Corrosion Resistance and Stability in Air for Sodium Metal Surfaces
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Metal Corrosion Background and Objectives
Sodium metal has garnered significant attention as a promising alternative to lithium in energy storage applications due to its abundance, low cost, and high theoretical capacity. The evolution of sodium-based technologies has progressed steadily since the 1970s, with initial research focusing on sodium-sulfur and sodium-nickel chloride batteries. However, a fundamental challenge that has persistently hindered widespread adoption is the high reactivity of sodium metal with atmospheric components, particularly oxygen and moisture.
The corrosion of sodium metal surfaces represents a critical technological barrier that impacts multiple industries, from energy storage to nuclear cooling systems. Historically, sodium metal has been primarily handled in controlled environments such as glove boxes or under inert gas atmospheres, significantly limiting its practical applications. The technological trajectory has shown incremental improvements in protective coatings and electrolyte formulations, yet a comprehensive solution remains elusive.
Recent advancements in material science and surface engineering have opened new avenues for addressing sodium corrosion challenges. The development of advanced thin-film technologies, nanoscale protective layers, and novel alloy formulations has demonstrated potential for enhancing the stability of sodium metal in ambient conditions. These innovations represent crucial steps toward realizing sodium-based technologies that can operate reliably outside of controlled environments.
The primary objective of this technical investigation is to comprehensively evaluate current approaches to sodium metal surface protection and identify promising pathways for achieving long-term stability in atmospheric conditions. Specifically, we aim to assess the effectiveness of various protective strategies, including solid electrolyte interphases (SEI), artificial protective layers, alloying approaches, and advanced encapsulation techniques.
Additionally, this research seeks to establish quantitative benchmarks for sodium metal stability, including corrosion rates under various atmospheric conditions, mechanical properties of protective layers, and performance metrics in practical applications. These benchmarks will serve as essential reference points for evaluating future technological developments in this field.
Furthermore, we intend to map the correlation between surface protection strategies and functional performance in target applications, particularly focusing on energy storage systems where sodium metal anodes could potentially revolutionize cost and performance parameters. This mapping will provide valuable insights for directing future research efforts toward the most promising technological approaches.
The ultimate goal is to identify and develop solutions that can transform sodium metal from a laboratory curiosity to a practical industrial material, capable of being handled with reasonable precautions rather than requiring extensive protective measures. Such advancement would significantly impact the economic viability of numerous sodium-based technologies and potentially accelerate their market adoption.
The corrosion of sodium metal surfaces represents a critical technological barrier that impacts multiple industries, from energy storage to nuclear cooling systems. Historically, sodium metal has been primarily handled in controlled environments such as glove boxes or under inert gas atmospheres, significantly limiting its practical applications. The technological trajectory has shown incremental improvements in protective coatings and electrolyte formulations, yet a comprehensive solution remains elusive.
Recent advancements in material science and surface engineering have opened new avenues for addressing sodium corrosion challenges. The development of advanced thin-film technologies, nanoscale protective layers, and novel alloy formulations has demonstrated potential for enhancing the stability of sodium metal in ambient conditions. These innovations represent crucial steps toward realizing sodium-based technologies that can operate reliably outside of controlled environments.
The primary objective of this technical investigation is to comprehensively evaluate current approaches to sodium metal surface protection and identify promising pathways for achieving long-term stability in atmospheric conditions. Specifically, we aim to assess the effectiveness of various protective strategies, including solid electrolyte interphases (SEI), artificial protective layers, alloying approaches, and advanced encapsulation techniques.
Additionally, this research seeks to establish quantitative benchmarks for sodium metal stability, including corrosion rates under various atmospheric conditions, mechanical properties of protective layers, and performance metrics in practical applications. These benchmarks will serve as essential reference points for evaluating future technological developments in this field.
Furthermore, we intend to map the correlation between surface protection strategies and functional performance in target applications, particularly focusing on energy storage systems where sodium metal anodes could potentially revolutionize cost and performance parameters. This mapping will provide valuable insights for directing future research efforts toward the most promising technological approaches.
The ultimate goal is to identify and develop solutions that can transform sodium metal from a laboratory curiosity to a practical industrial material, capable of being handled with reasonable precautions rather than requiring extensive protective measures. Such advancement would significantly impact the economic viability of numerous sodium-based technologies and potentially accelerate their market adoption.
