Design of Composite Electrolytes Compatible with Sodium Metal Anodes
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Battery Electrolyte Development Background and Objectives
The development of sodium-ion batteries (SIBs) has gained significant momentum in recent years as a promising alternative to lithium-ion batteries (LIBs). This surge in interest stems from the abundant and widespread availability of sodium resources, which are approximately 1000 times more plentiful than lithium in the Earth's crust. The lower cost and greater sustainability of sodium-based energy storage systems position them as a strategic solution for large-scale applications, particularly in grid energy storage where cost considerations often outweigh energy density requirements.
The evolution of sodium battery technology can be traced back to the 1970s and 1980s, when researchers first began exploring sodium-based electrochemical systems. However, the rapid commercialization of lithium-ion batteries in the 1990s shifted focus away from sodium-based alternatives. The renewed interest in sodium batteries over the past decade has been driven by concerns about lithium resource limitations and geopolitical supply chain vulnerabilities.
A critical challenge in sodium battery development has been the electrolyte design, particularly those compatible with sodium metal anodes. Sodium metal represents the ultimate anode material for sodium batteries due to its high theoretical capacity (1166 mAh/g) and low redox potential (-2.71V vs. standard hydrogen electrode). However, the high reactivity of sodium metal with conventional electrolytes leads to poor cycling efficiency, dendrite formation, and safety concerns.
Composite electrolytes have emerged as a promising approach to address these challenges. These systems combine multiple components—typically solid inorganic conductors, polymers, and liquid electrolytes—to create materials with synergistic properties that can effectively interface with sodium metal anodes while maintaining high ionic conductivity and mechanical stability.
The technical objectives for composite electrolyte development include achieving room temperature ionic conductivity exceeding 10^-4 S/cm, establishing stable sodium metal interfaces with Coulombic efficiency >99.5%, preventing dendrite propagation through mechanical and chemical mechanisms, and ensuring compatibility with high-voltage cathode materials to maximize energy density.
Recent advancements in materials science, computational modeling, and characterization techniques have accelerated progress in this field. The integration of artificial intelligence for materials discovery has further expanded the design space for novel composite electrolyte formulations, enabling researchers to navigate complex multi-component systems more efficiently.
The ultimate goal of this technical research is to develop composite electrolyte systems that enable safe, long-cycle-life sodium metal batteries with energy densities approaching those of lithium-ion systems but at significantly lower cost. Such achievements would position sodium batteries as a viable technology for next-generation energy storage applications, particularly in stationary storage markets where cost-effectiveness is paramount.
The evolution of sodium battery technology can be traced back to the 1970s and 1980s, when researchers first began exploring sodium-based electrochemical systems. However, the rapid commercialization of lithium-ion batteries in the 1990s shifted focus away from sodium-based alternatives. The renewed interest in sodium batteries over the past decade has been driven by concerns about lithium resource limitations and geopolitical supply chain vulnerabilities.
A critical challenge in sodium battery development has been the electrolyte design, particularly those compatible with sodium metal anodes. Sodium metal represents the ultimate anode material for sodium batteries due to its high theoretical capacity (1166 mAh/g) and low redox potential (-2.71V vs. standard hydrogen electrode). However, the high reactivity of sodium metal with conventional electrolytes leads to poor cycling efficiency, dendrite formation, and safety concerns.
Composite electrolytes have emerged as a promising approach to address these challenges. These systems combine multiple components—typically solid inorganic conductors, polymers, and liquid electrolytes—to create materials with synergistic properties that can effectively interface with sodium metal anodes while maintaining high ionic conductivity and mechanical stability.
The technical objectives for composite electrolyte development include achieving room temperature ionic conductivity exceeding 10^-4 S/cm, establishing stable sodium metal interfaces with Coulombic efficiency >99.5%, preventing dendrite propagation through mechanical and chemical mechanisms, and ensuring compatibility with high-voltage cathode materials to maximize energy density.
Recent advancements in materials science, computational modeling, and characterization techniques have accelerated progress in this field. The integration of artificial intelligence for materials discovery has further expanded the design space for novel composite electrolyte formulations, enabling researchers to navigate complex multi-component systems more efficiently.
The ultimate goal of this technical research is to develop composite electrolyte systems that enable safe, long-cycle-life sodium metal batteries with energy densities approaching those of lithium-ion systems but at significantly lower cost. Such achievements would position sodium batteries as a viable technology for next-generation energy storage applications, particularly in stationary storage markets where cost-effectiveness is paramount.
