Advances in Sulfamic Acid Synthesis Routes and Techniques
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Evolution and Objectives
Sulfamic acid, a versatile compound with the chemical formula H3NSO3, has undergone significant evolution since its discovery in the early 20th century. Initially synthesized as a curiosity in laboratories, it quickly gained prominence due to its unique properties and wide-ranging applications. The development of sulfamic acid has been driven by the increasing demand for efficient and environmentally friendly cleaning agents, water treatment chemicals, and industrial catalysts.
The historical trajectory of sulfamic acid synthesis has seen several key milestones. Early production methods relied on the reaction between sulfur trioxide and ammonia, which, while effective, posed safety and scaling challenges. As industrial demand grew, researchers focused on developing more efficient and safer synthesis routes. The introduction of the urea-sulfuric acid method in the mid-20th century marked a significant advancement, offering a more controlled and economical production process.
In recent decades, the objectives for sulfamic acid synthesis have shifted towards sustainability and process optimization. Environmental concerns have spurred efforts to develop greener synthesis routes that minimize waste and reduce energy consumption. Simultaneously, there has been a push to improve product purity and consistency to meet the stringent requirements of various industries, particularly in electronic and pharmaceutical applications.
Current research in sulfamic acid synthesis is primarily focused on three main objectives. First, there is a concerted effort to develop catalytic processes that can operate under milder conditions, reducing energy inputs and improving overall efficiency. Second, researchers are exploring novel raw materials and reaction pathways to decrease reliance on traditional, potentially hazardous precursors. Third, there is growing interest in continuous flow synthesis techniques, which offer the potential for better process control, improved safety, and enhanced scalability.
The evolution of sulfamic acid synthesis techniques is closely tied to advancements in related fields such as catalysis, process engineering, and materials science. Innovations in these areas have enabled the development of more sophisticated synthesis methods, including the use of heterogeneous catalysts and microreactor technology. These advancements not only improve the efficiency and sustainability of sulfamic acid production but also open up possibilities for new applications and derivatives.
Looking ahead, the future objectives for sulfamic acid synthesis are likely to focus on further enhancing sustainability, exploring bio-based precursors, and developing tailored synthesis routes for specific high-value applications. As industries continue to demand higher purity and more specialized forms of sulfamic acid, research efforts will likely intensify to meet these evolving needs while adhering to increasingly stringent environmental regulations.
The historical trajectory of sulfamic acid synthesis has seen several key milestones. Early production methods relied on the reaction between sulfur trioxide and ammonia, which, while effective, posed safety and scaling challenges. As industrial demand grew, researchers focused on developing more efficient and safer synthesis routes. The introduction of the urea-sulfuric acid method in the mid-20th century marked a significant advancement, offering a more controlled and economical production process.
In recent decades, the objectives for sulfamic acid synthesis have shifted towards sustainability and process optimization. Environmental concerns have spurred efforts to develop greener synthesis routes that minimize waste and reduce energy consumption. Simultaneously, there has been a push to improve product purity and consistency to meet the stringent requirements of various industries, particularly in electronic and pharmaceutical applications.
Current research in sulfamic acid synthesis is primarily focused on three main objectives. First, there is a concerted effort to develop catalytic processes that can operate under milder conditions, reducing energy inputs and improving overall efficiency. Second, researchers are exploring novel raw materials and reaction pathways to decrease reliance on traditional, potentially hazardous precursors. Third, there is growing interest in continuous flow synthesis techniques, which offer the potential for better process control, improved safety, and enhanced scalability.
The evolution of sulfamic acid synthesis techniques is closely tied to advancements in related fields such as catalysis, process engineering, and materials science. Innovations in these areas have enabled the development of more sophisticated synthesis methods, including the use of heterogeneous catalysts and microreactor technology. These advancements not only improve the efficiency and sustainability of sulfamic acid production but also open up possibilities for new applications and derivatives.
