Failure Mechanisms and Regeneration Pathways in Sodium Anodes
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Anode Technology Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The development of sodium anode technology can be traced back to the 1970s, but significant progress has been made only in the last decade. The evolution of this technology has been driven by the increasing demand for sustainable and cost-effective energy storage solutions, particularly for grid-scale applications where energy density is less critical than cost considerations.
The sodium anode represents a critical component in SIBs, functioning as the negative electrode where sodium ions are stored during charging. Unlike lithium, sodium does not form stable alloys with graphite, which has been the conventional anode material for lithium-ion batteries. This fundamental difference has necessitated the exploration of alternative anode materials and structures specifically designed for sodium-ion systems.
The technical evolution of sodium anodes has progressed through several key phases, from initial investigations of hard carbons to more recent developments in alloy-based anodes, conversion-type anodes, and organic sodium anodes. Each advancement has aimed to address the inherent challenges associated with sodium's larger ionic radius (1.02 Å compared to lithium's 0.76 Å) and different electrochemical properties.
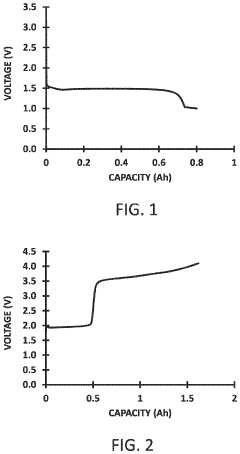
A significant challenge in sodium anode technology has been the understanding and mitigation of failure mechanisms. Sodium metal anodes, while offering high theoretical capacity (1166 mAh/g), suffer from severe dendrite formation, volume expansion, and unstable solid-electrolyte interphase (SEI) formation. These issues lead to safety concerns, reduced cycling efficiency, and shortened battery lifespan.
The primary technical objectives in sodium anode research currently focus on several key areas: developing mechanically stable host materials that can accommodate sodium's volume changes during cycling; designing electrolyte systems that form stable and ion-conductive SEI layers; understanding the fundamental mechanisms of sodium dendrite formation and growth; and exploring regeneration pathways to restore anode functionality after degradation.
Recent research has increasingly focused on the concept of "self-healing" or regenerative sodium anodes, where the electrode can recover from degradation mechanisms through carefully designed chemical or electrochemical processes. This approach represents a paradigm shift from merely preventing failure to actively managing and reversing degradation pathways.
The ultimate goal of sodium anode technology development is to achieve performance metrics comparable to lithium-ion systems while maintaining the cost advantages inherent to sodium-based chemistry. This includes achieving high coulombic efficiency (>99.9%), extended cycle life (>1000 cycles), fast charging capabilities, and improved safety characteristics, all while using earth-abundant materials and scalable manufacturing processes.
The sodium anode represents a critical component in SIBs, functioning as the negative electrode where sodium ions are stored during charging. Unlike lithium, sodium does not form stable alloys with graphite, which has been the conventional anode material for lithium-ion batteries. This fundamental difference has necessitated the exploration of alternative anode materials and structures specifically designed for sodium-ion systems.
The technical evolution of sodium anodes has progressed through several key phases, from initial investigations of hard carbons to more recent developments in alloy-based anodes, conversion-type anodes, and organic sodium anodes. Each advancement has aimed to address the inherent challenges associated with sodium's larger ionic radius (1.02 Å compared to lithium's 0.76 Å) and different electrochemical properties.
A significant challenge in sodium anode technology has been the understanding and mitigation of failure mechanisms. Sodium metal anodes, while offering high theoretical capacity (1166 mAh/g), suffer from severe dendrite formation, volume expansion, and unstable solid-electrolyte interphase (SEI) formation. These issues lead to safety concerns, reduced cycling efficiency, and shortened battery lifespan.
The primary technical objectives in sodium anode research currently focus on several key areas: developing mechanically stable host materials that can accommodate sodium's volume changes during cycling; designing electrolyte systems that form stable and ion-conductive SEI layers; understanding the fundamental mechanisms of sodium dendrite formation and growth; and exploring regeneration pathways to restore anode functionality after degradation.
Recent research has increasingly focused on the concept of "self-healing" or regenerative sodium anodes, where the electrode can recover from degradation mechanisms through carefully designed chemical or electrochemical processes. This approach represents a paradigm shift from merely preventing failure to actively managing and reversing degradation pathways.