Market Analysis for Sodium-based Energy Storage Solutions
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion technologies, driven by increasing demand for sustainable and cost-effective energy storage solutions. Current market projections indicate the sodium-ion battery market could reach $500 million by 2025, with a compound annual growth rate exceeding 20% through 2030. This acceleration is primarily fueled by concerns over lithium supply chain vulnerabilities and price volatility, with lithium carbonate prices having fluctuated dramatically in recent years.
Sodium-based energy storage solutions offer compelling economic advantages, with raw material costs approximately 30-40% lower than lithium-based counterparts. Sodium is the sixth most abundant element in the Earth's crust, with reserves distributed more equitably across global regions compared to lithium, reducing geopolitical supply risks. This abundance translates to potentially lower and more stable pricing for energy storage systems at grid and utility scales.
Market segmentation reveals distinct application sectors for sodium-based technologies. Grid-scale energy storage represents the largest potential market segment, particularly in regions with expanding renewable energy infrastructure requiring load balancing and frequency regulation. The electric vehicle sector presents a secondary market opportunity, especially for cost-sensitive applications where energy density requirements are less stringent, such as urban delivery vehicles and public transportation.
Regional market analysis shows Asia-Pacific leading sodium battery development and commercialization, with China accounting for over 50% of global research output and patent filings in this field. European markets show increasing interest driven by sustainability initiatives and circular economy regulations, while North American adoption remains more cautious but growing.
Key market barriers include performance limitations compared to established lithium technologies, particularly regarding energy density and cycle life. Current sodium-ion batteries typically deliver 120-150 Wh/kg compared to 250-300 Wh/kg for advanced lithium-ion cells. However, the development of composite electrolytes compatible with sodium metal anodes represents a critical technological advancement that could significantly narrow this performance gap.
Consumer and industrial adoption trends indicate growing receptiveness to sodium-based technologies, particularly in applications where cost sensitivity outweighs energy density requirements. Market surveys show 65% of utility-scale storage developers would consider sodium-ion alternatives if they could achieve cost reductions of 20% or more compared to lithium systems while maintaining acceptable performance metrics.
Sodium-based energy storage solutions offer compelling economic advantages, with raw material costs approximately 30-40% lower than lithium-based counterparts. Sodium is the sixth most abundant element in the Earth's crust, with reserves distributed more equitably across global regions compared to lithium, reducing geopolitical supply risks. This abundance translates to potentially lower and more stable pricing for energy storage systems at grid and utility scales.
Market segmentation reveals distinct application sectors for sodium-based technologies. Grid-scale energy storage represents the largest potential market segment, particularly in regions with expanding renewable energy infrastructure requiring load balancing and frequency regulation. The electric vehicle sector presents a secondary market opportunity, especially for cost-sensitive applications where energy density requirements are less stringent, such as urban delivery vehicles and public transportation.
Regional market analysis shows Asia-Pacific leading sodium battery development and commercialization, with China accounting for over 50% of global research output and patent filings in this field. European markets show increasing interest driven by sustainability initiatives and circular economy regulations, while North American adoption remains more cautious but growing.
Key market barriers include performance limitations compared to established lithium technologies, particularly regarding energy density and cycle life. Current sodium-ion batteries typically deliver 120-150 Wh/kg compared to 250-300 Wh/kg for advanced lithium-ion cells. However, the development of composite electrolytes compatible with sodium metal anodes represents a critical technological advancement that could significantly narrow this performance gap.
Consumer and industrial adoption trends indicate growing receptiveness to sodium-based technologies, particularly in applications where cost sensitivity outweighs energy density requirements. Market surveys show 65% of utility-scale storage developers would consider sodium-ion alternatives if they could achieve cost reductions of 20% or more compared to lithium systems while maintaining acceptable performance metrics.
Current Challenges in Na-Metal Anode Compatibility
Despite the promising potential of sodium-ion batteries as a cost-effective alternative to lithium-ion systems, the compatibility between sodium metal anodes and electrolytes presents significant challenges. The highly reactive nature of sodium metal creates a complex interface with conventional electrolytes, leading to several critical issues that impede commercial viability.
