and compatibility with electrode materials.
The technical objectives for sodium battery electrolyte optimization include achieving high ionic conductivity (>1 mS/cm at room temperature), wide electrochemical stability windows (>4V), improved interfacial stability with sodium metal anodes, suppression of dendrite formation, and enhanced safety characteristics. Additionally, the electrolyte must be economically viable for large-scale production and environmentally benign.
Recent breakthroughs in fluorinated solvents, ether-based electrolytes, and concentrated electrolyte systems have shown promising results in stabilizing the sodium metal interface. These developments suggest that properly engineered electrolyte compositions could overcome the historical limitations of sodium metal anodes.
The ultimate goal of this research direction is to develop sodium battery systems that can achieve energy densities approaching those of lithium-ion batteries (>250 Wh/kg) while maintaining significantly lower costs (<$100/kWh) and improved sustainability profiles. Such advancements would position sodium batteries as viable alternatives for grid-scale energy storage applications and potentially certain electric vehicle segments where cost considerations outweigh the need for maximum energy density.
Market Analysis for Sodium-based Energy Storage Solutions
The global energy storage market is witnessing a significant shift towards sodium-based technologies as a viable alternative to lithium-ion batteries. This transition is primarily driven by the increasing scarcity and rising costs of lithium resources, coupled with geopolitical concerns surrounding lithium supply chains. Sodium, being the sixth most abundant element in the Earth's crust, offers a sustainable and cost-effective solution for large-scale energy storage applications.
Market projections indicate that the sodium-based energy storage sector is poised for substantial growth, with an estimated market value expected to reach several billion dollars by 2030. This growth trajectory is supported by increasing investments in research and development, as well as strategic partnerships between technology developers and energy companies seeking to diversify their energy storage portfolios.
The demand for sodium-based energy storage solutions is particularly strong in grid-scale applications, where cost considerations often outweigh energy density requirements. Utility companies and grid operators are increasingly exploring sodium-ion and sodium-metal battery technologies for load leveling, peak shaving, and renewable energy integration. The ability to store large amounts of energy at a lower cost per kilowatt-hour makes these technologies especially attractive for stationary storage applications.
Regionally, Asia-Pacific leads the market development, with China at the forefront of commercializing sodium-ion batteries. European markets are following closely, driven by stringent environmental regulations and ambitious renewable energy targets. North America is also showing growing interest, particularly in applications where the lower energy density of sodium-based systems is not a limiting factor.
Consumer electronics and electric vehicle segments present emerging opportunities for sodium-based technologies, particularly as advancements in electrolyte composition for sodium metal anodes continue to improve performance metrics. While these markets currently favor lithium-ion technologies due to higher energy density requirements, certain niches within these sectors are beginning to consider sodium alternatives for specific applications where cost is a primary concern.
Market challenges include the need for further technological advancements to improve energy density and cycle life, as well as the establishment of dedicated manufacturing infrastructure. The optimization of electrolyte composition for sodium metal anodes represents a critical area of development that could significantly enhance the commercial viability of these systems by addressing issues related to dendrite formation and interface stability.
Industry analysts predict that as research progresses in electrolyte optimization, sodium-based energy storage solutions will capture an increasing share of the global energy storage market, particularly in applications where the cost-performance ratio is more important than achieving maximum energy density.
Market projections indicate that the sodium-based energy storage sector is poised for substantial growth, with an estimated market value expected to reach several billion dollars by 2030. This growth trajectory is supported by increasing investments in research and development, as well as strategic partnerships between technology developers and energy companies seeking to diversify their energy storage portfolios.
The demand for sodium-based energy storage solutions is particularly strong in grid-scale applications, where cost considerations often outweigh energy density requirements. Utility companies and grid operators are increasingly exploring sodium-ion and sodium-metal battery technologies for load leveling, peak shaving, and renewable energy integration. The ability to store large amounts of energy at a lower cost per kilowatt-hour makes these technologies especially attractive for stationary storage applications.