The ultimate goal of sodium anode technology development is to achieve performance metrics comparable to lithium-ion systems while maintaining the cost advantages inherent to sodium-based chemistry. This includes achieving high coulombic efficiency (>99.9%), extended cycle life (>1000 cycles), fast charging capabilities, and improved safety characteristics, all while using earth-abundant materials and scalable manufacturing processes.
Market Analysis for Sodium-based Battery Systems
The sodium-based battery market is experiencing significant growth as an alternative to lithium-ion technologies, driven by increasing concerns about lithium supply chain vulnerabilities and cost fluctuations. Current market projections indicate the global sodium-ion battery market could reach $1.2 billion by 2025, with a compound annual growth rate exceeding 23% between 2023-2030. This acceleration is particularly evident in grid storage applications, where cost-effectiveness outweighs energy density limitations.
Consumer electronics represents a secondary but expanding market segment, particularly in regions with limited lithium resources. China has emerged as the dominant player, with CATL and other manufacturers scaling production capacity for sodium-ion batteries. European and North American markets are following this trend with increasing investment in research and commercialization efforts.
Market demand analysis reveals three primary drivers fueling sodium-based battery adoption. First, resource abundance and geographical distribution of sodium significantly reduce geopolitical supply risks compared to lithium. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, with more evenly distributed reserves globally.
Second, cost advantages are substantial, with sodium-based systems potentially 30-40% less expensive than comparable lithium-ion batteries when considering full lifecycle costs. Raw material costs for sodium carbonate remain consistently lower than lithium carbonate, with less volatile pricing patterns observed over the past five years.
Third, sustainability considerations are increasingly influencing market dynamics. Sodium extraction generally has a lower environmental footprint than lithium mining operations, aligning with corporate ESG goals and regulatory requirements in key markets.
However, market penetration faces significant barriers related to the technical challenges of sodium anodes, particularly their failure mechanisms. Dendrite formation, volume expansion issues, and electrolyte decomposition remain critical obstacles to widespread commercial adoption. The market currently demonstrates a bifurcation between companies pursuing incremental improvements to existing technologies versus those developing novel approaches to address fundamental anode stability issues.
Industry forecasts suggest that breakthrough innovations in sodium anode regeneration pathways could potentially accelerate market adoption by 2-3 years. Sectors with less stringent energy density requirements, such as stationary storage, are expected to reach commercial viability first, creating essential market momentum for broader applications.
Consumer electronics represents a secondary but expanding market segment, particularly in regions with limited lithium resources. China has emerged as the dominant player, with CATL and other manufacturers scaling production capacity for sodium-ion batteries. European and North American markets are following this trend with increasing investment in research and commercialization efforts.
Market demand analysis reveals three primary drivers fueling sodium-based battery adoption. First, resource abundance and geographical distribution of sodium significantly reduce geopolitical supply risks compared to lithium. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, with more evenly distributed reserves globally.
Second, cost advantages are substantial, with sodium-based systems potentially 30-40% less expensive than comparable lithium-ion batteries when considering full lifecycle costs. Raw material costs for sodium carbonate remain consistently lower than lithium carbonate, with less volatile pricing patterns observed over the past five years.
Third, sustainability considerations are increasingly influencing market dynamics. Sodium extraction generally has a lower environmental footprint than lithium mining operations, aligning with corporate ESG goals and regulatory requirements in key markets.
However, market penetration faces significant barriers related to the technical challenges of sodium anodes, particularly their failure mechanisms. Dendrite formation, volume expansion issues, and electrolyte decomposition remain critical obstacles to widespread commercial adoption. The market currently demonstrates a bifurcation between companies pursuing incremental improvements to existing technologies versus those developing novel approaches to address fundamental anode stability issues.
Industry forecasts suggest that breakthrough innovations in sodium anode regeneration pathways could potentially accelerate market adoption by 2-3 years. Sectors with less stringent energy density requirements, such as stationary storage, are expected to reach commercial viability first, creating essential market momentum for broader applications.
