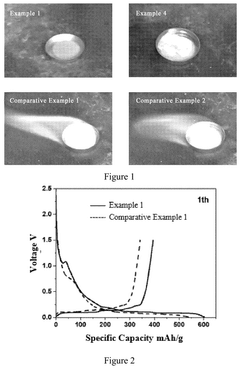
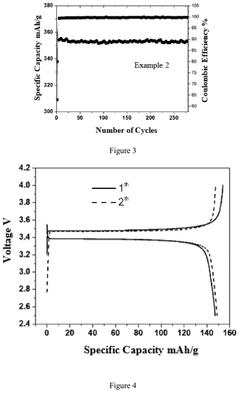
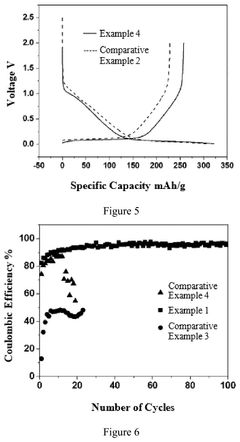
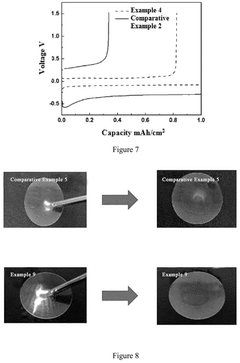
Electrochemical Behavior of Sodium Metal in Carbonate Electrolytes
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Battery Electrochemistry Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) due to the abundance and low cost of sodium resources. The development of SIBs can be traced back to the 1970s, when they were first investigated alongside LIBs. However, the rapid commercialization of LIBs in the 1990s shifted research focus away from sodium-based systems. In recent years, concerns about lithium resource limitations and increasing demand for energy storage solutions have reignited interest in SIB technology.
The electrochemical behavior of sodium metal in carbonate electrolytes represents a critical research area for advancing sodium-based energy storage systems. Unlike lithium, sodium has distinct electrochemical properties, including a lower reduction potential (-2.71 V vs. SHE compared to -3.04 V for lithium) and larger ionic radius (1.02 Å vs. 0.76 Å for lithium), which significantly influence its interactions with electrolyte components.
Carbonate-based electrolytes have been widely adopted in commercial lithium-ion batteries due to their excellent electrochemical stability and compatibility with electrode materials. Understanding how these electrolytes perform with sodium metal is essential for developing high-performance sodium batteries. The solid electrolyte interphase (SEI) formation on sodium metal surfaces in carbonate electrolytes follows different mechanisms compared to lithium systems, often resulting in less stable interfaces.
The evolution of sodium battery technology has shown several distinct phases, from initial fundamental studies to more recent practical applications. Current research trends indicate growing interest in developing advanced electrolyte formulations specifically tailored for sodium systems, rather than simply adapting lithium-based solutions. This includes exploring electrolyte additives that can stabilize the sodium-electrolyte interface and mitigate issues such as dendrite formation.
The technical objectives in this field include understanding the fundamental mechanisms of sodium metal deposition and dissolution in carbonate electrolytes, identifying the composition and formation process of the SEI layer, and developing strategies to enhance coulombic efficiency and cycling stability. Additionally, researchers aim to mitigate safety concerns associated with sodium metal anodes, particularly dendrite growth that can lead to short circuits.
Recent advancements in characterization techniques, including in-situ and operando methods, have enabled more detailed investigations of interfacial phenomena in sodium battery systems. These tools provide unprecedented insights into the dynamic processes occurring at the sodium metal-electrolyte interface during battery operation, helping to guide rational design of next-generation sodium battery technologies.
The electrochemical behavior of sodium metal in carbonate electrolytes represents a critical research area for advancing sodium-based energy storage systems. Unlike lithium, sodium has distinct electrochemical properties, including a lower reduction potential (-2.71 V vs. SHE compared to -3.04 V for lithium) and larger ionic radius (1.02 Å vs. 0.76 Å for lithium), which significantly influence its interactions with electrolyte components.
Carbonate-based electrolytes have been widely adopted in commercial lithium-ion batteries due to their excellent electrochemical stability and compatibility with electrode materials. Understanding how these electrolytes perform with sodium metal is essential for developing high-performance sodium batteries. The solid electrolyte interphase (SEI) formation on sodium metal surfaces in carbonate electrolytes follows different mechanisms compared to lithium systems, often resulting in less stable interfaces.