Regionally, Asia-Pacific leads the market development, with China at the forefront of commercializing sodium-ion batteries. European markets are following closely, driven by stringent environmental regulations and ambitious renewable energy targets. North America is also showing growing interest, particularly in applications where the lower energy density of sodium-based systems is not a limiting factor.
Consumer electronics and electric vehicle segments present emerging opportunities for sodium-based technologies, particularly as advancements in electrolyte composition for sodium metal anodes continue to improve performance metrics. While these markets currently favor lithium-ion technologies due to higher energy density requirements, certain niches within these sectors are beginning to consider sodium alternatives for specific applications where cost is a primary concern.
Market challenges include the need for further technological advancements to improve energy density and cycle life, as well as the establishment of dedicated manufacturing infrastructure. The optimization of electrolyte composition for sodium metal anodes represents a critical area of development that could significantly enhance the commercial viability of these systems by addressing issues related to dendrite formation and interface stability.
Industry analysts predict that as research progresses in electrolyte optimization, sodium-based energy storage solutions will capture an increasing share of the global energy storage market, particularly in applications where the cost-performance ratio is more important than achieving maximum energy density.
Current Challenges in Sodium Metal Anode Electrolytes
Despite significant advancements in sodium-ion battery technology, the development of effective electrolytes for sodium metal anodes remains a critical challenge. Current electrolyte formulations face several fundamental issues that hinder the commercial viability of sodium metal batteries. The primary challenge stems from sodium's high reactivity with conventional electrolyte components, leading to continuous side reactions and the formation of unstable solid electrolyte interphase (SEI) layers.
Conventional carbonate-based electrolytes, which perform adequately in lithium-ion systems, demonstrate poor compatibility with sodium metal. These electrolytes decompose rapidly upon contact with sodium, creating porous and non-uniform SEI layers that fail to prevent further electrolyte consumption. This ongoing parasitic reaction significantly reduces coulombic efficiency and battery lifespan.
Another major obstacle is sodium dendrite formation, which occurs more readily than with lithium due to sodium's lower surface energy and faster diffusion kinetics. Current electrolytes struggle to promote uniform sodium deposition, instead facilitating dendritic growth that can penetrate separators and cause catastrophic short circuits. The mechanical properties of SEI layers formed in existing electrolytes lack sufficient strength to suppress dendrite propagation.
The solvation structure of sodium ions in electrolytes presents additional complications. Unlike lithium ions, sodium ions exhibit different coordination behaviors with solvent molecules and anions, affecting ion transport mechanisms and interfacial processes. Current electrolyte designs have not fully optimized these solvation structures to facilitate efficient sodium plating/stripping.
Temperature sensitivity further complicates electrolyte development. Many promising electrolyte formulations show acceptable performance within narrow temperature ranges but deteriorate rapidly outside these windows. This temperature instability limits practical applications, particularly in regions with variable climates or in applications requiring operation across wide temperature ranges.
Electrolyte additives that effectively stabilize lithium metal interfaces often perform poorly with sodium, necessitating the development of sodium-specific additives. Current research has identified several promising candidates, including fluorinated compounds and certain sodium salts, but these solutions remain insufficient for long-term cycling stability.
The cost and sustainability of electrolyte components also present challenges. While sodium itself offers cost advantages over lithium, some specialized electrolyte components required for sodium metal protection are expensive or environmentally problematic, potentially negating sodium's inherent economic benefits.
Conventional carbonate-based electrolytes, which perform adequately in lithium-ion systems, demonstrate poor compatibility with sodium metal. These electrolytes decompose rapidly upon contact with sodium, creating porous and non-uniform SEI layers that fail to prevent further electrolyte consumption. This ongoing parasitic reaction significantly reduces coulombic efficiency and battery lifespan.
Another major obstacle is sodium dendrite formation, which occurs more readily than with lithium due to sodium's lower surface energy and faster diffusion kinetics. Current electrolytes struggle to promote uniform sodium deposition, instead facilitating dendritic growth that can penetrate separators and cause catastrophic short circuits. The mechanical properties of SEI layers formed in existing electrolytes lack sufficient strength to suppress dendrite propagation.