Current Challenges in Sodium Anode Development
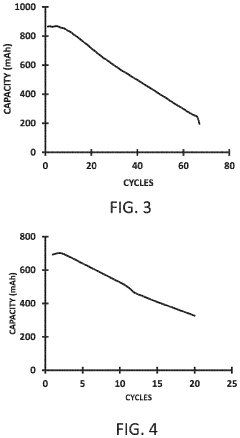
Despite significant advancements in sodium-ion battery technology, sodium metal anodes continue to face substantial challenges that impede their commercial viability. The primary obstacle remains the high reactivity of sodium metal with conventional electrolytes, resulting in continuous SEI (Solid Electrolyte Interphase) formation that consumes both electrolyte and active sodium. This parasitic reaction significantly reduces coulombic efficiency and battery lifespan, making long-term cycling stability difficult to achieve.
Dendrite formation presents another critical challenge, occurring more aggressively in sodium systems compared to lithium counterparts due to sodium's lower surface energy and faster diffusion kinetics. These dendrites not only reduce active material utilization but also create serious safety hazards through potential short-circuiting events that can lead to thermal runaway and catastrophic failure.
Volume expansion during cycling constitutes a third major hurdle, with sodium anodes experiencing approximately 230% volumetric change between charged and discharged states. This extreme dimensional instability causes mechanical stress that fractures the SEI layer, exposing fresh sodium surfaces to further reactions and accelerating capacity fade through continuous electrolyte consumption.
The inherent softness of sodium metal (Mohs hardness ~0.5) exacerbates these issues, as the material easily deforms under minimal pressure, complicating manufacturing processes and battery assembly. This mechanical fragility also contributes to non-uniform current distribution during cycling, promoting localized hotspots for dendrite nucleation and growth.
Sodium's high sensitivity to atmospheric components presents significant handling challenges, requiring stringent manufacturing environments with moisture and oxygen levels below 0.1 ppm. This necessity substantially increases production costs and complexity compared to graphite-based alternatives.
Electrolyte compatibility remains problematic, as most conventional electrolyte formulations developed for lithium systems perform poorly with sodium anodes. The larger ionic radius of sodium (102 pm vs. 76 pm for lithium) alters solvation structures and interfacial chemistries, necessitating sodium-specific electrolyte designs that remain underdeveloped.
Temperature sensitivity further complicates sodium anode implementation, with performance degrading rapidly outside the narrow 20-40°C operating window. At lower temperatures, sodium plating becomes highly irregular, while elevated temperatures accelerate side reactions and SEI degradation, creating a challenging thermal management requirement for practical applications.
Dendrite formation presents another critical challenge, occurring more aggressively in sodium systems compared to lithium counterparts due to sodium's lower surface energy and faster diffusion kinetics. These dendrites not only reduce active material utilization but also create serious safety hazards through potential short-circuiting events that can lead to thermal runaway and catastrophic failure.
Volume expansion during cycling constitutes a third major hurdle, with sodium anodes experiencing approximately 230% volumetric change between charged and discharged states. This extreme dimensional instability causes mechanical stress that fractures the SEI layer, exposing fresh sodium surfaces to further reactions and accelerating capacity fade through continuous electrolyte consumption.
The inherent softness of sodium metal (Mohs hardness ~0.5) exacerbates these issues, as the material easily deforms under minimal pressure, complicating manufacturing processes and battery assembly. This mechanical fragility also contributes to non-uniform current distribution during cycling, promoting localized hotspots for dendrite nucleation and growth.
Sodium's high sensitivity to atmospheric components presents significant handling challenges, requiring stringent manufacturing environments with moisture and oxygen levels below 0.1 ppm. This necessity substantially increases production costs and complexity compared to graphite-based alternatives.
Electrolyte compatibility remains problematic, as most conventional electrolyte formulations developed for lithium systems perform poorly with sodium anodes. The larger ionic radius of sodium (102 pm vs. 76 pm for lithium) alters solvation structures and interfacial chemistries, necessitating sodium-specific electrolyte designs that remain underdeveloped.
Temperature sensitivity further complicates sodium anode implementation, with performance degrading rapidly outside the narrow 20-40°C operating window. At lower temperatures, sodium plating becomes highly irregular, while elevated temperatures accelerate side reactions and SEI degradation, creating a challenging thermal management requirement for practical applications.