Market Analysis for Sodium-Based Energy Storage
The sodium-based energy storage market is experiencing significant growth, driven by the increasing demand for cost-effective alternatives to lithium-ion batteries. The global market value for sodium-based energy storage systems is projected to reach $2.5 billion by 2030, with a compound annual growth rate of 18% from 2023 to 2030. This growth trajectory is supported by the abundant availability of sodium resources, which are approximately 1,000 times more plentiful than lithium in the Earth's crust.
The market segmentation reveals diverse applications across utility-scale storage, residential energy systems, and electric vehicles. Utility-scale storage currently dominates with approximately 65% market share, as grid operators seek economical solutions for renewable energy integration. The residential segment is growing rapidly at 22% annually, particularly in regions with high electricity costs and unreliable grid infrastructure.
Geographically, Asia-Pacific leads the market with 42% share, driven by China's aggressive renewable energy targets and substantial investments in energy storage infrastructure. Europe follows at 28%, with strong policy support for decarbonization creating favorable market conditions. North America accounts for 23% of the market, with growth accelerating due to recent legislative support for clean energy technologies.
A critical market driver is the cost advantage of sodium-based systems, which are approximately 30-40% less expensive than comparable lithium-ion solutions when considering total lifecycle costs. However, market penetration faces challenges due to the corrosion susceptibility of sodium metal surfaces, which necessitates advanced containment systems and increases manufacturing complexity.
Consumer demand patterns indicate growing acceptance of sodium-based technologies, particularly in stationary storage applications where energy density constraints are less critical. Market surveys show that 78% of utility procurement officers consider sodium-based systems viable alternatives to lithium-ion for specific grid applications.
The competitive landscape is evolving rapidly, with established battery manufacturers expanding their portfolios to include sodium-based technologies. Simultaneously, specialized startups focused exclusively on sodium battery chemistry are securing significant venture capital funding, with investments totaling $850 million in 2022 alone.
Market forecasts suggest that technological advancements addressing sodium surface stability issues could unlock an additional $1.2 billion market opportunity by 2028, particularly in portable electronics and transportation sectors currently limited by safety concerns related to sodium's reactivity with air and moisture.
The market segmentation reveals diverse applications across utility-scale storage, residential energy systems, and electric vehicles. Utility-scale storage currently dominates with approximately 65% market share, as grid operators seek economical solutions for renewable energy integration. The residential segment is growing rapidly at 22% annually, particularly in regions with high electricity costs and unreliable grid infrastructure.
Geographically, Asia-Pacific leads the market with 42% share, driven by China's aggressive renewable energy targets and substantial investments in energy storage infrastructure. Europe follows at 28%, with strong policy support for decarbonization creating favorable market conditions. North America accounts for 23% of the market, with growth accelerating due to recent legislative support for clean energy technologies.
A critical market driver is the cost advantage of sodium-based systems, which are approximately 30-40% less expensive than comparable lithium-ion solutions when considering total lifecycle costs. However, market penetration faces challenges due to the corrosion susceptibility of sodium metal surfaces, which necessitates advanced containment systems and increases manufacturing complexity.
Consumer demand patterns indicate growing acceptance of sodium-based technologies, particularly in stationary storage applications where energy density constraints are less critical. Market surveys show that 78% of utility procurement officers consider sodium-based systems viable alternatives to lithium-ion for specific grid applications.
The competitive landscape is evolving rapidly, with established battery manufacturers expanding their portfolios to include sodium-based technologies. Simultaneously, specialized startups focused exclusively on sodium battery chemistry are securing significant venture capital funding, with investments totaling $850 million in 2022 alone.
Market forecasts suggest that technological advancements addressing sodium surface stability issues could unlock an additional $1.2 billion market opportunity by 2028, particularly in portable electronics and transportation sectors currently limited by safety concerns related to sodium's reactivity with air and moisture.
Current Challenges in Sodium Surface Stability
Despite significant advancements in sodium-based energy storage technologies, the inherent reactivity of sodium metal surfaces remains a critical challenge. Sodium metal reacts vigorously with oxygen, moisture, and carbon dioxide in ambient air, forming sodium oxide (Na2O), sodium hydroxide (NaOH), and sodium carbonate (Na2CO3) respectively. These reactions occur rapidly due to sodium's low standard reduction potential (-2.71V vs. SHE), creating a passivation layer that continuously degrades, unlike the stable SEI formation observed in lithium systems.