The solvation structure of sodium ions in electrolytes presents additional complications. Unlike lithium ions, sodium ions exhibit different coordination behaviors with solvent molecules and anions, affecting ion transport mechanisms and interfacial processes. Current electrolyte designs have not fully optimized these solvation structures to facilitate efficient sodium plating/stripping.
Temperature sensitivity further complicates electrolyte development. Many promising electrolyte formulations show acceptable performance within narrow temperature ranges but deteriorate rapidly outside these windows. This temperature instability limits practical applications, particularly in regions with variable climates or in applications requiring operation across wide temperature ranges.
Electrolyte additives that effectively stabilize lithium metal interfaces often perform poorly with sodium, necessitating the development of sodium-specific additives. Current research has identified several promising candidates, including fluorinated compounds and certain sodium salts, but these solutions remain insufficient for long-term cycling stability.
The cost and sustainability of electrolyte components also present challenges. While sodium itself offers cost advantages over lithium, some specialized electrolyte components required for sodium metal protection are expensive or environmentally problematic, potentially negating sodium's inherent economic benefits.
State-of-the-Art Electrolyte Formulations for Sodium Anodes
01 Fluorinated electrolyte additives for sodium batteries
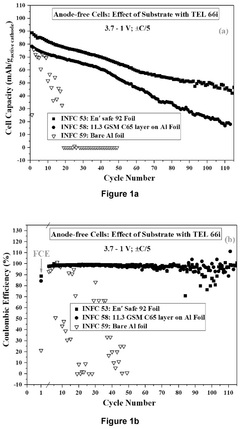
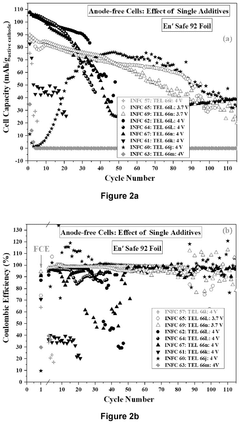
Fluorinated compounds can be incorporated into electrolytes for sodium metal anodes to form stable solid electrolyte interphase (SEI) layers. These additives, including fluoroethylene carbonate (FEC) and fluorinated ethers, help prevent dendrite formation and improve cycling stability. The fluorine-containing components react with the sodium metal surface to create a protective layer that reduces side reactions and extends battery life while maintaining high ionic conductivity.- Solvent engineering for sodium metal anodes: Optimizing solvent compositions in electrolytes is crucial for sodium metal anodes. Various solvents including ethers, carbonates, and their combinations can be tailored to form stable solid electrolyte interphases (SEI) on sodium metal surfaces. These carefully selected solvent systems help minimize side reactions, reduce dendrite formation, and improve cycling efficiency of sodium metal anodes. The right solvent composition can significantly enhance the electrochemical performance and stability of sodium-based battery systems.
- Salt additives and concentration effects: The type and concentration of salt additives in electrolytes significantly impact sodium metal anode performance. High-concentration electrolytes and salt additives can modify the SEI layer composition and structure, leading to improved sodium ion transport and reduced side reactions. Fluorinated salts and other specialized additives can enhance the stability of the sodium metal interface, suppress dendrite growth, and extend battery cycle life. Optimizing salt concentration is essential for balancing ionic conductivity and interfacial stability.
- Polymer and gel electrolyte systems: Polymer and gel-based electrolyte systems offer unique advantages for sodium metal anodes. These systems can provide mechanical stability to prevent dendrite penetration while maintaining good ionic conductivity. Polymer electrolytes can be designed with specific functional groups that interact favorably with sodium ions and the metal surface. The incorporation of polymers or gel networks into liquid electrolytes creates hybrid systems that combine the benefits of both liquid and solid electrolytes, resulting in improved safety and electrochemical performance.
