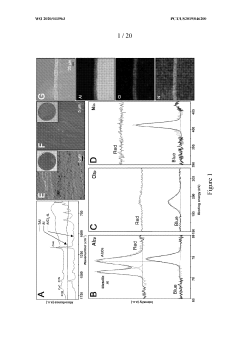
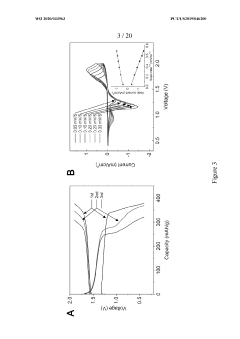
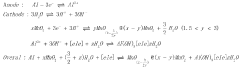Surface Passivation Layers and Their Effects on Sodium Metal Anode Life
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Metal Anode Passivation Background and Objectives
Sodium metal anodes have emerged as a promising alternative to lithium-based energy storage systems due to sodium's natural abundance, lower cost, and similar electrochemical properties. The evolution of sodium battery technology can be traced back to the 1970s, but significant advancements have only materialized in the last decade with improved understanding of interfacial chemistry and materials science.
The formation and stability of surface passivation layers on sodium metal anodes represent a critical factor determining battery performance and longevity. Unlike lithium, sodium's larger ionic radius and different chemical reactivity create unique challenges in forming stable solid electrolyte interphase (SEI) layers. Historical attempts to address these issues have progressed from simple electrolyte modifications to sophisticated engineered interfaces.
Recent technological trends indicate a shift toward multi-functional passivation strategies that combine organic and inorganic components to simultaneously address multiple degradation mechanisms. The integration of artificial SEI layers, electrolyte additives, and advanced characterization techniques has accelerated progress in this field, with publications in this area increasing by approximately 300% between 2015 and 2022.
The primary technical objectives for sodium metal anode passivation research include developing passivation layers that can effectively suppress dendrite formation, minimize irreversible sodium consumption, and maintain mechanical integrity during repeated cycling. Additionally, these layers must demonstrate compatibility with various electrolyte systems while operating across wide temperature ranges to ensure practical applicability.
Another critical goal is to achieve passivation layers that can self-heal during cycling, accommodating the volumetric changes inherent to sodium metal anodes. This requires fundamental understanding of the dynamic processes occurring at the electrode-electrolyte interface and the development of adaptive materials that respond to local environmental changes.
From an industrial perspective, the objectives extend to creating scalable and cost-effective passivation technologies that can be integrated into existing manufacturing processes. The ideal passivation solution must balance performance enhancement with practical implementation considerations, including processing complexity, material availability, and environmental impact.
The field aims to establish standardized protocols for evaluating passivation layer performance, enabling meaningful comparisons between different approaches. This includes developing in-situ and operando characterization methods that can monitor passivation layer evolution in real-time, providing insights into degradation mechanisms and failure modes.
Ultimately, the technological trajectory points toward multidisciplinary approaches that combine surface chemistry, materials science, and electrochemistry to design passivation strategies tailored to specific battery applications, from high-energy density systems to rapid-charging capabilities.
The formation and stability of surface passivation layers on sodium metal anodes represent a critical factor determining battery performance and longevity. Unlike lithium, sodium's larger ionic radius and different chemical reactivity create unique challenges in forming stable solid electrolyte interphase (SEI) layers. Historical attempts to address these issues have progressed from simple electrolyte modifications to sophisticated engineered interfaces.
Recent technological trends indicate a shift toward multi-functional passivation strategies that combine organic and inorganic components to simultaneously address multiple degradation mechanisms. The integration of artificial SEI layers, electrolyte additives, and advanced characterization techniques has accelerated progress in this field, with publications in this area increasing by approximately 300% between 2015 and 2022.
The primary technical objectives for sodium metal anode passivation research include developing passivation layers that can effectively suppress dendrite formation, minimize irreversible sodium consumption, and maintain mechanical integrity during repeated cycling. Additionally, these layers must demonstrate compatibility with various electrolyte systems while operating across wide temperature ranges to ensure practical applicability.
Another critical goal is to achieve passivation layers that can self-heal during cycling, accommodating the volumetric changes inherent to sodium metal anodes. This requires fundamental understanding of the dynamic processes occurring at the electrode-electrolyte interface and the development of adaptive materials that respond to local environmental changes.