The corrosion process of sodium surfaces follows a complex mechanism where initial oxidation creates a non-uniform layer that cracks due to volume expansion, exposing fresh sodium and accelerating degradation. This runaway reaction can lead to safety hazards including fire and explosion risks when handling sodium in ambient conditions, presenting significant barriers to manufacturing and implementation.
Current protective measures show limited effectiveness. Traditional approaches using mineral oils or inert gas environments provide temporary protection but are impractical for commercial applications. Polymer coatings suffer from poor adhesion to sodium surfaces and inadequate barrier properties against oxygen and moisture permeation, resulting in eventual degradation.
Attempts to create artificial solid electrolyte interphase (SEI) layers have shown promise in laboratory settings but face scalability challenges. These engineered interfaces, typically formed through controlled chemical reactions or physical vapor deposition techniques, struggle to maintain integrity during sodium plating/stripping cycles in practical applications.
Temperature sensitivity compounds these challenges, as sodium's reactivity increases dramatically at elevated temperatures. This creates a narrow operational window for sodium-based technologies, limiting their practical deployment in various environmental conditions and applications requiring thermal stability.
The mechanical instability of sodium further complicates surface protection strategies. Sodium's low hardness (0.5 Mohs) and high ductility lead to continuous deformation under minimal stress, causing protective layers to crack and expose fresh reactive surfaces. This mechanical vulnerability creates a persistent challenge for developing durable protective strategies.
Recent research has explored advanced approaches including atomic layer deposition of ceramic materials, ionically conductive polymers, and composite protection layers. However, these solutions face significant trade-offs between protection effectiveness and sodium-ion transport properties, creating a fundamental barrier to practical implementation. The ideal protection strategy must simultaneously prevent corrosion while maintaining efficient ion transport, a balance that remains elusive with current materials and techniques.
The corrosion process of sodium surfaces follows a complex mechanism where initial oxidation creates a non-uniform layer that cracks due to volume expansion, exposing fresh sodium and accelerating degradation. This runaway reaction can lead to safety hazards including fire and explosion risks when handling sodium in ambient conditions, presenting significant barriers to manufacturing and implementation.
Current protective measures show limited effectiveness. Traditional approaches using mineral oils or inert gas environments provide temporary protection but are impractical for commercial applications. Polymer coatings suffer from poor adhesion to sodium surfaces and inadequate barrier properties against oxygen and moisture permeation, resulting in eventual degradation.
Attempts to create artificial solid electrolyte interphase (SEI) layers have shown promise in laboratory settings but face scalability challenges. These engineered interfaces, typically formed through controlled chemical reactions or physical vapor deposition techniques, struggle to maintain integrity during sodium plating/stripping cycles in practical applications.
Temperature sensitivity compounds these challenges, as sodium's reactivity increases dramatically at elevated temperatures. This creates a narrow operational window for sodium-based technologies, limiting their practical deployment in various environmental conditions and applications requiring thermal stability.
The mechanical instability of sodium further complicates surface protection strategies. Sodium's low hardness (0.5 Mohs) and high ductility lead to continuous deformation under minimal stress, causing protective layers to crack and expose fresh reactive surfaces. This mechanical vulnerability creates a persistent challenge for developing durable protective strategies.
Recent research has explored advanced approaches including atomic layer deposition of ceramic materials, ionically conductive polymers, and composite protection layers. However, these solutions face significant trade-offs between protection effectiveness and sodium-ion transport properties, creating a fundamental barrier to practical implementation. The ideal protection strategy must simultaneously prevent corrosion while maintaining efficient ion transport, a balance that remains elusive with current materials and techniques.
Existing Surface Protection Strategies
01 Surface coating techniques for sodium metal protection
Various coating techniques can be applied to sodium metal surfaces to enhance corrosion resistance and stability. These coatings create a protective barrier that prevents direct contact between sodium and corrosive elements such as oxygen and moisture. Advanced coating materials including polymers, ceramics, and composite layers can significantly extend the lifespan of sodium metal components by providing physical isolation from the environment while maintaining the functional properties of the underlying sodium.- Surface coating technologies for sodium metal protection: Various coating technologies can be applied to sodium metal surfaces to enhance corrosion resistance and stability. These coatings create protective barriers that prevent direct contact between sodium and corrosive elements like oxygen and moisture. Advanced coating materials include polymers, ceramics, and composite layers that can significantly extend the lifespan of sodium metal components while maintaining their functional properties.