The evolution of sodium battery technology has shown several distinct phases, from initial fundamental studies to more recent practical applications. Current research trends indicate growing interest in developing advanced electrolyte formulations specifically tailored for sodium systems, rather than simply adapting lithium-based solutions. This includes exploring electrolyte additives that can stabilize the sodium-electrolyte interface and mitigate issues such as dendrite formation.
The technical objectives in this field include understanding the fundamental mechanisms of sodium metal deposition and dissolution in carbonate electrolytes, identifying the composition and formation process of the SEI layer, and developing strategies to enhance coulombic efficiency and cycling stability. Additionally, researchers aim to mitigate safety concerns associated with sodium metal anodes, particularly dendrite growth that can lead to short circuits.
Recent advancements in characterization techniques, including in-situ and operando methods, have enabled more detailed investigations of interfacial phenomena in sodium battery systems. These tools provide unprecedented insights into the dynamic processes occurring at the sodium metal-electrolyte interface during battery operation, helping to guide rational design of next-generation sodium battery technologies.
Market Analysis for Sodium-based Energy Storage
The global energy storage market is witnessing a significant shift towards sodium-based technologies as a viable alternative to lithium-ion batteries. This transition is primarily driven by the increasing cost and supply constraints associated with lithium resources. The sodium-based energy storage market, currently valued at approximately $1.2 billion, is projected to grow at a compound annual growth rate of 18% through 2030, potentially reaching $5.6 billion by the end of the decade.
The demand for sodium-based energy storage solutions is particularly strong in grid-scale applications, where cost considerations often outweigh energy density requirements. Utility companies and grid operators are increasingly deploying sodium-based systems for load leveling, peak shaving, and renewable energy integration. This segment represents nearly 60% of the current market share and is expected to maintain dominance in the foreseeable future.
Geographically, Asia-Pacific leads the market adoption, with China and South Korea making substantial investments in sodium-based energy storage research and manufacturing capabilities. Europe follows closely, driven by stringent environmental regulations and ambitious renewable energy targets. North America, despite its traditional focus on lithium technologies, is showing growing interest in sodium alternatives, particularly for stationary storage applications.
Consumer electronics and electric vehicle sectors remain challenging markets for sodium-based technologies due to lower energy density compared to lithium-ion batteries. However, niche applications in low-cost electronic devices and short-range urban mobility solutions are emerging as promising entry points for sodium battery technologies.
The carbonate electrolyte segment within sodium-based energy storage is experiencing particular attention due to its compatibility with existing manufacturing infrastructure originally designed for lithium-ion production. This compatibility significantly reduces the capital investment required for transitioning to sodium-based technologies, making it an attractive option for battery manufacturers looking to diversify their product portfolios.
Price sensitivity analysis indicates that sodium-based systems become economically competitive with lithium-ion alternatives when raw material costs for lithium exceed certain thresholds. Current market conditions, with lithium prices experiencing volatility, have created favorable conditions for sodium technology adoption in price-sensitive applications.
Customer acceptance surveys reveal growing awareness and interest in sodium-based energy storage solutions, particularly among industrial and utility customers. However, concerns regarding long-term performance, cycle life, and system integration remain significant barriers to widespread adoption that technology developers must address to accelerate market penetration.
The demand for sodium-based energy storage solutions is particularly strong in grid-scale applications, where cost considerations often outweigh energy density requirements. Utility companies and grid operators are increasingly deploying sodium-based systems for load leveling, peak shaving, and renewable energy integration. This segment represents nearly 60% of the current market share and is expected to maintain dominance in the foreseeable future.
Geographically, Asia-Pacific leads the market adoption, with China and South Korea making substantial investments in sodium-based energy storage research and manufacturing capabilities. Europe follows closely, driven by stringent environmental regulations and ambitious renewable energy targets. North America, despite its traditional focus on lithium technologies, is showing growing interest in sodium alternatives, particularly for stationary storage applications.
Consumer electronics and electric vehicle sectors remain challenging markets for sodium-based technologies due to lower energy density compared to lithium-ion batteries. However, niche applications in low-cost electronic devices and short-range urban mobility solutions are emerging as promising entry points for sodium battery technologies.