The formation of unstable solid electrolyte interphase (SEI) layers represents one of the most significant obstacles. Unlike lithium metal anodes, sodium metal forms SEI layers that are typically more fragile and less uniform. These unstable interfaces fail to effectively prevent continuous electrolyte decomposition, resulting in low Coulombic efficiency and rapid capacity fading during cycling.
Dendrite growth poses another severe challenge for sodium metal anodes. The uneven deposition of sodium during charging creates needle-like structures that can penetrate the separator, causing internal short circuits and potential safety hazards. This dendrite formation is particularly aggressive with sodium compared to lithium due to sodium's lower surface energy and faster diffusion kinetics.
Volume expansion during cycling further complicates the electrode-electrolyte interface. Sodium metal undergoes significant volumetric changes during plating and stripping processes, creating mechanical stress that damages the SEI layer. This continuous breaking and reforming of the protective interface accelerates electrolyte consumption and degrades battery performance over time.
Electrolyte decomposition occurs rapidly when in contact with sodium metal, leading to gas evolution and increased internal pressure within cells. Conventional carbonate-based electrolytes are particularly susceptible to reductive decomposition, forming unstable organic compounds that contribute to cell failure. This decomposition not only consumes electrolyte but also increases internal resistance.
The high chemical reactivity of sodium with trace moisture and impurities presents additional compatibility challenges. Even minute amounts of water or oxygen can trigger parasitic reactions that compromise the electrode-electrolyte interface. These reactions generate sodium hydroxide and other compounds that accelerate corrosion and degrade cell components.
Temperature sensitivity further exacerbates these compatibility issues. At elevated temperatures, the reactivity between sodium metal and electrolytes increases dramatically, accelerating all degradation mechanisms. Conversely, at low temperatures, sodium's electrochemical activity decreases significantly, limiting power capability and increasing the likelihood of uneven deposition.
Current research efforts focus on developing composite electrolytes that can address these multiple challenges simultaneously, requiring innovative approaches that combine organic and inorganic components to create stable interfaces with sodium metal anodes.
The formation of unstable solid electrolyte interphase (SEI) layers represents one of the most significant obstacles. Unlike lithium metal anodes, sodium metal forms SEI layers that are typically more fragile and less uniform. These unstable interfaces fail to effectively prevent continuous electrolyte decomposition, resulting in low Coulombic efficiency and rapid capacity fading during cycling.
Dendrite growth poses another severe challenge for sodium metal anodes. The uneven deposition of sodium during charging creates needle-like structures that can penetrate the separator, causing internal short circuits and potential safety hazards. This dendrite formation is particularly aggressive with sodium compared to lithium due to sodium's lower surface energy and faster diffusion kinetics.
Volume expansion during cycling further complicates the electrode-electrolyte interface. Sodium metal undergoes significant volumetric changes during plating and stripping processes, creating mechanical stress that damages the SEI layer. This continuous breaking and reforming of the protective interface accelerates electrolyte consumption and degrades battery performance over time.
Electrolyte decomposition occurs rapidly when in contact with sodium metal, leading to gas evolution and increased internal pressure within cells. Conventional carbonate-based electrolytes are particularly susceptible to reductive decomposition, forming unstable organic compounds that contribute to cell failure. This decomposition not only consumes electrolyte but also increases internal resistance.
The high chemical reactivity of sodium with trace moisture and impurities presents additional compatibility challenges. Even minute amounts of water or oxygen can trigger parasitic reactions that compromise the electrode-electrolyte interface. These reactions generate sodium hydroxide and other compounds that accelerate corrosion and degrade cell components.
Temperature sensitivity further exacerbates these compatibility issues. At elevated temperatures, the reactivity between sodium metal and electrolytes increases dramatically, accelerating all degradation mechanisms. Conversely, at low temperatures, sodium's electrochemical activity decreases significantly, limiting power capability and increasing the likelihood of uneven deposition.
Current research efforts focus on developing composite electrolytes that can address these multiple challenges simultaneously, requiring innovative approaches that combine organic and inorganic components to create stable interfaces with sodium metal anodes.