- Interface engineering and protective coatings: Engineering the interface between sodium metal anodes and electrolytes is critical for battery performance. Protective coatings and artificial SEI layers can be applied to sodium metal surfaces to control reactivity and improve stability. These engineered interfaces help regulate sodium ion transport, prevent continuous electrolyte decomposition, and inhibit dendrite formation. Various coating materials including inorganic compounds, polymers, and composite structures can be tailored to create optimal interfaces that enhance the cycling efficiency and lifespan of sodium metal anodes.
- Ionic liquid-based electrolytes: Ionic liquid-based electrolytes offer promising alternatives for sodium metal batteries due to their wide electrochemical stability windows, low volatility, and high thermal stability. These electrolytes can be designed with specific cation and anion combinations to optimize interactions with sodium metal surfaces. The unique properties of ionic liquids help suppress dendrite formation and reduce side reactions at the electrode-electrolyte interface. By incorporating ionic liquids into electrolyte formulations, the safety and performance of sodium metal anodes can be significantly improved, especially at elevated temperatures.
02 Ether-based electrolyte systems
Ether-based solvents such as glymes (mono-, di-, and tetraglyme) provide excellent compatibility with sodium metal anodes due to their high donor number and ability to solvate sodium ions effectively. These electrolytes demonstrate reduced reactivity with sodium metal compared to carbonate-based systems, resulting in improved coulombic efficiency and cycle life. The flexible coordination structure of ethers allows for faster sodium ion transport while minimizing parasitic reactions at the electrode-electrolyte interface.Expand Specific Solutions03 Sodium salt concentration and composition
The type and concentration of sodium salts in the electrolyte significantly impact sodium metal anode performance. Salts such as NaPF6, NaFSI, NaTFSI, and NaClO4 offer different ionic conductivity and interfacial stability properties. High-concentration or localized high-concentration electrolytes (HCEs/LHCEs) reduce free solvent molecules, minimizing parasitic reactions with sodium metal. The anion chemistry plays a crucial role in SEI formation and stability, with larger anions often providing better passivation of the reactive sodium surface.Expand Specific Solutions04 Polymer and gel electrolyte systems
Polymer and gel-based electrolytes offer improved safety and mechanical stability for sodium metal batteries. These systems incorporate polymers such as polyethylene oxide (PEO), PVDF, or PMMA with sodium salts to create flexible electrolyte membranes that can accommodate volume changes during cycling. The semi-solid nature of these electrolytes helps suppress sodium dendrite growth while maintaining adequate ionic conductivity. Some formulations include ceramic fillers to enhance mechanical properties and sodium ion transport numbers.Expand Specific Solutions05 Ionic liquid-based electrolytes
Ionic liquids serve as promising electrolyte components for sodium metal anodes due to their negligible vapor pressure, wide electrochemical stability window, and thermal stability. When combined with sodium salts, ionic liquids such as pyrrolidinium or imidazolium-based compounds can effectively suppress dendrite formation and improve the interfacial stability between the electrolyte and sodium metal. These non-flammable electrolytes enhance safety while providing good ionic conductivity and long-term cycling performance.Expand Specific Solutions
Leading Research Groups and Companies in Sodium Battery Field
The sodium metal anode electrolyte optimization landscape is currently in the early growth phase, with a market projected to expand significantly as sodium-ion batteries emerge as a cost-effective alternative to lithium-ion technology. Key players represent diverse sectors including academic institutions (Cornell University, Central South University), research organizations (Centre National de la Recherche Scientifique), and commercial entities (Faradion, CATL, Sila Nanotechnologies). Technical maturity varies considerably across the ecosystem, with established companies like BASF and BMW investing in sodium battery technology while specialized startups like BroadBit Batteries and Sicona Battery Technologies focus on innovative electrolyte formulations. Academic-industry partnerships are accelerating development, though commercial deployment remains limited compared to traditional battery technologies, indicating significant growth potential in this emerging field.
Faradion Ltd.