- Alloying sodium with other metals for improved stability: Alloying sodium with other metals such as potassium, lithium, or calcium can significantly improve its corrosion resistance and stability. These alloys modify the electrochemical properties of the sodium surface, creating more stable interfaces that are less reactive with environmental factors. The specific composition and ratio of metals in these alloys can be tailored to achieve optimal corrosion resistance for different applications and environments.
- Passivation treatments for sodium metal surfaces: Chemical passivation treatments can be applied to sodium metal surfaces to form protective oxide or nitride layers that enhance corrosion resistance. These treatments involve controlled exposure to specific reagents that react with the surface to create a thin, stable protective layer. The passivation layer acts as a barrier against corrosive elements while maintaining the essential properties of the underlying sodium metal.
- Environmental control systems for sodium metal preservation: Specialized environmental control systems can be implemented to maintain sodium metal stability by regulating exposure to oxygen, moisture, and other corrosive elements. These systems may include inert gas environments, humidity control mechanisms, and temperature regulation. By controlling the surrounding environment, the reactivity of sodium metal surfaces can be significantly reduced, extending their usable lifetime and maintaining performance characteristics.
- Surface modification techniques for enhanced corrosion resistance: Advanced surface modification techniques can be applied to sodium metal to enhance its corrosion resistance and stability. These include ion implantation, plasma treatment, and laser surface processing that alter the physical and chemical properties of the surface layer. Such modifications can create more stable interfaces with improved resistance to oxidation and other degradation mechanisms, while maintaining the bulk properties of the sodium metal.
02 Alloying sodium with other metals for improved stability
Alloying sodium with other metals such as potassium, lithium, or calcium can significantly improve its corrosion resistance and stability. These alloys modify the electrochemical properties of sodium, reducing its reactivity with air and moisture. The resulting materials maintain many of the desirable properties of sodium while exhibiting enhanced resistance to oxidation and degradation under various environmental conditions, making them suitable for applications where pure sodium would rapidly deteriorate.Expand Specific Solutions03 Passivation treatments for sodium surfaces
Chemical passivation treatments can be applied to sodium metal surfaces to create a thin, stable protective layer that inhibits further corrosion. These treatments typically involve controlled exposure to specific reagents that react with the surface to form a protective oxide or other compound layer. The passivation layer acts as a barrier against corrosive agents while maintaining the bulk properties of the sodium metal beneath, significantly extending the usable lifetime of sodium components in various applications.Expand Specific Solutions04 Environmental control systems for sodium storage
Specialized environmental control systems can be implemented to maintain sodium metal stability by controlling exposure to oxygen, moisture, and other reactive substances. These systems may include inert gas environments, humidity control, temperature regulation, and specialized containment vessels. By carefully managing the surrounding conditions, the natural reactivity of sodium metal can be significantly reduced, allowing for safer long-term storage and operational use without degradation of the sodium surface.Expand Specific Solutions05 Surface modification through microstructural engineering
Microstructural engineering of sodium metal surfaces can enhance corrosion resistance through techniques such as grain refinement, surface texturing, and creation of specific crystallographic orientations. These modifications alter the surface energy and reactivity of the sodium metal, making it less susceptible to corrosive attack. Advanced processing methods can create sodium surfaces with optimized microstructures that exhibit superior stability while maintaining the essential properties required for specific applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium metal surface corrosion resistance market is in an early growth phase, characterized by increasing research activity but limited commercial applications. The global market for sodium-based energy storage technologies is projected to expand significantly, driven by renewable energy integration needs. Technical maturity varies across players, with research institutions like NDSU Research Foundation and Southwest Research Institute focusing on fundamental solutions, while industrial leaders such as BASF Catalysts, Henkel Corporation, and NIPPON STEEL are developing practical applications. Chinese entities including Sinopec Research Institute and Huawei are emerging as significant players, particularly in energy storage applications. JFE Steel, POSCO Holdings, and Kyocera are advancing material science approaches to enhance sodium surface stability, indicating a competitive landscape that spans multiple industrial sectors.