Looking ahead, the future objectives for sulfamic acid synthesis are likely to focus on further enhancing sustainability, exploring bio-based precursors, and developing tailored synthesis routes for specific high-value applications. As industries continue to demand higher purity and more specialized forms of sulfamic acid, research efforts will likely intensify to meet these evolving needs while adhering to increasingly stringent environmental regulations.
Industrial Demand Analysis
The global demand for sulfamic acid has been steadily increasing due to its versatile applications across various industries. The market for sulfamic acid is primarily driven by its use as a cleaning agent, particularly in the household and industrial sectors. The growing emphasis on hygiene and sanitation, especially in the wake of global health concerns, has further boosted the demand for sulfamic acid-based cleaning products.
In the industrial sector, sulfamic acid plays a crucial role in metal finishing and descaling operations. The expanding manufacturing and metalworking industries, particularly in emerging economies, have contributed significantly to the market growth. Additionally, the water treatment industry has been a major consumer of sulfamic acid, utilizing it for scale removal and pH adjustment in various water systems.
The agricultural sector represents another significant market for sulfamic acid, where it is used in the production of herbicides and pesticides. As global food demand continues to rise, the need for effective crop protection solutions has led to increased adoption of sulfamic acid-based agricultural chemicals.
The pulp and paper industry has also been a consistent consumer of sulfamic acid, employing it in bleaching processes and as a chlorine stabilizer. With the growing demand for paper products, especially in developing regions, this sector continues to drive the sulfamic acid market.
Recent trends indicate a rising interest in environmentally friendly and biodegradable alternatives to traditional cleaning agents. This has prompted research into developing more sustainable synthesis routes for sulfamic acid, potentially expanding its market reach to eco-conscious consumers and industries.
The pharmaceutical industry has shown increasing interest in sulfamic acid as a reagent in drug synthesis. As the global pharmaceutical market expands, this sector is expected to become a more significant driver of sulfamic acid demand in the coming years.
Geographically, Asia-Pacific has emerged as the largest consumer of sulfamic acid, driven by rapid industrialization, urbanization, and population growth. North America and Europe follow, with mature markets focused on high-quality, specialized applications of sulfamic acid.
Despite the positive growth trajectory, the sulfamic acid market faces challenges such as price volatility of raw materials and stringent environmental regulations in some regions. These factors have spurred innovation in synthesis techniques, aiming to develop more cost-effective and environmentally sustainable production methods.
In the industrial sector, sulfamic acid plays a crucial role in metal finishing and descaling operations. The expanding manufacturing and metalworking industries, particularly in emerging economies, have contributed significantly to the market growth. Additionally, the water treatment industry has been a major consumer of sulfamic acid, utilizing it for scale removal and pH adjustment in various water systems.
The agricultural sector represents another significant market for sulfamic acid, where it is used in the production of herbicides and pesticides. As global food demand continues to rise, the need for effective crop protection solutions has led to increased adoption of sulfamic acid-based agricultural chemicals.
The pulp and paper industry has also been a consistent consumer of sulfamic acid, employing it in bleaching processes and as a chlorine stabilizer. With the growing demand for paper products, especially in developing regions, this sector continues to drive the sulfamic acid market.
Recent trends indicate a rising interest in environmentally friendly and biodegradable alternatives to traditional cleaning agents. This has prompted research into developing more sustainable synthesis routes for sulfamic acid, potentially expanding its market reach to eco-conscious consumers and industries.
The pharmaceutical industry has shown increasing interest in sulfamic acid as a reagent in drug synthesis. As the global pharmaceutical market expands, this sector is expected to become a more significant driver of sulfamic acid demand in the coming years.
Geographically, Asia-Pacific has emerged as the largest consumer of sulfamic acid, driven by rapid industrialization, urbanization, and population growth. North America and Europe follow, with mature markets focused on high-quality, specialized applications of sulfamic acid.