Technical Solution: Faradion has pioneered a comprehensive approach to electrolyte optimization for sodium metal anodes centered around their patented "NaSICON-compatible" electrolyte formulations. Their technology employs a strategic blend of glyme-based solvents (particularly diglyme and tetraglyme) with carefully selected sodium salts to create a stable interface with sodium metal. Faradion's research has demonstrated that their electrolytes form a sodium-fluoride rich SEI layer that effectively prevents continuous electrolyte decomposition while maintaining high sodium-ion conductivity. The company utilizes sodium perchlorate (NaClO4) and sodium hexafluorophosphate (NaPF6) salts at optimized concentrations (0.5-1.0M), often in combination, to balance conductivity and interfacial stability requirements. Their electrolyte systems incorporate small amounts (<5%) of fluorinated additives that decompose preferentially on the sodium surface to create a protective film that inhibits dendrite formation. Faradion has published results showing their electrolyte formulations enable sodium metal anodes with coulombic efficiencies approaching 98% over hundreds of cycles, with significantly reduced dendrite formation compared to conventional electrolytes.
Strengths: Faradion's electrolytes demonstrate excellent compatibility with their proprietary cathode materials, creating a holistic cell chemistry approach. Their formulations show good low-temperature performance and relatively low cost compared to lithium-based systems. Weaknesses: The glyme-based solvents may have higher volatility than some alternatives, potentially creating safety concerns. The system may be more sensitive to moisture contamination during manufacturing, requiring stringent production controls.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a comprehensive electrolyte optimization strategy for sodium metal anodes focusing on multi-component solvent systems. Their approach combines fluoroethylene carbonate (FEC) as a primary additive with ether-based solvents to form stable solid electrolyte interphases (SEI) on sodium metal surfaces. The company has pioneered the use of sodium bis(fluorosulfonyl)imide (NaFSI) salt in combination with sodium hexafluorophosphate (NaPF6) at optimized concentrations (0.8-1.2M) to enhance ionic conductivity while minimizing corrosion issues. CATL's research has demonstrated that their electrolyte formulations can effectively suppress sodium dendrite growth through the formation of a uniform, sodium-ion conductive SEI layer. Their electrolytes incorporate trace amounts of functional additives such as fluorinated compounds and sodium nitrate to further enhance the mechanical properties and chemical stability of the SEI layer, resulting in sodium metal anodes with coulombic efficiencies exceeding 99% over hundreds of cycles.
Strengths: CATL's formulations demonstrate superior cycling stability with high coulombic efficiency, effectively addressing the dendrite formation issue. Their multi-component approach allows for customization based on specific cell designs and operating conditions. Weaknesses: The complex formulations may increase production costs and quality control challenges. Some additives used may have environmental concerns and limited availability at scale.
Critical Patents and Research on Sodium Electrolyte Interfaces
An electrolyte composition for a highly stable and reversible sodium-metal anode for high-energy rechargeable batteries
PatentPendingIN202211027550A
Innovation
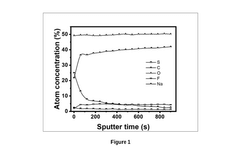
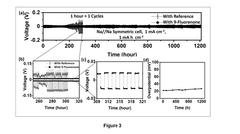
- An electrolyte composition comprising an organic aromatic additive, 9-Fluorenone, which solubilizes in ether-based electrolytes and electrochemically decomposes to form a protective layer, stabilizing the sodium-metal anode and reducing sodium deposition overpotential, enabling dendrite-free and uniform sodium deposition.
Electrolyte compositions
PatentPendingUS20250226448A1
Innovation
- Development of a non-aqueous electrolyte composition comprising sodium-containing salts and a solvent system with specific additives such as glyme-based solvents and additives like sulfur-, boron-, nitrile-, and phosphorous-containing compounds, within defined weight percentages, to enhance electrochemical performance and stability.
Sustainability and Resource Considerations for Sodium Technologies
The sustainability profile of sodium-based battery technologies represents a significant advantage over traditional lithium-ion systems. Sodium is the sixth most abundant element in the Earth's crust, comprising approximately 2.8% of the planet's surface composition, compared to lithium's mere 0.002%. This abundance translates directly into reduced extraction impacts and substantially lower raw material costs, with sodium commodities typically priced at 1/30th the cost of lithium equivalents.