The carbonate electrolyte segment within sodium-based energy storage is experiencing particular attention due to its compatibility with existing manufacturing infrastructure originally designed for lithium-ion production. This compatibility significantly reduces the capital investment required for transitioning to sodium-based technologies, making it an attractive option for battery manufacturers looking to diversify their product portfolios.
Price sensitivity analysis indicates that sodium-based systems become economically competitive with lithium-ion alternatives when raw material costs for lithium exceed certain thresholds. Current market conditions, with lithium prices experiencing volatility, have created favorable conditions for sodium technology adoption in price-sensitive applications.
Customer acceptance surveys reveal growing awareness and interest in sodium-based energy storage solutions, particularly among industrial and utility customers. However, concerns regarding long-term performance, cycle life, and system integration remain significant barriers to widespread adoption that technology developers must address to accelerate market penetration.
Current Challenges in Sodium-Carbonate Electrolyte Systems
Despite significant advancements in sodium-ion battery technology, the electrochemical behavior of sodium metal in carbonate electrolytes presents several persistent challenges that impede commercial viability. The primary obstacle remains the high reactivity of sodium metal with conventional carbonate-based electrolytes, leading to continuous electrolyte decomposition and formation of unstable solid electrolyte interphase (SEI) layers. Unlike lithium systems, sodium's larger ionic radius (1.02Å vs. 0.76Å for Li+) results in different solvation structures and desolvation energetics, fundamentally altering interfacial reactions.
The instability of the sodium-electrolyte interface manifests as poor coulombic efficiency, typically below 80% in standard carbonate formulations compared to >95% achievable in optimized lithium systems. This inefficiency stems from parasitic reactions that continuously consume both sodium metal and electrolyte components during cycling, resulting in capacity fade and shortened battery lifespan.
Dendrite formation represents another critical challenge, occurring more aggressively in sodium systems than lithium counterparts. The lower surface energy of sodium metal (approximately 0.26 J/m² versus 0.52 J/m² for lithium) promotes non-uniform deposition morphologies. These dendrites can penetrate separators, causing internal short circuits and serious safety hazards, particularly at higher current densities above 1 mA/cm².
Carbonate solvents (EC, DMC, PC) undergo reductive decomposition at potentials well above the Na+/Na redox potential (2.71V vs. SHE), forming complex organic and inorganic products at the electrode interface. Recent spectroscopic studies have identified sodium alkyl carbonates, sodium alkoxides, and various polymerized species as major components of the SEI layer, which lacks the beneficial NaF-rich composition found in fluoride-containing electrolyte systems.
Temperature sensitivity further complicates sodium-carbonate systems, with performance deteriorating significantly below 10°C due to increased electrolyte viscosity and reduced ionic conductivity. At elevated temperatures (>40°C), accelerated side reactions and SEI dissolution create a self-reinforcing cycle of degradation.
The co-intercalation phenomenon, where solvent molecules insert alongside sodium ions into graphitic structures, disrupts electrode integrity and contributes to capacity fading. This behavior, less prominent in lithium systems, necessitates alternative anode materials or specialized electrolyte formulations for sodium batteries.
Recent research has identified trace moisture (even at ppm levels) as particularly problematic in sodium systems, forming highly reactive sodium hydroxide that aggressively attacks both the electrolyte and electrode materials, creating a cascade of degradation reactions that are difficult to mitigate in practical manufacturing environments.
The instability of the sodium-electrolyte interface manifests as poor coulombic efficiency, typically below 80% in standard carbonate formulations compared to >95% achievable in optimized lithium systems. This inefficiency stems from parasitic reactions that continuously consume both sodium metal and electrolyte components during cycling, resulting in capacity fade and shortened battery lifespan.
Dendrite formation represents another critical challenge, occurring more aggressively in sodium systems than lithium counterparts. The lower surface energy of sodium metal (approximately 0.26 J/m² versus 0.52 J/m² for lithium) promotes non-uniform deposition morphologies. These dendrites can penetrate separators, causing internal short circuits and serious safety hazards, particularly at higher current densities above 1 mA/cm².