State-of-the-Art Composite Electrolyte Designs
01 Polymer-based composite electrolytes
Polymer-based composite electrolytes combine polymers with other materials to enhance compatibility and performance in battery systems. These composites typically incorporate inorganic fillers or additives to improve ionic conductivity while maintaining mechanical stability. The polymer matrix provides flexibility and processability, while the additives enhance electrochemical properties. These composite electrolytes show improved compatibility with electrode materials and can operate across wider temperature ranges.- Polymer-based composite electrolytes: Polymer-based composite electrolytes combine polymer matrices with various additives to enhance ionic conductivity and mechanical stability. These electrolytes typically incorporate polymers such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF) with ceramic fillers or ionic liquids. The polymer matrix provides mechanical support while the additives improve ion transport properties. These composites offer improved compatibility with electrode materials and can mitigate interface resistance issues in battery applications.
- Ceramic-polymer composite electrolytes: Ceramic-polymer composite electrolytes combine the high ionic conductivity of ceramic materials with the flexibility and processability of polymers. These composites typically contain ceramic particles such as Li7La3Zr2O12 (LLZO) or Li1.3Al0.3Ti1.7(PO4)3 (LATP) dispersed within a polymer matrix. The ceramic components enhance lithium-ion conductivity while the polymer improves mechanical properties and electrode contact. These electrolytes demonstrate improved compatibility with lithium metal anodes and can suppress dendrite formation.
- Gel polymer electrolytes for enhanced compatibility: Gel polymer electrolytes (GPEs) consist of a polymer matrix swollen with liquid electrolyte, combining the advantages of both solid and liquid systems. These electrolytes offer improved electrode-electrolyte contact and compatibility while maintaining good ionic conductivity. GPEs typically use polymers such as PVDF-HFP or PAN with conventional liquid electrolytes containing lithium salts. The gel nature allows for better interfacial contact with electrodes while reducing leakage and safety concerns associated with pure liquid electrolytes.
- Interface engineering for composite electrolytes: Interface engineering techniques improve the compatibility between composite electrolytes and electrode materials. These approaches include surface modifications, buffer layers, and gradient compositions to reduce interfacial resistance. Methods such as atomic layer deposition, solution-based coatings, or in-situ formed interphases can be employed to enhance ion transport across interfaces. Properly engineered interfaces prevent side reactions and ensure stable long-term cycling performance in battery systems using composite electrolytes.
- Additives for improving composite electrolyte compatibility: Various additives can be incorporated into composite electrolytes to enhance their compatibility with electrode materials. These additives include ionic liquids, flame retardants, plasticizers, and lithium salt complexes. Ionic liquids improve the ionic conductivity and electrochemical stability window, while plasticizers enhance flexibility and electrode contact. Certain additives can form stable solid electrolyte interphase (SEI) layers that protect the electrolyte from degradation during cycling, leading to improved battery performance and longevity.
02 Ceramic-polymer composite electrolytes
Ceramic-polymer composite electrolytes combine the high ionic conductivity of ceramic materials with the flexibility and processability of polymers. These hybrid electrolytes address compatibility issues at electrode interfaces by creating stable interphases. The ceramic components enhance mechanical strength and thermal stability while reducing interfacial resistance. This combination results in improved electrochemical performance and better compatibility with various electrode materials in battery systems.Expand Specific Solutions03 Liquid electrolyte additives for compatibility enhancement
Additives in liquid electrolytes can significantly improve compatibility with electrode materials. These additives form protective films at electrode-electrolyte interfaces, preventing unwanted side reactions and enhancing cycling stability. Various compounds including fluorinated carbonates, boron-based additives, and lithium salts can be incorporated to modify interfacial properties. The additives help maintain electrolyte stability during operation and improve overall battery performance by reducing degradation mechanisms.Expand Specific Solutions04 Gel polymer electrolytes for interface compatibility
Gel polymer electrolytes provide enhanced compatibility at electrode interfaces by combining the cohesive properties of polymers with the high conductivity of liquid electrolytes. These systems form stable interfaces with both anode and cathode materials, reducing interfacial resistance. The gel structure accommodates volume changes during cycling while maintaining good contact with electrode surfaces. This approach addresses compatibility issues that often arise with purely solid or liquid electrolyte systems.Expand Specific Solutions05 Solid-state composite electrolytes with improved compatibility