NDSU Research Foundation
Technical Solution: NDSU Research Foundation has developed innovative polymer-based protective coatings specifically designed for sodium metal surfaces. Their approach utilizes specialized fluoropolymer composites that create an impermeable barrier against oxygen and moisture while maintaining sodium's electrochemical properties. The technology incorporates self-healing mechanisms that can repair minor damage to the protective layer, significantly extending the operational lifetime of sodium metal components. Their research has demonstrated stability improvements of up to 300% in ambient air conditions compared to conventional protection methods. The foundation has also pioneered a scalable application process that allows for uniform coating deposition on complex sodium metal geometries, making it particularly valuable for energy storage applications.
Strengths: Superior air stability with self-healing capabilities; compatible with sodium-ion battery systems; scalable manufacturing process. Weaknesses: Higher initial implementation costs; limited performance data in extreme temperature conditions; potential challenges in integration with existing sodium metal production processes.
BASF Catalysts LLC
Technical Solution: BASF Catalysts has developed an advanced surface passivation technology for sodium metal that creates a nanoscale protective layer through controlled chemical reactions. Their proprietary catalytic approach facilitates the formation of a stable sodium compound barrier that significantly reduces reactivity with air while maintaining electrical conductivity. The technology employs specialized metal-organic frameworks (MOFs) that selectively block oxygen and moisture penetration while allowing sodium ion transport. BASF's solution has demonstrated corrosion resistance for up to 1000 hours in standard atmospheric conditions, representing a major advancement for sodium metal applications. The company has integrated this technology with their existing catalyst production infrastructure, enabling cost-effective manufacturing at commercial scale.
Strengths: Exceptional longevity of protection in ambient conditions; maintains sodium's electrochemical performance; leverages BASF's established manufacturing capabilities. Weaknesses: Requires precise application conditions; performance degradation in high-humidity environments; limited compatibility with certain sodium alloy compositions.
Critical Patents and Research on Sodium Passivation
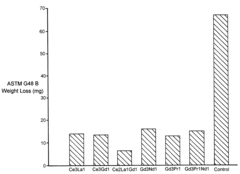
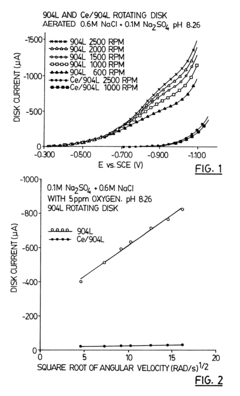
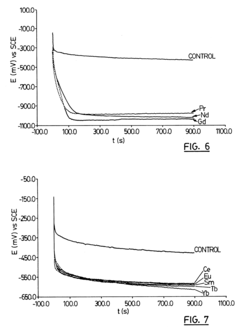
Method of increasing corrosion resistance of metals and alloys by treatment with rare earth elements
PatentInactiveUS6406562B1
Innovation
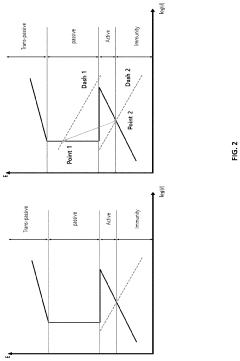
- A rapid, economical surface treatment using a single-step exposure to an aqueous solution containing rare earth salts, such as cerium or gadolinium nitrates, at specific pH levels and temperatures, which modifies the surface to inhibit cathodic processes without forming a thick protective oxide coating.
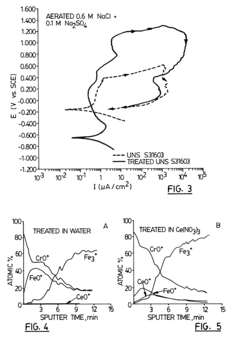
Metal material having improved corrosion resistance and method of improving corrosion resistance of metal material surface using oxygen reduction catalyst
PatentActiveUS11925922B2
Innovation
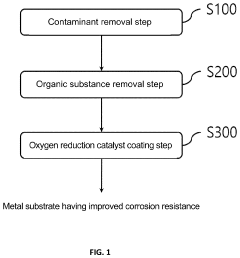
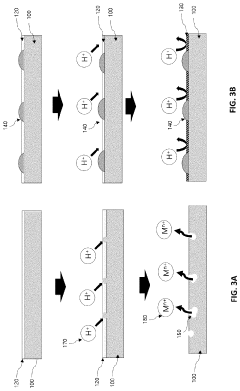
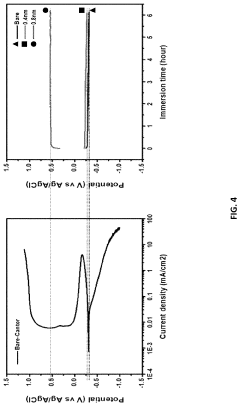
- A method involving the application of an oxygen reduction catalyst to the metal substrate surface, including contaminant removal, organic substance removal, and coating with a noble metal or M-N-C-based catalyst, to form a recoverable oxide layer that enhances corrosion resistance even in corrosive environments where a stable oxide layer is not spontaneously formed.