Carbonate solvents (EC, DMC, PC) undergo reductive decomposition at potentials well above the Na+/Na redox potential (2.71V vs. SHE), forming complex organic and inorganic products at the electrode interface. Recent spectroscopic studies have identified sodium alkyl carbonates, sodium alkoxides, and various polymerized species as major components of the SEI layer, which lacks the beneficial NaF-rich composition found in fluoride-containing electrolyte systems.
Temperature sensitivity further complicates sodium-carbonate systems, with performance deteriorating significantly below 10°C due to increased electrolyte viscosity and reduced ionic conductivity. At elevated temperatures (>40°C), accelerated side reactions and SEI dissolution create a self-reinforcing cycle of degradation.
The co-intercalation phenomenon, where solvent molecules insert alongside sodium ions into graphitic structures, disrupts electrode integrity and contributes to capacity fading. This behavior, less prominent in lithium systems, necessitates alternative anode materials or specialized electrolyte formulations for sodium batteries.
Recent research has identified trace moisture (even at ppm levels) as particularly problematic in sodium systems, forming highly reactive sodium hydroxide that aggressively attacks both the electrolyte and electrode materials, creating a cascade of degradation reactions that are difficult to mitigate in practical manufacturing environments.
Current Approaches to Sodium Metal Electrode Stabilization
01 Electrochemical behavior of sodium metal in carbonate-based electrolytes
Sodium metal exhibits specific electrochemical behaviors when used in carbonate-based electrolytes. These behaviors include the formation of solid electrolyte interphase (SEI) layers, cycling efficiency, and voltage characteristics. The interaction between sodium metal and carbonate solvents affects the stability and performance of the electrochemical system, particularly in terms of sodium ion transport and electrode-electrolyte interface properties.- Electrochemical behavior of sodium metal in carbonate electrolytes: Sodium metal exhibits specific electrochemical behaviors when used in carbonate-based electrolytes. These behaviors include the formation of solid electrolyte interphase (SEI) layers, voltage profiles during cycling, and capacity retention characteristics. Understanding these behaviors is crucial for developing efficient sodium-based battery systems as they directly impact battery performance, safety, and longevity.
- Carbonate electrolyte compositions for sodium batteries: Various carbonate electrolyte compositions have been developed specifically for sodium-based battery systems. These compositions typically include combinations of cyclic carbonates (such as ethylene carbonate, propylene carbonate) and linear carbonates (such as dimethyl carbonate, diethyl carbonate) with sodium salts. The specific ratio and selection of carbonates significantly affect the ionic conductivity, viscosity, and electrochemical stability window of the electrolyte system.
- Interface stabilization and SEI formation in sodium-carbonate systems: The interface between sodium metal and carbonate electrolytes is highly reactive, leading to continuous electrolyte decomposition and sodium consumption. Various approaches have been developed to stabilize this interface, including electrolyte additives, protective coatings, and engineered solid electrolyte interphase (SEI) layers. These stabilization methods aim to create a passivation layer that prevents further reactions while maintaining efficient sodium ion transport.
- Sodium salt types and their impact on electrochemical performance: Different sodium salts used in carbonate electrolytes, such as NaPF6, NaClO4, and NaTFSI, significantly influence the electrochemical behavior of sodium metal anodes. The choice of salt affects ionic conductivity, the nature of the SEI layer formation, and overall cell performance. The anion chemistry plays a crucial role in determining the stability of the electrolyte against decomposition and the reversibility of sodium plating/stripping processes.
- Temperature effects on sodium-carbonate electrolyte systems: Temperature significantly impacts the electrochemical behavior of sodium metal in carbonate electrolytes. At elevated temperatures, increased ionic conductivity can improve performance, but may also accelerate side reactions and electrolyte decomposition. At lower temperatures, higher viscosity and reduced ionic mobility can limit battery performance. Understanding these temperature dependencies is essential for developing sodium battery systems that can operate reliably across a wide temperature range.