Current Solutions for Sodium Anode Failure Mitigation
01 Dendrite formation and prevention mechanisms
Sodium anodes in batteries often fail due to dendrite formation during charging cycles. These dendrites can penetrate separators, causing short circuits and battery failure. Prevention mechanisms include using specialized electrolyte additives, structured separators, and surface coatings that suppress dendrite growth. These approaches help maintain uniform sodium deposition and extend anode lifespan by preventing the irregular growth patterns that lead to failure.- Dendrite formation and prevention mechanisms: Sodium anodes in batteries often fail due to dendrite formation during charging cycles. These dendrites can grow through the electrolyte, causing short circuits and battery failure. Prevention mechanisms include using specialized electrolyte additives, protective surface coatings, and structured interfaces that inhibit dendrite nucleation and growth. These approaches help maintain the integrity of the sodium anode and extend battery life by preventing internal short circuits.
- Solid electrolyte interface (SEI) degradation and stabilization: The solid electrolyte interface (SEI) layer on sodium anodes is critical for battery performance but often degrades over time. This degradation leads to continuous electrolyte consumption, increased impedance, and eventual anode failure. Stabilization approaches include engineered SEI formulations, functional additives that promote stable SEI formation, and surface treatments that enhance the mechanical and chemical stability of the interface, allowing for improved cycling performance and longevity.
- Volume expansion management and structural integrity: Sodium anodes undergo significant volume changes during charge-discharge cycles, leading to mechanical stress, pulverization, and loss of electrical contact. Managing this volume expansion is crucial for maintaining structural integrity. Approaches include designing flexible electrode architectures, incorporating buffer materials, using porous structures that accommodate expansion, and developing composite anodes with improved mechanical properties to withstand repeated cycling without degradation.
- Electrolyte compatibility and optimization: The compatibility between sodium anodes and electrolytes significantly impacts anode performance and failure rates. Incompatible electrolytes can cause accelerated corrosion, parasitic reactions, and unstable interfaces. Optimization strategies include developing sodium-specific electrolyte formulations, incorporating stabilizing additives, adjusting salt concentrations, and using solvent blends that minimize side reactions while promoting efficient sodium ion transport, thereby extending anode lifespan and improving overall battery performance.
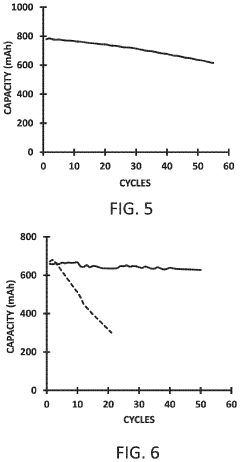
- Regeneration and self-healing mechanisms: Regeneration pathways for sodium anodes focus on self-healing mechanisms that can restore functionality after degradation. These include engineered additives that facilitate the repair of damaged SEI layers, reversible binding agents that can reconstruct anode surfaces, electrochemical protocols that dissolve and redeposit sodium in controlled ways, and dynamic interface components that adapt to changing conditions within the battery. These approaches aim to extend anode lifespan by addressing damage as it occurs rather than preventing it entirely.