Solid-state composite electrolytes are designed to improve compatibility with electrode materials while eliminating flammability concerns associated with liquid electrolytes. These composites typically combine inorganic solid electrolytes with polymers or other binding materials to enhance mechanical properties and reduce interfacial resistance. The composite structure helps accommodate volume changes during cycling and creates stable interfaces with both cathode and anode materials, resulting in improved electrochemical performance and longer cycle life.Expand Specific Solutions
Leading Organizations in Na-Battery Electrolyte Research
The sodium metal anode composite electrolyte market is in an early growth phase, characterized by increasing R&D investments but limited commercial deployment. The global market is projected to expand significantly as sodium-ion battery technology gains traction as a cost-effective alternative to lithium-ion batteries. Technologically, the field shows moderate maturity with key players at different development stages. Companies like PolyPlus Battery and LG Energy Solution are leading with advanced electrolyte formulations, while SAMSUNG SDI, Toyota Motor Corp, and Faradion are developing proprietary solutions. Academic institutions including Ulsan National Institute of Science & Technology and Cornell University are contributing fundamental research. The competitive landscape features collaboration between established battery manufacturers and specialized materials companies, with increasing patent activity signaling growing commercial interest.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has developed a proprietary Protected Anode Technology for sodium metal batteries that incorporates a composite solid electrolyte interface. Their approach uses a multi-layered electrolyte system with a solid ceramic membrane that serves as a protective layer between the sodium metal anode and the liquid or gel electrolyte. This membrane allows for efficient sodium ion transport while preventing dendrite formation and electrolyte degradation. The company's composite electrolyte combines inorganic solid-state components with polymer-based materials to create a stable interface with sodium metal. Their technology includes specialized coatings that enhance the wettability between the sodium metal and the solid electrolyte, addressing one of the key challenges in sodium metal battery development. PolyPlus has demonstrated cells with energy densities exceeding 400 Wh/kg using their protected sodium anode technology, significantly higher than conventional lithium-ion batteries.
Strengths: Superior protection against sodium dendrite formation; excellent ionic conductivity at room temperature; demonstrated long cycle life in full cells. Weaknesses: Manufacturing complexity of multi-layer electrolyte systems; potential challenges in scaling production; higher cost compared to conventional electrolyte systems.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed an advanced composite electrolyte system specifically designed for sodium metal batteries. Their approach combines a sodium-ion conducting ceramic (typically NASICON-type materials) with a polymer matrix to create a flexible yet mechanically robust electrolyte interface. The company utilizes a gradient-structured composite where the concentration of ceramic particles varies across the thickness, optimizing both the mechanical properties and ionic conductivity. Their proprietary surface modification techniques for the ceramic particles enhance compatibility with the polymer matrix and improve the interfacial stability with sodium metal. LG Energy Solution's composite electrolyte incorporates flame-retardant additives to address safety concerns associated with sodium metal batteries. The company has reported achieving ionic conductivities of 1-3 mS/cm at room temperature with their composite electrolytes, while maintaining stable cycling performance over hundreds of cycles with minimal capacity degradation.
Strengths: Excellent balance of mechanical strength and ionic conductivity; enhanced safety features through flame-retardant additives; compatibility with existing manufacturing processes. Weaknesses: Potential challenges in achieving uniform dispersion of ceramic particles in polymer matrix; temperature sensitivity affecting performance in extreme conditions; trade-off between mechanical properties and ionic conductivity.
Critical Patents and Research on Na-Compatible Electrolytes
Composite Electrolytes for Low Temperature Sodium Batteries
PatentInactiveUS20150364787A1
Innovation
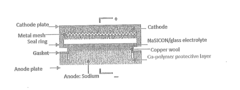
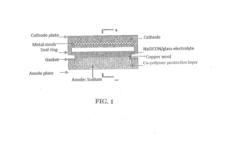
- A solid electrolyte composite is developed using a sodium-super-ionic-conductor framework (NaxAyBzP3−zOw) combined with a glass material, specifically Na2O—B2O3, to create a NaSICON/glass composite electrolyte that reduces grain boundary resistivity and enhances stability, suitable for use in sodium-ion batteries operating at 120° C. to 250° C.
An electrolyte composition for a highly stable and reversible sodium-metal anode for high-energy rechargeable batteries
PatentPendingIN202211027550A
Innovation
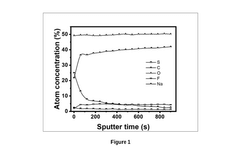
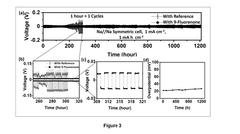
- An electrolyte composition comprising an organic aromatic additive, 9-Fluorenone, which solubilizes in ether-based electrolytes and electrochemically decomposes to form a protective layer, stabilizing the sodium-metal anode and reducing sodium deposition overpotential, enabling dendrite-free and uniform sodium deposition.