02 Electrolyte additives for improved sodium metal performance
Various additives can be incorporated into carbonate electrolytes to enhance the performance of sodium metal electrodes. These additives help to form more stable SEI layers, reduce side reactions, and improve cycling efficiency. They can include fluorinated compounds, nitrogen-containing compounds, and other functional additives that modify the electrode-electrolyte interface and enhance the electrochemical stability of sodium metal in carbonate-based systems.Expand Specific Solutions03 Carbonate solvent compositions for sodium batteries
Specific carbonate solvent compositions can be tailored for sodium battery applications. These may include mixtures of cyclic carbonates (such as ethylene carbonate, propylene carbonate) and linear carbonates (such as dimethyl carbonate, diethyl carbonate). The ratio and type of carbonate solvents significantly affect the electrochemical behavior of sodium metal, including its plating/stripping efficiency, dendrite formation, and overall battery performance.Expand Specific Solutions04 Sodium salt concentration effects in carbonate electrolytes
The concentration of sodium salts in carbonate electrolytes plays a crucial role in determining the electrochemical behavior of sodium metal anodes. Higher salt concentrations can modify the solvation structure of sodium ions, affect the viscosity and ionic conductivity of the electrolyte, and influence the formation and composition of the SEI layer. Optimizing salt concentration is essential for achieving stable sodium metal cycling in carbonate-based electrolytes.Expand Specific Solutions05 Temperature dependence of sodium metal behavior in carbonate electrolytes
The electrochemical behavior of sodium metal in carbonate electrolytes shows significant temperature dependence. At different operating temperatures, the reaction kinetics, electrolyte stability, SEI formation, and sodium ion transport properties change. Understanding these temperature effects is crucial for designing sodium-based battery systems that can operate reliably across a wide temperature range, particularly for applications requiring operation in extreme conditions.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrochemical behavior of sodium metal in carbonate electrolytes represents an emerging field in energy storage technology, currently in its growth phase. The market for sodium-based battery technologies is expanding rapidly, projected to reach significant scale as a cost-effective alternative to lithium-ion systems. Technical maturity varies across competitors, with academic institutions like Beijing University of Chemical Technology, Central South University, and Cornell University establishing fundamental research foundations. Commercial development is advancing through specialized companies like Faradion Ltd., Lina Energy, and Form Energy, while established players including CATL, Samsung SDI, and Saft Groupe are integrating sodium technologies into their portfolios. The industry benefits from collaborative efforts between research institutions and industrial partners, with significant progress in addressing challenges related to sodium's reactivity with carbonate electrolytes.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced electrolyte formulations specifically addressing sodium metal's electrochemical behavior in carbonate-based systems. Their proprietary technology employs fluoroethylene carbonate (FEC) additives at optimized concentrations (typically 5-10%) to form stable solid electrolyte interphase (SEI) layers on sodium metal anodes. This approach significantly reduces dendrite formation and parasitic reactions between sodium metal and conventional carbonate solvents like ethylene carbonate (EC) and dimethyl carbonate (DMC). CATL's research demonstrates that their modified carbonate electrolytes enable sodium metal cells to achieve over 500 cycles with capacity retention exceeding 80%, compared to rapid failure in unmodified systems. Additionally, they've incorporated sodium salt innovations, moving beyond traditional NaPF6 to custom-designed salts that further enhance SEI stability and sodium ion transport properties in carbonate environments.
Strengths: Superior cycle life in sodium metal systems compared to competitors; scalable manufacturing compatibility with existing lithium-ion production infrastructure; reduced dendrite formation through proprietary additives. Weaknesses: Higher cost due to specialty additives; potential temperature sensitivity of fluorinated compounds; still exhibits capacity fade beyond 500 cycles compared to conventional lithium-ion systems.
Saft Groupe SA
Technical Solution: Saft has developed a sophisticated approach to sodium metal electrochemistry in carbonate electrolytes through their "Advanced Sodium Interface Protection" (ASIP) technology. Their system employs a multi-component electrolyte formulation based on a carefully optimized mixture of ethylene carbonate (EC) and diethyl carbonate (DEC) as primary solvents (typically in a 1:1 to 1:2 ratio), supplemented with proprietary additives that specifically target the sodium metal interface. A key innovation in Saft's approach is the incorporation of sodium bis(trifluoromethanesulfonyl)imide (NaTFSI) salt at concentrations between 0.8-1.2M, which demonstrates superior anodic stability compared to conventional NaPF6. Their research shows that this salt chemistry, combined with 2-3% vinylene carbonate (VC) and trace amounts of organophosphorus compounds, creates a more robust and flexible SEI layer on sodium surfaces. This protective interface can accommodate the volumetric changes during sodium plating/stripping while maintaining ionic conductivity. Saft's technology enables sodium metal half-cells to achieve over 300 cycles with capacity retention above 85% in carbonate-based electrolytes - a significant improvement over conventional formulations that typically fail within 50-100 cycles.