Environmental impact assessments of sodium metal anode technologies reveal promising sustainability metrics. The carbon footprint associated with sodium extraction and processing is estimated to be 60-70% lower than comparable lithium operations. This reduction stems primarily from simpler extraction processes that require less energy input and fewer chemical reagents, as sodium can often be derived from seawater or abundant mineral deposits without extensive purification steps.
Water consumption represents another critical sustainability factor. Conventional lithium extraction in salt flats can consume between 500,000 to 2 million liters of water per ton of lithium produced. In contrast, sodium extraction methodologies typically require 70-80% less water, significantly reducing pressure on water resources in production regions.
The geographical distribution of sodium resources presents additional sustainability advantages. Unlike lithium, which is concentrated in the "Lithium Triangle" of South America and a few other locations, sodium resources are globally distributed, reducing geopolitical supply risks and transportation-related emissions. This distribution enables more localized production chains and reduces the carbon footprint associated with material transportation.
End-of-life considerations also favor sodium technologies. Preliminary recycling studies indicate that sodium components from spent batteries can be recovered using simpler hydrometallurgical processes than those required for lithium. Recovery rates of up to 90% have been demonstrated in laboratory settings, though commercial-scale recycling infrastructure remains underdeveloped.
When optimizing electrolyte compositions for sodium metal anodes, sustainability considerations should influence formulation choices. Bio-derived solvents and less toxic salt alternatives can significantly reduce the environmental impact without compromising electrochemical performance. For instance, replacing fluorinated components with more environmentally benign alternatives could reduce the persistence of harmful compounds in the environment while maintaining adequate electrochemical stability windows.
Resource efficiency in electrolyte design also extends to minimizing the use of critical materials. Electrolyte additives based on abundant elements rather than rare metals or compounds can ensure long-term scalability while maintaining performance metrics. This approach aligns with circular economy principles and reduces vulnerability to supply chain disruptions.
Environmental impact assessments of sodium metal anode technologies reveal promising sustainability metrics. The carbon footprint associated with sodium extraction and processing is estimated to be 60-70% lower than comparable lithium operations. This reduction stems primarily from simpler extraction processes that require less energy input and fewer chemical reagents, as sodium can often be derived from seawater or abundant mineral deposits without extensive purification steps.
Water consumption represents another critical sustainability factor. Conventional lithium extraction in salt flats can consume between 500,000 to 2 million liters of water per ton of lithium produced. In contrast, sodium extraction methodologies typically require 70-80% less water, significantly reducing pressure on water resources in production regions.
The geographical distribution of sodium resources presents additional sustainability advantages. Unlike lithium, which is concentrated in the "Lithium Triangle" of South America and a few other locations, sodium resources are globally distributed, reducing geopolitical supply risks and transportation-related emissions. This distribution enables more localized production chains and reduces the carbon footprint associated with material transportation.
End-of-life considerations also favor sodium technologies. Preliminary recycling studies indicate that sodium components from spent batteries can be recovered using simpler hydrometallurgical processes than those required for lithium. Recovery rates of up to 90% have been demonstrated in laboratory settings, though commercial-scale recycling infrastructure remains underdeveloped.
When optimizing electrolyte compositions for sodium metal anodes, sustainability considerations should influence formulation choices. Bio-derived solvents and less toxic salt alternatives can significantly reduce the environmental impact without compromising electrochemical performance. For instance, replacing fluorinated components with more environmentally benign alternatives could reduce the persistence of harmful compounds in the environment while maintaining adequate electrochemical stability windows.
Resource efficiency in electrolyte design also extends to minimizing the use of critical materials. Electrolyte additives based on abundant elements rather than rare metals or compounds can ensure long-term scalability while maintaining performance metrics. This approach aligns with circular economy principles and reduces vulnerability to supply chain disruptions.
Safety Standards and Testing Protocols for Sodium Battery Systems
The development of sodium battery systems necessitates comprehensive safety standards and testing protocols to ensure their reliable and safe operation. Current safety frameworks for sodium-based batteries are largely adapted from lithium-ion battery standards, requiring significant modifications to address the unique properties of sodium metal anodes and their electrolyte compositions.