From an industrial perspective, the objectives extend to creating scalable and cost-effective passivation technologies that can be integrated into existing manufacturing processes. The ideal passivation solution must balance performance enhancement with practical implementation considerations, including processing complexity, material availability, and environmental impact.
The field aims to establish standardized protocols for evaluating passivation layer performance, enabling meaningful comparisons between different approaches. This includes developing in-situ and operando characterization methods that can monitor passivation layer evolution in real-time, providing insights into degradation mechanisms and failure modes.
Ultimately, the technological trajectory points toward multidisciplinary approaches that combine surface chemistry, materials science, and electrochemistry to design passivation strategies tailored to specific battery applications, from high-energy density systems to rapid-charging capabilities.
Market Analysis for Sodium-based Battery Technologies
The sodium-based battery market is experiencing significant growth as an alternative to lithium-ion technologies, driven by increasing concerns about lithium supply constraints and cost volatility. Current market projections indicate that the global sodium-ion battery market could reach $1.2 billion by 2025, with a compound annual growth rate exceeding 25% between 2023 and 2030. This growth trajectory is particularly notable in grid storage applications, where cost considerations often outweigh energy density requirements.
The market demand for sodium metal anodes specifically is emerging as a critical segment within this broader market. Sodium metal batteries offer theoretical energy densities approaching 1,100 Wh/kg, positioning them as potential competitors to lithium metal systems while utilizing more abundant resources. Current market penetration remains limited, primarily confined to research and development phases, with commercial deployment expected to accelerate from 2025 onward.
Regional analysis reveals China leading sodium battery technology development with over 40% of global patents, followed by the European Union and United States. China's dominance stems from strategic investments in alternative battery technologies and control of processing facilities. The European Battery Alliance has identified sodium-based technologies as a strategic priority, allocating substantial funding through Horizon Europe programs to accelerate commercialization.
Market segmentation shows distinct application trajectories. Stationary energy storage represents the largest immediate opportunity, with projected market share of 65% for sodium-based technologies by 2030 in grid-level applications. Electric mobility applications face greater barriers due to energy density limitations, though specialized vehicle segments including two-wheelers and urban delivery vehicles show promising adoption potential in price-sensitive markets.
Consumer electronics presents a smaller but growing opportunity, particularly for devices where cost sensitivity outweighs size constraints. The industrial sector shows increasing interest in sodium battery technologies for backup power systems and remote operations equipment, with projected adoption rates increasing as surface passivation challenges are overcome.
Key market drivers include raw material economics (sodium being approximately 95% less expensive than lithium per kilogram), supply chain resilience considerations, and environmental sustainability advantages. The surface passivation layer technology specifically addresses cycle life limitations that have historically constrained market adoption, potentially unlocking significant market expansion if durability metrics can approach those of commercial lithium-ion systems.
The market demand for sodium metal anodes specifically is emerging as a critical segment within this broader market. Sodium metal batteries offer theoretical energy densities approaching 1,100 Wh/kg, positioning them as potential competitors to lithium metal systems while utilizing more abundant resources. Current market penetration remains limited, primarily confined to research and development phases, with commercial deployment expected to accelerate from 2025 onward.
Regional analysis reveals China leading sodium battery technology development with over 40% of global patents, followed by the European Union and United States. China's dominance stems from strategic investments in alternative battery technologies and control of processing facilities. The European Battery Alliance has identified sodium-based technologies as a strategic priority, allocating substantial funding through Horizon Europe programs to accelerate commercialization.
Market segmentation shows distinct application trajectories. Stationary energy storage represents the largest immediate opportunity, with projected market share of 65% for sodium-based technologies by 2030 in grid-level applications. Electric mobility applications face greater barriers due to energy density limitations, though specialized vehicle segments including two-wheelers and urban delivery vehicles show promising adoption potential in price-sensitive markets.
Consumer electronics presents a smaller but growing opportunity, particularly for devices where cost sensitivity outweighs size constraints. The industrial sector shows increasing interest in sodium battery technologies for backup power systems and remote operations equipment, with projected adoption rates increasing as surface passivation challenges are overcome.