Strengths: Excellent compatibility with existing manufacturing infrastructure; superior safety performance through enhanced thermal stability; good low-temperature performance compared to competing technologies. Weaknesses: Higher electrolyte cost due to specialty salt and additives; still exhibits capacity fade beyond 300 cycles; performance at high current densities remains challenging.
Key Mechanisms of Sodium-Carbonate Interfacial Reactions
Electrolyte, additive thereof, secondary cell, and application thereof
PatentActiveUS12107223B2
Innovation
- An organic electrolyte composition comprising a lithium or sodium salt, a phosphate ester, and a fluoroether, without carbonate ester, with specific concentration ranges and structural formulas, providing improved safety, thermal stability, and electrochemical performance.
Electrochemical cell
PatentWO2016183638A1
Innovation
- A sodium electrochemical cell with a sodium-ion ionic liquid electrolyte having a sodium-ion concentration of at least 75% of its saturation limit, subjected to a polarisation cycle, forming a low-resistance solid-electrolyte interphase (SEI) layer at the negative electrode, enabling high current densities and stability at room temperature.
Safety Considerations in Sodium Metal Battery Systems
The safety aspects of sodium metal battery systems require comprehensive consideration due to the reactive nature of sodium metal, especially in carbonate electrolyte environments. Sodium metal exhibits high chemical reactivity with moisture and oxygen, creating potential fire and explosion hazards when exposed to air. This reactivity is particularly pronounced in carbonate-based electrolytes, where side reactions can lead to gas evolution and pressure build-up within cells.
Temperature management represents a critical safety parameter in sodium metal battery systems. The low melting point of sodium (97.8°C) compared to lithium (180.5°C) creates unique thermal runaway risks. When sodium metal reaches its melting point in a battery system, the increased mobility and surface area dramatically accelerate reaction rates with electrolytes, potentially triggering catastrophic failure cascades.
Dendrite formation during cycling poses another significant safety concern. The electrochemical behavior of sodium in carbonate electrolytes often results in non-uniform deposition, leading to dendrite growth that can penetrate separators and cause internal short circuits. These dendrites exhibit particularly aggressive growth patterns in certain carbonate formulations, necessitating specialized electrolyte additives or physical barriers.
Electrolyte decomposition products from sodium-carbonate interactions can include flammable gases such as carbon dioxide, ethylene, and methane. The generation of these gases not only creates pressure hazards but also forms a potentially combustible atmosphere within the cell. Safety engineering must account for both gas management and flame retardation strategies specific to sodium-based systems.
The solid electrolyte interphase (SEI) formed on sodium metal in carbonate electrolytes often lacks the stability observed in lithium systems. This unstable interface can lead to continuous electrolyte consumption, impedance growth, and thermal events. Engineering stable SEI formation through electrolyte formulation represents a primary safety enhancement approach for sodium metal batteries.
Transportation and storage regulations for sodium metal batteries require special considerations beyond those established for lithium-ion systems. The unique reactivity profile of sodium with carbonates necessitates specific protocols for thermal isolation, moisture protection, and impact resistance during shipping and warehousing operations.
Temperature management represents a critical safety parameter in sodium metal battery systems. The low melting point of sodium (97.8°C) compared to lithium (180.5°C) creates unique thermal runaway risks. When sodium metal reaches its melting point in a battery system, the increased mobility and surface area dramatically accelerate reaction rates with electrolytes, potentially triggering catastrophic failure cascades.
Dendrite formation during cycling poses another significant safety concern. The electrochemical behavior of sodium in carbonate electrolytes often results in non-uniform deposition, leading to dendrite growth that can penetrate separators and cause internal short circuits. These dendrites exhibit particularly aggressive growth patterns in certain carbonate formulations, necessitating specialized electrolyte additives or physical barriers.
Electrolyte decomposition products from sodium-carbonate interactions can include flammable gases such as carbon dioxide, ethylene, and methane. The generation of these gases not only creates pressure hazards but also forms a potentially combustible atmosphere within the cell. Safety engineering must account for both gas management and flame retardation strategies specific to sodium-based systems.