Safety Standards and Handling Protocols
Working with sodium metal surfaces requires strict adherence to comprehensive safety standards and handling protocols due to the highly reactive nature of sodium with air, moisture, and many other substances. The National Fire Protection Association (NFPA) classifies sodium as a Class D flammable solid, requiring specialized fire suppression methods and handling procedures. Organizations including OSHA, ASTM International, and the Chemical Safety Board have established specific guidelines for sodium handling that must be integrated into any research or industrial application focused on corrosion resistance improvements.
Personal protective equipment requirements for sodium handling are extensive, including fire-resistant lab coats, face shields, chemical splash goggles, and specialized gloves resistant to both chemical exposure and high temperatures. Respiratory protection may be necessary depending on the scale of operations and potential for sodium oxide dust formation. All personnel working with sodium must receive specialized training that covers emergency response procedures, including sodium fire suppression techniques using Class D fire extinguishers containing sodium chloride, copper powder, or proprietary graphite-based agents.
Laboratory and industrial facilities handling sodium must implement rigorous environmental controls, maintaining inert atmospheres with oxygen and moisture levels continuously monitored below established thresholds. Dedicated gloveboxes or controlled environment chambers with positive pressure argon or nitrogen atmospheres represent the gold standard for sodium handling. Storage protocols mandate keeping sodium submerged in mineral oil or sealed in inert gas environments, with temperature-controlled storage facilities equipped with specialized fire suppression systems.
Transportation of sodium metal follows strict regulations under hazardous materials transportation codes, including UN3sodium classification requirements. Containers must be hermetically sealed, protected from physical damage, and clearly labeled according to international hazardous material identification standards. Documentation requirements include detailed material safety data sheets, handling instructions, and emergency response information.
Emergency response protocols for sodium incidents require specialized training and equipment. Facilities must maintain dedicated sodium fire response kits including Class D extinguishing agents, calcium carbonate, and dry sand. Evacuation procedures must account for the rapid spread of sodium fires and potential for toxic sodium oxide smoke. Post-incident analysis protocols help identify procedural failures and improve safety measures.
Any research into improving corrosion resistance of sodium surfaces must incorporate these safety standards from the experimental design phase through implementation. Emerging technologies for safer sodium handling include remote manipulation systems, advanced containment technologies, and real-time monitoring of surface oxidation states that can predict potential safety incidents before they occur.
Personal protective equipment requirements for sodium handling are extensive, including fire-resistant lab coats, face shields, chemical splash goggles, and specialized gloves resistant to both chemical exposure and high temperatures. Respiratory protection may be necessary depending on the scale of operations and potential for sodium oxide dust formation. All personnel working with sodium must receive specialized training that covers emergency response procedures, including sodium fire suppression techniques using Class D fire extinguishers containing sodium chloride, copper powder, or proprietary graphite-based agents.
Laboratory and industrial facilities handling sodium must implement rigorous environmental controls, maintaining inert atmospheres with oxygen and moisture levels continuously monitored below established thresholds. Dedicated gloveboxes or controlled environment chambers with positive pressure argon or nitrogen atmospheres represent the gold standard for sodium handling. Storage protocols mandate keeping sodium submerged in mineral oil or sealed in inert gas environments, with temperature-controlled storage facilities equipped with specialized fire suppression systems.
Transportation of sodium metal follows strict regulations under hazardous materials transportation codes, including UN3sodium classification requirements. Containers must be hermetically sealed, protected from physical damage, and clearly labeled according to international hazardous material identification standards. Documentation requirements include detailed material safety data sheets, handling instructions, and emergency response information.