Key market drivers include raw material economics (sodium being approximately 95% less expensive than lithium per kilogram), supply chain resilience considerations, and environmental sustainability advantages. The surface passivation layer technology specifically addresses cycle life limitations that have historically constrained market adoption, potentially unlocking significant market expansion if durability metrics can approach those of commercial lithium-ion systems.
Current Challenges in Surface Passivation for Sodium Anodes
Despite significant advancements in sodium-ion battery technology, surface passivation for sodium metal anodes remains a critical challenge that impedes commercial viability. The highly reactive nature of sodium metal creates complex interfacial chemistry when in contact with electrolytes, resulting in unstable solid electrolyte interphase (SEI) layers. Unlike lithium metal anodes, sodium's larger ionic radius (102 pm vs. 76 pm for lithium) and different chemical properties create unique passivation challenges that cannot be addressed by simply transferring lithium-based solutions.
Current passivation layers suffer from poor mechanical stability due to the significant volume changes during sodium plating/stripping cycles. The softer nature of sodium metal (0.69 GPa hardness compared to lithium's 0.91 GPa) exacerbates this issue, causing more severe morphological instabilities during cycling. This leads to continuous SEI breakdown and reformation, consuming both electrolyte and active sodium.
Sodium's higher chemical reactivity with conventional electrolyte components produces less favorable decomposition products compared to lithium systems. The resulting SEI layers typically exhibit higher ionic resistance, lower uniformity, and poorer adhesion to the sodium metal surface. Research has shown that sodium fluoride (NaF) components in the SEI, while beneficial for stability, are more difficult to form consistently than their lithium counterparts.
The inhomogeneous sodium deposition problem presents another major obstacle. Current passivation strategies struggle to promote uniform sodium nucleation and growth, leading to dendrite formation that eventually causes internal short circuits. Studies indicate that sodium dendrites can penetrate passivation layers more aggressively than lithium dendrites due to different growth mechanisms and mechanical properties.
Electrolyte compatibility issues further complicate passivation efforts. Many additives and formulations that effectively passivate lithium surfaces perform poorly with sodium, creating unstable interfaces that continue to evolve during cycling. The limited selection of sodium-compatible electrolyte systems that can form effective passivation layers restricts design flexibility.
Temperature sensitivity represents another significant challenge. Sodium-based SEI layers typically exhibit greater thermal instability than lithium counterparts, degrading more rapidly at elevated temperatures and forming different decomposition products at low temperatures. This narrow operational window limits practical applications in real-world environments where temperature fluctuations are common.
Manufacturing scalability of effective passivation techniques remains problematic. Laboratory-scale approaches like atomic layer deposition or specialized pre-treatment methods have shown promise but face significant barriers to industrial implementation. The cost-effectiveness and process compatibility of these techniques with existing battery manufacturing infrastructure require substantial improvement before commercial adoption becomes viable.
Current passivation layers suffer from poor mechanical stability due to the significant volume changes during sodium plating/stripping cycles. The softer nature of sodium metal (0.69 GPa hardness compared to lithium's 0.91 GPa) exacerbates this issue, causing more severe morphological instabilities during cycling. This leads to continuous SEI breakdown and reformation, consuming both electrolyte and active sodium.
Sodium's higher chemical reactivity with conventional electrolyte components produces less favorable decomposition products compared to lithium systems. The resulting SEI layers typically exhibit higher ionic resistance, lower uniformity, and poorer adhesion to the sodium metal surface. Research has shown that sodium fluoride (NaF) components in the SEI, while beneficial for stability, are more difficult to form consistently than their lithium counterparts.
The inhomogeneous sodium deposition problem presents another major obstacle. Current passivation strategies struggle to promote uniform sodium nucleation and growth, leading to dendrite formation that eventually causes internal short circuits. Studies indicate that sodium dendrites can penetrate passivation layers more aggressively than lithium dendrites due to different growth mechanisms and mechanical properties.
Electrolyte compatibility issues further complicate passivation efforts. Many additives and formulations that effectively passivate lithium surfaces perform poorly with sodium, creating unstable interfaces that continue to evolve during cycling. The limited selection of sodium-compatible electrolyte systems that can form effective passivation layers restricts design flexibility.