Material Sustainability and Supply Chain Considerations
The sustainability of materials used in composite electrolytes for sodium metal anodes represents a critical consideration for large-scale implementation. Sodium-based battery technologies offer inherent advantages over lithium-based systems from a sustainability perspective, as sodium is approximately 1,000 times more abundant in the Earth's crust than lithium. This abundance translates to lower extraction costs and reduced geopolitical supply risks, making sodium-based systems particularly attractive for grid-scale energy storage applications where cost considerations often outweigh energy density requirements.
The supply chain for composite electrolyte components presents both opportunities and challenges. Polymer matrices commonly used in these electrolytes, such as PEO (polyethylene oxide), PVDF (polyvinylidene fluoride), and PMMA (polymethyl methacrylate), are derived from petrochemical feedstocks with established production infrastructure. However, the environmental impact of these petroleum-based polymers has prompted research into bio-derived alternatives, including cellulose-based polymers and chitosan, which offer improved end-of-life management options.
Ceramic fillers utilized in composite electrolytes, including NASICON-type materials, β-alumina, and various metal oxides (Al2O3, SiO2, TiO2), require careful consideration regarding their production methods. Energy-intensive high-temperature synthesis routes traditionally employed for these materials contribute significantly to the carbon footprint of the final electrolyte system. Recent advances in low-temperature sol-gel processing and microwave-assisted synthesis offer more sustainable production pathways with reduced energy requirements.
The salt components in sodium-based electrolytes, primarily NaPF6, NaClO4, and NaTFSI, present specific sustainability challenges. The production of fluorinated salts involves environmentally problematic compounds and generates hazardous by-products. Research into alternative sodium salts with reduced environmental impact, such as sodium bis(oxalato)borate (NaBOB), represents an important direction for improving the overall sustainability profile of these electrolyte systems.
Recycling considerations must be integrated into the design phase of composite electrolytes. Current end-of-life management for battery components remains underdeveloped, particularly for the complex multi-material nature of composite electrolytes. Designing for disassembly and material recovery will be essential for establishing closed-loop material flows and reducing dependence on primary resource extraction.
Regional availability of raw materials introduces strategic considerations for supply chain resilience. While sodium resources are globally distributed, specialized components such as high-purity ceramic fillers and advanced polymer matrices may face supply constraints. Developing diversified supply networks and exploring material substitution options will be crucial for mitigating supply chain vulnerabilities in the scaling of sodium-based battery technologies.
The supply chain for composite electrolyte components presents both opportunities and challenges. Polymer matrices commonly used in these electrolytes, such as PEO (polyethylene oxide), PVDF (polyvinylidene fluoride), and PMMA (polymethyl methacrylate), are derived from petrochemical feedstocks with established production infrastructure. However, the environmental impact of these petroleum-based polymers has prompted research into bio-derived alternatives, including cellulose-based polymers and chitosan, which offer improved end-of-life management options.
Ceramic fillers utilized in composite electrolytes, including NASICON-type materials, β-alumina, and various metal oxides (Al2O3, SiO2, TiO2), require careful consideration regarding their production methods. Energy-intensive high-temperature synthesis routes traditionally employed for these materials contribute significantly to the carbon footprint of the final electrolyte system. Recent advances in low-temperature sol-gel processing and microwave-assisted synthesis offer more sustainable production pathways with reduced energy requirements.
The salt components in sodium-based electrolytes, primarily NaPF6, NaClO4, and NaTFSI, present specific sustainability challenges. The production of fluorinated salts involves environmentally problematic compounds and generates hazardous by-products. Research into alternative sodium salts with reduced environmental impact, such as sodium bis(oxalato)borate (NaBOB), represents an important direction for improving the overall sustainability profile of these electrolyte systems.
Recycling considerations must be integrated into the design phase of composite electrolytes. Current end-of-life management for battery components remains underdeveloped, particularly for the complex multi-material nature of composite electrolytes. Designing for disassembly and material recovery will be essential for establishing closed-loop material flows and reducing dependence on primary resource extraction.
Regional availability of raw materials introduces strategic considerations for supply chain resilience. While sodium resources are globally distributed, specialized components such as high-purity ceramic fillers and advanced polymer matrices may face supply constraints. Developing diversified supply networks and exploring material substitution options will be crucial for mitigating supply chain vulnerabilities in the scaling of sodium-based battery technologies.