02 Solid electrolyte interface (SEI) degradation and stabilization
The solid electrolyte interface (SEI) layer on sodium anodes is critical for battery performance but often degrades during cycling. This degradation exposes fresh sodium surfaces to electrolyte, causing continuous reactions that consume active material and reduce capacity. Stabilization approaches include electrolyte engineering with film-forming additives, artificial SEI layers, and protective coatings that resist cracking during volume changes, enabling more stable cycling and improved regeneration pathways.Expand Specific Solutions03 Volume expansion management and structural integrity
Sodium anodes undergo significant volume changes during charge-discharge cycles, leading to mechanical stress and structural degradation. This expansion can cause pulverization of the anode, loss of electrical contact, and increased impedance. Advanced designs incorporate flexible matrices, porous structures, and composite materials that accommodate volume changes while maintaining structural integrity. These approaches prevent cracking and delamination, preserving electrochemical performance over extended cycling.Expand Specific Solutions04 Electrolyte decomposition and reformulation strategies
Electrolyte decomposition at the sodium anode interface contributes significantly to capacity fade and failure. Reactive sodium metal can continuously consume electrolyte components, forming unstable byproducts that impede ion transport. Reformulation strategies include concentrated electrolytes, localized high-concentration electrolytes, and solvent systems with reduced reactivity toward sodium. These approaches minimize parasitic reactions, improve coulombic efficiency, and create more favorable conditions for anode regeneration during cycling.Expand Specific Solutions05 Self-healing and regeneration technologies
Advanced self-healing mechanisms can restore sodium anode functionality after degradation. These technologies include reversible polymer binders that reform broken connections, liquid metal infusion systems that repair cracks, and electrochemical protocols that redistribute sodium during rest periods. Some approaches incorporate sacrificial components that preferentially react with contaminants, protecting the main anode structure. These regeneration pathways extend battery life by actively counteracting the various failure mechanisms that typically lead to capacity loss.Expand Specific Solutions
Key Industry Players in Sodium Battery Research
The sodium anode technology market is currently in an early growth phase, characterized by intensive R&D efforts across academic institutions and commercial entities. The global market size for sodium-based battery technologies is expanding rapidly, driven by the need for alternatives to lithium-ion batteries. While technical challenges remain, significant progress in addressing failure mechanisms and regeneration pathways is evident from research by key players. Academic institutions like Xiamen University, Cornell University, and Central South University are advancing fundamental understanding, while companies including CATL, Faradion, and Bloom Energy are commercializing solutions. Established industrial players such as Bosch and Johnson Matthey are investing in the technology, indicating growing commercial viability despite remaining challenges in cycle life and stability.
Xiamen University
Technical Solution: Xiamen University's research team has made significant breakthroughs in understanding sodium anode failure mechanisms through their "Interfacial Engineering for Sodium Metal Anodes" program. Their approach focuses on the fundamental electrochemical processes occurring at the sodium metal/electrolyte interface. The team has identified that the primary failure modes include: (1) continuous electrolyte decomposition leading to impedance growth, (2) dendrite formation causing internal short circuits, and (3) pulverization of sodium metal during cycling. Their innovative solution involves a dual-phase artificial interphase consisting of an inorganic sodium-ion conducting layer combined with an elastic organic outer layer. This structure effectively suppresses dendrite growth while accommodating the volumetric changes during cycling. For regeneration pathways, the research team has developed "smart" electrolyte additives that activate upon detecting local current density spikes, depositing protective materials precisely where dendrite formation begins. Their work has demonstrated that controlling sodium ion solvation structures in the electrolyte is crucial for achieving stable cycling performance.
Strengths: Fundamental scientific understanding of failure mechanisms provides basis for rational design; innovative dual-phase interphase demonstrates superior stability in laboratory testing; approach addresses multiple failure modes simultaneously. Weaknesses: Technology remains primarily at laboratory scale; complex fabrication process may present challenges for commercial scale-up; requires highly specialized materials.
Xiamen Hithium New Energy Technology Co., Ltd.
Technical Solution: Hithium has developed a specialized approach to sodium anode stabilization through their "Nano-Composite Interface" (NCI) technology. This system addresses the primary failure mechanisms of sodium anodes—dendrite formation and pulverization—by creating a nanostructured protective layer on the anode surface. Their research has identified that sodium metal anodes typically fail due to uncontrolled reactivity with electrolytes and mechanical stress during volume changes. Hithium's solution incorporates a multi-functional artificial SEI composed of inorganic/organic hybrid materials that simultaneously provide mechanical strength and ionic conductivity. The company has also pioneered a "controlled pre-sodiation" technique that pre-conditions the anode structure to accommodate volume changes during cycling. For regeneration pathways, Hithium employs electrolyte additives that selectively decompose at damaged sites, rebuilding the protective interface layer in a self-healing process during battery operation.
Strengths: Nano-Composite Interface provides exceptional mechanical stability while maintaining high ionic conductivity; self-healing capability extends cycle life significantly; compatible with existing manufacturing processes. Weaknesses: Requires precise control of electrolyte composition; performance degradation still observed at extreme temperatures; higher initial impedance compared to conventional designs.