Temperature sensitivity represents another significant challenge. Sodium-based SEI layers typically exhibit greater thermal instability than lithium counterparts, degrading more rapidly at elevated temperatures and forming different decomposition products at low temperatures. This narrow operational window limits practical applications in real-world environments where temperature fluctuations are common.
Manufacturing scalability of effective passivation techniques remains problematic. Laboratory-scale approaches like atomic layer deposition or specialized pre-treatment methods have shown promise but face significant barriers to industrial implementation. The cost-effectiveness and process compatibility of these techniques with existing battery manufacturing infrastructure require substantial improvement before commercial adoption becomes viable.
State-of-the-Art Passivation Layer Solutions
01 Artificial SEI formation on sodium metal anodes
Artificial solid electrolyte interphase (SEI) layers can be formed on sodium metal anodes to enhance their stability and longevity. These protective layers act as barriers against electrolyte decomposition while allowing sodium ion transport. Various methods for creating artificial SEI layers include chemical pretreatment, physical vapor deposition, and solution-based coating techniques. These engineered interfaces significantly improve cycling performance and reduce dendrite formation.- Artificial SEI formation on sodium metal anodes: Artificial solid electrolyte interphase (SEI) layers can be formed on sodium metal anodes to enhance their stability and longevity. These protective layers act as barriers against electrolyte decomposition while allowing sodium ion transport. Various methods including chemical pretreatment, coating deposition, and in-situ formation techniques can create these artificial SEI layers, significantly improving cycling performance and reducing dendrite formation.
- Polymer-based protective coatings for sodium anodes: Polymer-based protective coatings serve as effective passivation layers for sodium metal anodes. These polymeric materials create a flexible barrier that accommodates volume changes during cycling while preventing direct contact between the sodium metal and electrolyte. The polymer coatings can be functionalized to enhance sodium ion conductivity while blocking unwanted side reactions, thereby extending anode life and improving battery performance.
- Inorganic protective layers for sodium anodes: Inorganic materials can form robust passivation layers on sodium metal anodes to enhance their stability. These layers, composed of materials such as metal oxides, fluorides, or nitrides, provide mechanical strength and chemical stability against electrolyte attack. The inorganic protective layers can be deposited through various techniques including atomic layer deposition, sputtering, or chemical vapor deposition, creating uniform and conformal coatings that significantly extend anode lifetime.
- Electrolyte additives for in-situ passivation layer formation: Specific electrolyte additives can promote the formation of stable passivation layers on sodium metal anodes during battery operation. These additives decompose preferentially on the sodium surface, creating protective films that prevent continuous electrolyte degradation. The in-situ formed passivation layers help suppress dendrite growth and reduce irreversible capacity loss, leading to improved cycling stability and extended anode life in sodium-based battery systems.
- Surface treatment methods for sodium metal anodes: Various surface treatment methods can be applied to sodium metal anodes to create effective passivation layers. These include mechanical polishing, plasma treatment, chemical etching, and thermal processing. Such treatments modify the surface properties of sodium metal, creating more favorable interfaces for stable SEI formation. The treated surfaces exhibit reduced reactivity with electrolytes, suppressed dendrite formation, and improved electrochemical performance, ultimately extending the operational life of sodium metal anodes.