The solid electrolyte interphase (SEI) formed on sodium metal in carbonate electrolytes often lacks the stability observed in lithium systems. This unstable interface can lead to continuous electrolyte consumption, impedance growth, and thermal events. Engineering stable SEI formation through electrolyte formulation represents a primary safety enhancement approach for sodium metal batteries.
Transportation and storage regulations for sodium metal batteries require special considerations beyond those established for lithium-ion systems. The unique reactivity profile of sodium with carbonates necessitates specific protocols for thermal isolation, moisture protection, and impact resistance during shipping and warehousing operations.
Sustainability Impact of Sodium Battery Technologies
The transition to sodium-based battery technologies represents a significant advancement in sustainable energy storage solutions. Unlike lithium-ion batteries that rely on scarce lithium resources, sodium batteries utilize sodium - the sixth most abundant element in the Earth's crust. This abundance translates to reduced environmental impact from mining operations and substantially lower raw material costs, making sodium batteries a promising alternative for large-scale energy storage applications.
The sustainability benefits extend beyond resource availability. The electrochemical behavior of sodium metal in carbonate electrolytes enables manufacturing processes that require less energy and produce fewer emissions compared to conventional lithium-ion battery production. Studies indicate that sodium battery production can reduce carbon footprint by approximately 20-30% compared to lithium-ion counterparts, primarily due to less energy-intensive material extraction and processing.
From a lifecycle perspective, sodium batteries offer additional environmental advantages. The carbonate electrolytes used in sodium metal batteries can be formulated with lower concentrations of environmentally harmful fluorinated compounds than those typically required in lithium systems. This reduction in fluorinated materials decreases potential groundwater contamination risks associated with improper disposal or recycling.
The end-of-life management of sodium batteries also presents sustainability benefits. The recycling processes for sodium batteries are potentially less complex than those for lithium-ion batteries, as they contain fewer toxic or difficult-to-separate components. Recovery rates for key materials could reach 80-90% with optimized recycling technologies, creating a more circular economy approach to battery production.
Economic sustainability is equally important when considering the broader impact of this technology. The lower cost structure of sodium batteries could democratize access to energy storage solutions in developing regions, supporting renewable energy integration in areas previously unable to afford large-scale storage systems. This accessibility could accelerate the global transition away from fossil fuels, particularly in emerging economies.
Water usage represents another critical sustainability metric. The production of sodium-based battery materials typically requires 35-40% less water compared to lithium extraction, particularly when considering the water-intensive evaporation processes used in lithium brine operations. This reduced water footprint is especially significant in water-stressed regions where battery manufacturing facilities might be located.
The sustainability benefits extend beyond resource availability. The electrochemical behavior of sodium metal in carbonate electrolytes enables manufacturing processes that require less energy and produce fewer emissions compared to conventional lithium-ion battery production. Studies indicate that sodium battery production can reduce carbon footprint by approximately 20-30% compared to lithium-ion counterparts, primarily due to less energy-intensive material extraction and processing.
From a lifecycle perspective, sodium batteries offer additional environmental advantages. The carbonate electrolytes used in sodium metal batteries can be formulated with lower concentrations of environmentally harmful fluorinated compounds than those typically required in lithium systems. This reduction in fluorinated materials decreases potential groundwater contamination risks associated with improper disposal or recycling.
The end-of-life management of sodium batteries also presents sustainability benefits. The recycling processes for sodium batteries are potentially less complex than those for lithium-ion batteries, as they contain fewer toxic or difficult-to-separate components. Recovery rates for key materials could reach 80-90% with optimized recycling technologies, creating a more circular economy approach to battery production.
Economic sustainability is equally important when considering the broader impact of this technology. The lower cost structure of sodium batteries could democratize access to energy storage solutions in developing regions, supporting renewable energy integration in areas previously unable to afford large-scale storage systems. This accessibility could accelerate the global transition away from fossil fuels, particularly in emerging economies.
Water usage represents another critical sustainability metric. The production of sodium-based battery materials typically requires 35-40% less water compared to lithium extraction, particularly when considering the water-intensive evaporation processes used in lithium brine operations. This reduced water footprint is especially significant in water-stressed regions where battery manufacturing facilities might be located.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!