International organizations including IEC, ISO, and UL have begun developing specific standards for sodium battery systems, with particular attention to thermal runaway prevention, electrolyte stability, and dendrite formation mitigation. These standards typically require rigorous testing of electrolyte compositions under various conditions to evaluate their safety performance when paired with sodium metal anodes.
Key safety testing protocols for sodium-based electrolytes include thermal stability assessments, which evaluate electrolyte decomposition temperatures and potential for exothermic reactions. These tests typically involve differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) to determine thermal runaway thresholds specific to sodium systems.
Electrochemical stability window testing represents another critical protocol, measuring the voltage range within which the electrolyte remains stable without decomposition. This is particularly important for sodium metal anodes, as electrolyte decomposition can lead to safety hazards and performance degradation. Cyclic voltammetry and impedance spectroscopy are commonly employed techniques for these assessments.
Abuse testing protocols have been established to evaluate system response under extreme conditions, including overcharging, short-circuiting, mechanical impact, and puncture tests. These protocols must be specifically calibrated for sodium systems, as their failure modes differ significantly from lithium-ion batteries due to sodium's distinct chemical properties and reactivity patterns.
Transportation safety regulations present additional considerations, with UN 38.3 testing requirements being adapted for sodium battery systems. These include altitude simulation, thermal cycling, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests—all requiring specific modifications to account for sodium metal anode and electrolyte interactions.
Cell-level safety certification typically requires nail penetration tests, crush tests, and external heating tests to evaluate the safety of the electrolyte-anode interface under mechanical failure conditions. The optimization of electrolyte composition directly impacts performance in these critical safety tests, with flame-retardant additives and ionic liquid components showing promising results in improving safety margins.
Industry-academic collaborations are currently working to standardize testing methodologies specifically for sodium metal anodes with various electrolyte formulations, aiming to establish benchmarks that can accelerate commercial development while ensuring public safety. These emerging protocols will be essential for the widespread adoption of sodium battery technology across various applications.
International organizations including IEC, ISO, and UL have begun developing specific standards for sodium battery systems, with particular attention to thermal runaway prevention, electrolyte stability, and dendrite formation mitigation. These standards typically require rigorous testing of electrolyte compositions under various conditions to evaluate their safety performance when paired with sodium metal anodes.
Key safety testing protocols for sodium-based electrolytes include thermal stability assessments, which evaluate electrolyte decomposition temperatures and potential for exothermic reactions. These tests typically involve differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) to determine thermal runaway thresholds specific to sodium systems.
Electrochemical stability window testing represents another critical protocol, measuring the voltage range within which the electrolyte remains stable without decomposition. This is particularly important for sodium metal anodes, as electrolyte decomposition can lead to safety hazards and performance degradation. Cyclic voltammetry and impedance spectroscopy are commonly employed techniques for these assessments.
Abuse testing protocols have been established to evaluate system response under extreme conditions, including overcharging, short-circuiting, mechanical impact, and puncture tests. These protocols must be specifically calibrated for sodium systems, as their failure modes differ significantly from lithium-ion batteries due to sodium's distinct chemical properties and reactivity patterns.
Transportation safety regulations present additional considerations, with UN 38.3 testing requirements being adapted for sodium battery systems. These include altitude simulation, thermal cycling, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests—all requiring specific modifications to account for sodium metal anode and electrolyte interactions.
Cell-level safety certification typically requires nail penetration tests, crush tests, and external heating tests to evaluate the safety of the electrolyte-anode interface under mechanical failure conditions. The optimization of electrolyte composition directly impacts performance in these critical safety tests, with flame-retardant additives and ionic liquid components showing promising results in improving safety margins.
Industry-academic collaborations are currently working to standardize testing methodologies specifically for sodium metal anodes with various electrolyte formulations, aiming to establish benchmarks that can accelerate commercial development while ensuring public safety. These emerging protocols will be essential for the widespread adoption of sodium battery technology across various applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!