Critical Patents in Sodium Anode Regeneration Technology
Lithium cells and methods of making and use thereof
PatentInactiveUS20210234149A1
Innovation
- The use of an over-lithiated cathode material with a lithiated metal oxide formula LixMO2, where X>1.2, in combination with a silicon anode, and a non-aqueous electrolyte solution, allows for improved charge capacity and cyclability by managing volume changes and enhancing the stability of the Solid Electrolyte Interphase (SEI).
Sustainability Impact of Sodium Battery Technology
The transition to sodium-based battery technologies represents a significant advancement in sustainable energy storage solutions. Unlike lithium-ion batteries that rely on scarce lithium resources, sodium batteries utilize sodium, the sixth most abundant element in the Earth's crust. This abundance translates to reduced environmental impact from mining operations and substantially lower raw material costs, making sodium batteries a more economically viable option for large-scale energy storage applications.
From a lifecycle perspective, sodium battery technology demonstrates promising sustainability credentials. The extraction of sodium from seawater or salt deposits requires significantly less energy and produces fewer greenhouse gas emissions compared to lithium extraction from hard rock mining or brine evaporation. This reduced carbon footprint extends throughout the manufacturing process, contributing to lower overall environmental impact.
Waste management considerations also favor sodium battery technology. The components of sodium batteries are generally less toxic than their lithium counterparts, potentially simplifying recycling processes and reducing end-of-life environmental hazards. Furthermore, the development of regeneration pathways for sodium anodes addresses one of the key sustainability challenges in battery technology: extending operational lifespan through self-healing mechanisms rather than replacement.
The economic implications of sustainable sodium battery technology are substantial. By reducing dependency on geopolitically concentrated lithium supplies, countries can enhance energy security while supporting domestic manufacturing capabilities. The lower cost structure of sodium batteries could accelerate the adoption of renewable energy storage solutions, particularly in developing economies where cost barriers have historically limited clean energy transitions.
From a social sustainability perspective, sodium battery technology offers opportunities for more equitable access to energy storage solutions. The reduced cost and increased availability of raw materials can democratize access to reliable energy storage, supporting electrification efforts in remote and underserved communities worldwide.
However, challenges remain in optimizing the sustainability profile of sodium batteries. Current failure mechanisms in sodium anodes, including dendrite formation and volume expansion issues, can limit battery lifespan and efficiency. Addressing these challenges through innovative regeneration pathways is essential to maximize the sustainability benefits of this technology and ensure its competitive position against established battery technologies.
From a lifecycle perspective, sodium battery technology demonstrates promising sustainability credentials. The extraction of sodium from seawater or salt deposits requires significantly less energy and produces fewer greenhouse gas emissions compared to lithium extraction from hard rock mining or brine evaporation. This reduced carbon footprint extends throughout the manufacturing process, contributing to lower overall environmental impact.
Waste management considerations also favor sodium battery technology. The components of sodium batteries are generally less toxic than their lithium counterparts, potentially simplifying recycling processes and reducing end-of-life environmental hazards. Furthermore, the development of regeneration pathways for sodium anodes addresses one of the key sustainability challenges in battery technology: extending operational lifespan through self-healing mechanisms rather than replacement.
The economic implications of sustainable sodium battery technology are substantial. By reducing dependency on geopolitically concentrated lithium supplies, countries can enhance energy security while supporting domestic manufacturing capabilities. The lower cost structure of sodium batteries could accelerate the adoption of renewable energy storage solutions, particularly in developing economies where cost barriers have historically limited clean energy transitions.
From a social sustainability perspective, sodium battery technology offers opportunities for more equitable access to energy storage solutions. The reduced cost and increased availability of raw materials can democratize access to reliable energy storage, supporting electrification efforts in remote and underserved communities worldwide.
However, challenges remain in optimizing the sustainability profile of sodium batteries. Current failure mechanisms in sodium anodes, including dendrite formation and volume expansion issues, can limit battery lifespan and efficiency. Addressing these challenges through innovative regeneration pathways is essential to maximize the sustainability benefits of this technology and ensure its competitive position against established battery technologies.
Safety Standards and Testing Protocols for Sodium Batteries
The development of sodium batteries necessitates comprehensive safety standards and testing protocols to ensure their reliable and safe operation. Current safety frameworks for sodium-based energy storage systems are evolving from those established for lithium-ion technologies, with significant adaptations required to address the unique characteristics of sodium anodes.