02 Polymer-based protective coatings for sodium anodes
Polymer-based protective coatings can be applied to sodium metal anodes to create effective passivation layers. These polymeric materials form flexible, ion-conductive interfaces that accommodate volume changes during cycling while preventing direct contact between sodium and the electrolyte. The polymer layers can be functionalized with specific groups to enhance sodium ion conductivity while maintaining mechanical integrity, resulting in improved anode lifespan and battery performance.Expand Specific Solutions03 Inorganic protective layers and ceramic coatings
Inorganic materials and ceramic coatings can be deposited on sodium metal anodes to create robust passivation layers. These materials, including metal oxides, nitrides, and fluorides, provide excellent mechanical stability and protection against electrolyte decomposition. The inorganic layers can be engineered with controlled thickness and composition to optimize sodium ion transport while maintaining structural integrity during cycling, significantly extending anode life.Expand Specific Solutions04 Electrolyte additives for in-situ passivation layer formation
Specific electrolyte additives can be incorporated to promote the in-situ formation of stable passivation layers on sodium metal anodes. These additives preferentially react with the sodium surface to create protective films with favorable properties for sodium ion transport. The resulting passivation layers help prevent continuous electrolyte decomposition and sodium dendrite growth, thereby enhancing the cycling stability and extending the operational life of sodium metal anodes.Expand Specific Solutions05 Composite and multi-layered protection strategies
Composite and multi-layered protection strategies combine different materials to create synergistic passivation layers on sodium metal anodes. These approaches typically involve sequential deposition of organic and inorganic components or gradient structures that provide multiple protective functions. The composite nature of these passivation layers offers enhanced mechanical stability, improved ionic conductivity, and better chemical resistance, resulting in superior cycling performance and extended anode life.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium metal anode technology for batteries is currently in an early growth phase, characterized by significant research activity but limited commercial deployment. The market size is expanding rapidly, driven by the need for cost-effective alternatives to lithium-ion batteries, with projections suggesting a multi-billion dollar opportunity by 2030. Technologically, surface passivation layers represent a critical innovation frontier, with varying degrees of maturity across key players. Leading organizations like BASF, LG Energy Solution, and Applied Materials are advancing industrial applications, while research institutions including Caltech, Max Planck Society, and Xiamen University are developing fundamental breakthroughs. Chinese companies such as BTR New Material Group and China Petroleum & Chemical Corp. are rapidly scaling capabilities, positioning themselves as significant competitors in this emerging field.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to sodium metal anode passivation through their "Controlled Interface Chemistry" technology. This system employs specially designed electrolyte formulations containing functional additives that decompose preferentially on the sodium surface to form a stable protective layer. Their proprietary fluorinated and phosphorylated compounds create a robust SEI containing NaF, Na3P, and Na2CO3 components that effectively suppress dendrite formation while maintaining high ionic conductivity. BASF's approach also includes surface-active polymers that adhere to the sodium metal surface, providing mechanical stability during volume changes. The company has further advanced this technology by incorporating nanostructured ceramic particles into the protective layer, creating a composite passivation film that combines the benefits of organic flexibility and inorganic stability. Their research demonstrates that these engineered interfaces can extend sodium metal anode cycling life by over 500% compared to conventional electrolytes.
Strengths: Extensive chemical expertise and manufacturing infrastructure enable large-scale production of specialized electrolyte additives; strong position in the chemical supply chain provides market advantage. Weaknesses: Their solutions may be more focused on chemical approaches rather than physical protection methods; some proprietary additives may face regulatory challenges.
Faradion Ltd.
Technical Solution: Faradion has pioneered a proprietary surface passivation technology specifically designed for sodium metal anodes in ambient temperature sodium-ion batteries. Their approach utilizes a multi-component electrolyte formulation containing fluorinated additives that spontaneously form a stable and ion-conductive solid electrolyte interphase (SEI) on sodium metal surfaces. This engineered SEI contains sodium fluoride compounds that effectively prevent continuous electrolyte decomposition while maintaining high sodium-ion conductivity. Faradion's technology also incorporates phosphorus-based compounds that create a flexible, self-healing passivation layer capable of accommodating the volumetric changes during sodium plating/stripping cycles. Their latest advancement includes a gradient-structured artificial SEI with varying chemical compositions from the sodium metal interface to the electrolyte-facing surface, optimizing both interfacial stability and ion transport properties.
Strengths: Specialized focus on sodium-ion technology gives them deep expertise in sodium metal interfaces; their solutions are designed specifically for commercial viability and cost-effectiveness. Weaknesses: As a smaller company, they have limited manufacturing capacity compared to larger competitors; their technology may face challenges in scaling to mass production.
Critical Patents and Research on Na-Metal Interface Engineering
Aqueous aluminum batteries and methods of making same
PatentWO2020033963A1
Innovation
- The introduction of a passivation layer, specifically an artificial solid electrolyte interface (ASEI), formed by contacting aluminum anodes with a composition of ionic liquids and aluminum halides, which prevents oxide passivation and enables reversible aluminum plating and stripping in aqueous electrolytes, allowing for the creation of rechargeable aluminum batteries.