Safety and Performance Testing Protocols
The development of comprehensive safety and performance testing protocols is essential for the successful implementation of composite electrolytes compatible with sodium metal anodes. These protocols must address the unique challenges posed by sodium-based systems, which differ significantly from their lithium counterparts in terms of reactivity, thermal stability, and electrochemical behavior.
Standard testing procedures should begin with basic safety assessments including thermal stability tests (DSC/TGA), flammability tests, and chemical compatibility evaluations between the composite electrolyte and sodium metal. Particular attention must be paid to the potential formation of dendrites, which can lead to short circuits and thermal runaway. Accelerated aging tests under controlled temperature conditions (typically ranging from -20°C to 60°C) are crucial for understanding long-term stability and safety implications.
Performance testing protocols should systematically evaluate ionic conductivity across a wide temperature range (-20°C to 80°C), interfacial resistance measurements via electrochemical impedance spectroscopy (EIS), and transference number determinations. These measurements provide critical insights into the fundamental electrochemical properties that govern battery performance. Sodium-specific testing considerations include monitoring for potential sodium plating/stripping efficiency, which directly impacts coulombic efficiency and cycle life.
Mechanical property assessments represent another critical dimension of testing protocols. Evaluations should include measurements of tensile strength, elongation at break, and puncture resistance to ensure the composite electrolyte can withstand the volume changes associated with sodium plating and stripping. Dynamic mechanical analysis (DMA) can provide valuable information about the viscoelastic properties that influence dendrite suppression capabilities.
Full-cell testing protocols must be established to evaluate real-world performance metrics including capacity retention, rate capability, and cycle life under various charge-discharge conditions. Standardized testing conditions should include different C-rates (0.1C to 2C), depth of discharge variations, and temperature cycling to simulate real-world usage scenarios. Post-mortem analysis techniques such as SEM, XPS, and FTIR should be incorporated to understand degradation mechanisms after cycling.
Industry-standard abuse testing protocols must be adapted specifically for sodium-based systems, including nail penetration tests, crush tests, and overcharge/overdischarge evaluations. These tests should be conducted at both cell and small pack levels to understand potential failure propagation mechanisms. The development of sodium-specific safety standards is essential, as current lithium-ion battery standards may not adequately address the unique characteristics of sodium metal anodes and their composite electrolyte systems.
Standard testing procedures should begin with basic safety assessments including thermal stability tests (DSC/TGA), flammability tests, and chemical compatibility evaluations between the composite electrolyte and sodium metal. Particular attention must be paid to the potential formation of dendrites, which can lead to short circuits and thermal runaway. Accelerated aging tests under controlled temperature conditions (typically ranging from -20°C to 60°C) are crucial for understanding long-term stability and safety implications.
Performance testing protocols should systematically evaluate ionic conductivity across a wide temperature range (-20°C to 80°C), interfacial resistance measurements via electrochemical impedance spectroscopy (EIS), and transference number determinations. These measurements provide critical insights into the fundamental electrochemical properties that govern battery performance. Sodium-specific testing considerations include monitoring for potential sodium plating/stripping efficiency, which directly impacts coulombic efficiency and cycle life.
Mechanical property assessments represent another critical dimension of testing protocols. Evaluations should include measurements of tensile strength, elongation at break, and puncture resistance to ensure the composite electrolyte can withstand the volume changes associated with sodium plating and stripping. Dynamic mechanical analysis (DMA) can provide valuable information about the viscoelastic properties that influence dendrite suppression capabilities.
Full-cell testing protocols must be established to evaluate real-world performance metrics including capacity retention, rate capability, and cycle life under various charge-discharge conditions. Standardized testing conditions should include different C-rates (0.1C to 2C), depth of discharge variations, and temperature cycling to simulate real-world usage scenarios. Post-mortem analysis techniques such as SEM, XPS, and FTIR should be incorporated to understand degradation mechanisms after cycling.
Industry-standard abuse testing protocols must be adapted specifically for sodium-based systems, including nail penetration tests, crush tests, and overcharge/overdischarge evaluations. These tests should be conducted at both cell and small pack levels to understand potential failure propagation mechanisms. The development of sodium-specific safety standards is essential, as current lithium-ion battery standards may not adequately address the unique characteristics of sodium metal anodes and their composite electrolyte systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!