Despite the positive growth trajectory, the sulfamic acid market faces challenges such as price volatility of raw materials and stringent environmental regulations in some regions. These factors have spurred innovation in synthesis techniques, aiming to develop more cost-effective and environmentally sustainable production methods.
Synthesis Challenges
The synthesis of sulfamic acid presents several significant challenges that researchers and manufacturers must overcome to improve production efficiency and product quality. One of the primary obstacles is the control of reaction conditions, particularly temperature and pressure. The exothermic nature of the synthesis process requires precise temperature management to prevent runaway reactions and ensure product purity. Maintaining optimal pressure conditions is equally crucial, as it affects the reaction kinetics and yield.
Another major challenge lies in the selection and handling of raw materials. The traditional synthesis route involves the reaction of urea with sulfuric acid or oleum. However, the quality and consistency of these precursors can significantly impact the final product. Impurities in the raw materials can lead to side reactions, reducing yield and compromising the purity of the sulfamic acid produced. Additionally, the corrosive nature of the reactants necessitates specialized equipment and safety measures, adding complexity to the manufacturing process.
The formation of unwanted by-products poses a persistent challenge in sulfamic acid synthesis. Side reactions can lead to the production of ammonium sulfate and other impurities, which not only reduce the yield but also complicate the purification process. Developing strategies to minimize these side reactions or efficiently separate the desired product from contaminants is an ongoing area of research.
Scale-up issues present another set of challenges when transitioning from laboratory-scale synthesis to industrial production. The heat transfer and mixing dynamics change significantly at larger scales, potentially affecting reaction rates and product quality. Engineers must carefully design reactor systems and process controls to maintain consistent performance across different production volumes.
Environmental concerns and regulatory compliance add another layer of complexity to sulfamic acid synthesis. The process generates waste streams that require proper treatment and disposal. Developing more environmentally friendly synthesis routes or implementing effective waste management strategies is crucial for sustainable production. Moreover, meeting increasingly stringent regulatory standards for product purity and environmental impact necessitates continuous improvement in synthesis techniques and quality control measures.
Lastly, the energy intensity of traditional sulfamic acid synthesis methods presents both economic and environmental challenges. The high temperatures and pressures required in conventional processes contribute to significant energy consumption and associated costs. Research into more energy-efficient synthesis routes, such as catalytic processes or alternative reaction pathways, is essential for improving the overall sustainability and economic viability of sulfamic acid production.
Another major challenge lies in the selection and handling of raw materials. The traditional synthesis route involves the reaction of urea with sulfuric acid or oleum. However, the quality and consistency of these precursors can significantly impact the final product. Impurities in the raw materials can lead to side reactions, reducing yield and compromising the purity of the sulfamic acid produced. Additionally, the corrosive nature of the reactants necessitates specialized equipment and safety measures, adding complexity to the manufacturing process.
The formation of unwanted by-products poses a persistent challenge in sulfamic acid synthesis. Side reactions can lead to the production of ammonium sulfate and other impurities, which not only reduce the yield but also complicate the purification process. Developing strategies to minimize these side reactions or efficiently separate the desired product from contaminants is an ongoing area of research.
Scale-up issues present another set of challenges when transitioning from laboratory-scale synthesis to industrial production. The heat transfer and mixing dynamics change significantly at larger scales, potentially affecting reaction rates and product quality. Engineers must carefully design reactor systems and process controls to maintain consistent performance across different production volumes.
Environmental concerns and regulatory compliance add another layer of complexity to sulfamic acid synthesis. The process generates waste streams that require proper treatment and disposal. Developing more environmentally friendly synthesis routes or implementing effective waste management strategies is crucial for sustainable production. Moreover, meeting increasingly stringent regulatory standards for product purity and environmental impact necessitates continuous improvement in synthesis techniques and quality control measures.
Lastly, the energy intensity of traditional sulfamic acid synthesis methods presents both economic and environmental challenges. The high temperatures and pressures required in conventional processes contribute to significant energy consumption and associated costs. Research into more energy-efficient synthesis routes, such as catalytic processes or alternative reaction pathways, is essential for improving the overall sustainability and economic viability of sulfamic acid production.