Method of passivating metallic surfaces by means of copolymers having phosphoric acid and/or phosphonic acid groups
PatentInactiveEP2049708A1
Innovation
- An acidic preparation with a pH <5, containing a copolymer composed of mono(meth)acrylic esters, phosphoric acid/phosphonic acid groups, and COOH groups, is used to passivate metallic surfaces, specifically formulated to minimize chalking and enhance optical properties.
Safety and Stability Considerations for Na-Metal Batteries
Safety considerations for sodium metal batteries represent a critical aspect of their development pathway toward commercial viability. Unlike lithium-ion batteries, sodium metal anodes present unique safety challenges due to sodium's higher reactivity with air and moisture, lower melting point (97.8°C compared to lithium's 180.5°C), and more vigorous reactions with electrolytes. These properties significantly increase the risk of thermal runaway events and potential battery fires under abuse conditions.
The stability of Na-metal batteries is intrinsically linked to the formation and maintenance of effective surface passivation layers. When these protective layers fail or become compromised, sodium can react exothermically with electrolyte components, generating heat and potentially triggering cascading failure mechanisms. Research indicates that conventional carbonate-based electrolytes form unstable SEI layers on sodium metal, leading to continuous electrolyte decomposition and safety hazards during cycling.
Temperature sensitivity presents another major concern for Na-metal battery systems. At elevated temperatures, sodium's low melting point can lead to localized melting, accelerated dendrite formation, and potential internal short circuits. Conversely, at low temperatures, the kinetics of sodium ion transport through the SEI layer can become severely limited, increasing cell impedance and the likelihood of sodium plating in non-uniform patterns that compromise safety.
Dendrite formation remains perhaps the most significant stability challenge for Na-metal batteries. These needle-like structures can penetrate separators, causing internal short circuits and catastrophic failure. Current research indicates that surface passivation layers play a crucial role in mitigating dendrite growth by promoting more uniform sodium deposition. Fluorinated additives and artificial SEI components have shown promise in improving the mechanical strength and ionic conductivity of these protective layers.
Gas generation during cycling represents another stability concern directly related to surface chemistry. Continuous electrolyte decomposition at the sodium metal interface can generate various gaseous products, leading to cell swelling, increased internal pressure, and potential rupture. Advanced electrolyte formulations incorporating FEC (fluoroethylene carbonate) and other functional additives have demonstrated reduced gas evolution through the formation of more stable passivation films.
Long-term calendar aging effects must also be considered when evaluating Na-metal battery safety. Even during storage, slow but continuous reactions between sodium metal and electrolyte components can degrade performance and compromise safety margins. The development of self-healing passivation layers that can maintain integrity during extended idle periods represents an important research direction for improving the practical viability of these battery systems.
The stability of Na-metal batteries is intrinsically linked to the formation and maintenance of effective surface passivation layers. When these protective layers fail or become compromised, sodium can react exothermically with electrolyte components, generating heat and potentially triggering cascading failure mechanisms. Research indicates that conventional carbonate-based electrolytes form unstable SEI layers on sodium metal, leading to continuous electrolyte decomposition and safety hazards during cycling.
Temperature sensitivity presents another major concern for Na-metal battery systems. At elevated temperatures, sodium's low melting point can lead to localized melting, accelerated dendrite formation, and potential internal short circuits. Conversely, at low temperatures, the kinetics of sodium ion transport through the SEI layer can become severely limited, increasing cell impedance and the likelihood of sodium plating in non-uniform patterns that compromise safety.
Dendrite formation remains perhaps the most significant stability challenge for Na-metal batteries. These needle-like structures can penetrate separators, causing internal short circuits and catastrophic failure. Current research indicates that surface passivation layers play a crucial role in mitigating dendrite growth by promoting more uniform sodium deposition. Fluorinated additives and artificial SEI components have shown promise in improving the mechanical strength and ionic conductivity of these protective layers.
Gas generation during cycling represents another stability concern directly related to surface chemistry. Continuous electrolyte decomposition at the sodium metal interface can generate various gaseous products, leading to cell swelling, increased internal pressure, and potential rupture. Advanced electrolyte formulations incorporating FEC (fluoroethylene carbonate) and other functional additives have demonstrated reduced gas evolution through the formation of more stable passivation films.