International organizations including IEC, UL, and ISO have begun developing specific standards for sodium battery technologies, though these remain in nascent stages compared to their lithium counterparts. Key safety standards focus on thermal stability, electrical safety, mechanical integrity, and environmental resilience of sodium battery systems.
Thermal runaway testing represents a critical safety protocol for sodium batteries, particularly given the reactive nature of sodium metal with moisture and oxygen. Calorimetry tests measuring heat generation during failure events reveal that sodium anodes exhibit different thermal profiles compared to lithium systems, necessitating modified safety thresholds and containment strategies.
Dendrite formation testing protocols have been established to evaluate the propensity of sodium anodes to develop dendritic structures during cycling. These protocols typically involve electrochemical impedance spectroscopy and post-mortem analysis to identify conditions that accelerate dendrite growth and subsequent short-circuiting risks.
Accelerated aging tests simulate long-term degradation mechanisms in sodium anodes, including SEI layer breakdown, electrolyte decomposition, and structural changes. These tests provide valuable data on failure progression and help establish operational safety limits for various sodium battery chemistries.
Environmental testing protocols assess sodium battery performance under extreme conditions, including high/low temperatures, humidity variations, and mechanical stress. These tests are particularly important given sodium's high reactivity with moisture, requiring robust encapsulation strategies to prevent catastrophic failures.
Abuse testing protocols evaluate sodium battery response to abnormal conditions such as overcharging, external short circuits, crush tests, and penetration scenarios. These tests have revealed that sodium batteries exhibit different failure modes compared to lithium systems, with implications for battery management system design and safety engineering.
Standardized regeneration assessment protocols are emerging to evaluate the effectiveness of various regeneration strategies for degraded sodium anodes. These protocols measure capacity recovery, impedance changes, and morphological improvements following regenerative treatments, providing quantitative metrics for comparing different approaches.
Regulatory frameworks are increasingly incorporating sodium-specific safety requirements, with transportation regulations being updated to address the unique hazards associated with sodium battery shipment and handling. These developments signal growing recognition of sodium batteries as a distinct technology requiring tailored safety approaches.
International organizations including IEC, UL, and ISO have begun developing specific standards for sodium battery technologies, though these remain in nascent stages compared to their lithium counterparts. Key safety standards focus on thermal stability, electrical safety, mechanical integrity, and environmental resilience of sodium battery systems.
Thermal runaway testing represents a critical safety protocol for sodium batteries, particularly given the reactive nature of sodium metal with moisture and oxygen. Calorimetry tests measuring heat generation during failure events reveal that sodium anodes exhibit different thermal profiles compared to lithium systems, necessitating modified safety thresholds and containment strategies.
Dendrite formation testing protocols have been established to evaluate the propensity of sodium anodes to develop dendritic structures during cycling. These protocols typically involve electrochemical impedance spectroscopy and post-mortem analysis to identify conditions that accelerate dendrite growth and subsequent short-circuiting risks.
Accelerated aging tests simulate long-term degradation mechanisms in sodium anodes, including SEI layer breakdown, electrolyte decomposition, and structural changes. These tests provide valuable data on failure progression and help establish operational safety limits for various sodium battery chemistries.
Environmental testing protocols assess sodium battery performance under extreme conditions, including high/low temperatures, humidity variations, and mechanical stress. These tests are particularly important given sodium's high reactivity with moisture, requiring robust encapsulation strategies to prevent catastrophic failures.
Abuse testing protocols evaluate sodium battery response to abnormal conditions such as overcharging, external short circuits, crush tests, and penetration scenarios. These tests have revealed that sodium batteries exhibit different failure modes compared to lithium systems, with implications for battery management system design and safety engineering.
Standardized regeneration assessment protocols are emerging to evaluate the effectiveness of various regeneration strategies for degraded sodium anodes. These protocols measure capacity recovery, impedance changes, and morphological improvements following regenerative treatments, providing quantitative metrics for comparing different approaches.
Regulatory frameworks are increasingly incorporating sodium-specific safety requirements, with transportation regulations being updated to address the unique hazards associated with sodium battery shipment and handling. These developments signal growing recognition of sodium batteries as a distinct technology requiring tailored safety approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!