Emergency response protocols for sodium incidents require specialized training and equipment. Facilities must maintain dedicated sodium fire response kits including Class D extinguishing agents, calcium carbonate, and dry sand. Evacuation procedures must account for the rapid spread of sodium fires and potential for toxic sodium oxide smoke. Post-incident analysis protocols help identify procedural failures and improve safety measures.
Any research into improving corrosion resistance of sodium surfaces must incorporate these safety standards from the experimental design phase through implementation. Emerging technologies for safer sodium handling include remote manipulation systems, advanced containment technologies, and real-time monitoring of surface oxidation states that can predict potential safety incidents before they occur.
Environmental Impact Assessment
The environmental implications of sodium metal surface technologies extend beyond their immediate applications, requiring thorough assessment of their ecological footprint throughout their lifecycle. Sodium metal surfaces, while offering promising energy storage solutions, present significant environmental challenges due to their high reactivity with air and moisture.
When sodium metal surfaces corrode or degrade in ambient conditions, they can release sodium hydroxide and hydrogen gas. These byproducts may contribute to localized pH changes in soil and water systems if improperly contained. The production processes for corrosion-resistant sodium surfaces often involve chemical treatments and coatings that may contain volatile organic compounds (VOCs) or other potentially harmful substances.
Life cycle assessment (LCA) studies indicate that advanced sodium metal technologies can reduce environmental impact compared to traditional lithium-based systems, primarily due to sodium's greater abundance and reduced mining requirements. However, these benefits must be weighed against the environmental risks associated with potential sodium leakage and the energy-intensive manufacturing processes required for protective coatings.
Water resource impacts deserve particular attention, as accidental exposure of sodium metal to water bodies can trigger rapid exothermic reactions. Protective measures and containment systems are essential to prevent such incidents, especially in regions with high precipitation or flood risks. Monitoring systems and emergency response protocols must be established in facilities utilizing sodium metal technologies.
Waste management represents another critical environmental consideration. End-of-life disposal of sodium-containing components requires specialized handling procedures to prevent environmental contamination. Recycling pathways for sodium metal products remain underdeveloped compared to other metal recovery systems, creating potential waste management challenges as these technologies scale up.
Air quality impacts primarily stem from manufacturing processes rather than the sodium metal itself when properly contained. However, in failure scenarios where sodium surfaces are exposed to air, the resulting reactions can release particulate matter and potentially harmful gases. Advanced containment systems and monitoring technologies have significantly reduced these risks in modern applications.
Regulatory frameworks governing sodium metal technologies vary globally, with more stringent environmental protection measures typically found in Europe and North America. Harmonization of these regulations would benefit both environmental protection efforts and technology development by establishing consistent safety and disposal standards across markets.
When sodium metal surfaces corrode or degrade in ambient conditions, they can release sodium hydroxide and hydrogen gas. These byproducts may contribute to localized pH changes in soil and water systems if improperly contained. The production processes for corrosion-resistant sodium surfaces often involve chemical treatments and coatings that may contain volatile organic compounds (VOCs) or other potentially harmful substances.
Life cycle assessment (LCA) studies indicate that advanced sodium metal technologies can reduce environmental impact compared to traditional lithium-based systems, primarily due to sodium's greater abundance and reduced mining requirements. However, these benefits must be weighed against the environmental risks associated with potential sodium leakage and the energy-intensive manufacturing processes required for protective coatings.
Water resource impacts deserve particular attention, as accidental exposure of sodium metal to water bodies can trigger rapid exothermic reactions. Protective measures and containment systems are essential to prevent such incidents, especially in regions with high precipitation or flood risks. Monitoring systems and emergency response protocols must be established in facilities utilizing sodium metal technologies.
Waste management represents another critical environmental consideration. End-of-life disposal of sodium-containing components requires specialized handling procedures to prevent environmental contamination. Recycling pathways for sodium metal products remain underdeveloped compared to other metal recovery systems, creating potential waste management challenges as these technologies scale up.
Air quality impacts primarily stem from manufacturing processes rather than the sodium metal itself when properly contained. However, in failure scenarios where sodium surfaces are exposed to air, the resulting reactions can release particulate matter and potentially harmful gases. Advanced containment systems and monitoring technologies have significantly reduced these risks in modern applications.
Regulatory frameworks governing sodium metal technologies vary globally, with more stringent environmental protection measures typically found in Europe and North America. Harmonization of these regulations would benefit both environmental protection efforts and technology development by establishing consistent safety and disposal standards across markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!