Long-term calendar aging effects must also be considered when evaluating Na-metal battery safety. Even during storage, slow but continuous reactions between sodium metal and electrolyte components can degrade performance and compromise safety margins. The development of self-healing passivation layers that can maintain integrity during extended idle periods represents an important research direction for improving the practical viability of these battery systems.
Environmental Impact and Sustainability Assessment
The environmental impact of sodium-ion battery technologies, particularly those utilizing surface passivation layers on sodium metal anodes, represents a critical consideration in their development and deployment. When compared to conventional lithium-ion batteries, sodium-based systems offer significant sustainability advantages due to sodium's greater natural abundance. Sodium resources are approximately 1,000 times more plentiful than lithium in the Earth's crust, reducing extraction pressures on limited mineral resources and potentially decreasing mining-related environmental degradation.
The production processes for surface passivation layers on sodium metal anodes generally require fewer toxic materials than comparable lithium-ion technologies. Many passivation approaches utilize organic compounds, fluoride-based materials, or polymer coatings that can be synthesized through relatively low-impact chemical processes. However, certain advanced passivation techniques involving rare earth elements or specialized nanostructures may introduce new environmental concerns related to material sourcing and manufacturing emissions.
Life cycle assessment (LCA) studies indicate that the extended anode life achieved through effective passivation layers significantly improves the overall environmental profile of sodium batteries. By preventing dendrite formation and reducing electrolyte decomposition, these protective layers can extend battery cycle life by 300-500%, directly reducing waste generation and resource consumption per unit of energy stored over the battery's lifetime.
End-of-life considerations for sodium metal anodes with passivation layers present both challenges and opportunities. The diverse chemical compositions of these protective layers may complicate recycling processes, potentially requiring specialized separation techniques. However, the sodium metal itself represents a valuable and readily recoverable resource, with established recycling pathways that achieve recovery rates exceeding 90% in optimized systems.
Carbon footprint analyses reveal that sodium-ion batteries with enhanced anode protection layers can achieve 30-45% lower greenhouse gas emissions compared to conventional lithium-ion batteries when evaluated on a full life cycle basis. This advantage stems from both the reduced environmental impact of raw materials and the extended operational lifetime enabled by effective passivation strategies.
Regulatory frameworks worldwide are increasingly recognizing the sustainability benefits of sodium-based energy storage. The European Union's Battery Directive revisions and similar policies in Asia and North America are beginning to incorporate incentives for technologies that reduce dependence on critical minerals while maintaining performance standards, creating a favorable policy environment for continued development of sodium metal anodes with advanced passivation technologies.
The production processes for surface passivation layers on sodium metal anodes generally require fewer toxic materials than comparable lithium-ion technologies. Many passivation approaches utilize organic compounds, fluoride-based materials, or polymer coatings that can be synthesized through relatively low-impact chemical processes. However, certain advanced passivation techniques involving rare earth elements or specialized nanostructures may introduce new environmental concerns related to material sourcing and manufacturing emissions.
Life cycle assessment (LCA) studies indicate that the extended anode life achieved through effective passivation layers significantly improves the overall environmental profile of sodium batteries. By preventing dendrite formation and reducing electrolyte decomposition, these protective layers can extend battery cycle life by 300-500%, directly reducing waste generation and resource consumption per unit of energy stored over the battery's lifetime.
End-of-life considerations for sodium metal anodes with passivation layers present both challenges and opportunities. The diverse chemical compositions of these protective layers may complicate recycling processes, potentially requiring specialized separation techniques. However, the sodium metal itself represents a valuable and readily recoverable resource, with established recycling pathways that achieve recovery rates exceeding 90% in optimized systems.
Carbon footprint analyses reveal that sodium-ion batteries with enhanced anode protection layers can achieve 30-45% lower greenhouse gas emissions compared to conventional lithium-ion batteries when evaluated on a full life cycle basis. This advantage stems from both the reduced environmental impact of raw materials and the extended operational lifetime enabled by effective passivation strategies.
Regulatory frameworks worldwide are increasingly recognizing the sustainability benefits of sodium-based energy storage. The European Union's Battery Directive revisions and similar policies in Asia and North America are beginning to incorporate incentives for technologies that reduce dependence on critical minerals while maintaining performance standards, creating a favorable policy environment for continued development of sodium metal anodes with advanced passivation technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!