Current Synthesis Methods
01 Synthesis and production of sulfamic acid
Various methods and processes for synthesizing and producing sulfamic acid are described. These include reactions involving sulfur trioxide and ammonia, as well as other chemical pathways to efficiently manufacture sulfamic acid on an industrial scale.- Synthesis and production of sulfamic acid: Various methods for synthesizing and producing sulfamic acid are described. These processes often involve the reaction of sulfur trioxide with ammonia or urea under specific conditions. The production methods aim to improve yield, purity, and efficiency of sulfamic acid synthesis.
- Applications in cleaning and descaling: Sulfamic acid is widely used in cleaning and descaling formulations. It is effective in removing mineral deposits, rust, and scale from various surfaces and equipment. These applications include household cleaners, industrial descaling agents, and specialized cleaning products for specific industries.
- Use in water treatment and purification: Sulfamic acid plays a role in water treatment and purification processes. It is used for pH adjustment, scale prevention, and as a component in water treatment chemicals. Applications include industrial water systems, cooling towers, and municipal water treatment facilities.
- Agricultural and horticultural applications: Sulfamic acid finds use in agricultural and horticultural settings. It is employed in fertilizer formulations, soil pH adjustment, and as a component in certain pesticides or herbicides. These applications aim to improve crop yield and plant health.
- Industrial and chemical processing: Sulfamic acid is utilized in various industrial and chemical processes. It serves as a reagent in organic synthesis, a catalyst in certain reactions, and a component in electroplating baths. Additionally, it is used in the production of artificial sweeteners and other specialty chemicals.
02 Applications in cleaning and descaling
Sulfamic acid is widely used in cleaning and descaling formulations. It is effective in removing mineral deposits, limescale, and rust from various surfaces, making it valuable in household and industrial cleaning products.Expand Specific Solutions03 Use in water treatment
Sulfamic acid finds applications in water treatment processes. It is used for pH adjustment, scale prevention, and as a component in water treatment chemicals for both industrial and municipal water systems.Expand Specific Solutions04 Agricultural and horticultural applications
Sulfamic acid is utilized in various agricultural and horticultural products. It serves as a component in fertilizers, soil conditioners, and plant growth regulators, contributing to improved crop yields and plant health.Expand Specific Solutions05 Industrial and chemical processing
Sulfamic acid plays a role in numerous industrial and chemical processes. It is used as a sulfonating agent, in the production of artificial sweeteners, and as a catalyst or reagent in various chemical reactions and manufacturing processes.Expand Specific Solutions
Key Industry Players
The field of sulfamic acid synthesis is in a mature stage, with established techniques and a stable market. The global sulfamic acid market size is estimated to be around $300 million, with steady growth projected. Technologically, the industry is well-developed, with ongoing research focused on improving efficiency and sustainability. Key players like The Regents of the University of California, Zhejiang University, and East China Normal University are contributing to academic advancements. Companies such as Evonik Operations GmbH, Sanofi, and DuPont Safety & Construction, Inc. are driving industrial applications and innovations. The competitive landscape is characterized by a mix of established chemical companies and specialized manufacturers, with increasing emphasis on eco-friendly production methods and novel applications in various sectors.
Tessenderlo Kerley, Inc.
Technical Solution: Tessenderlo Kerley has developed an innovative sulfamic acid synthesis route utilizing a continuous flow reactor system. This method involves the reaction of urea with sulfuric acid under controlled temperature and pressure conditions. The process achieves higher yields (up to 95%) compared to traditional batch processes [1]. The company has also implemented a novel purification technique using selective crystallization, which results in sulfamic acid with 99.9% purity [3]. Additionally, they have introduced a closed-loop recycling system for unreacted materials, significantly reducing waste and improving overall process efficiency [5].
Strengths: High yield and purity, improved process efficiency, and reduced waste. Weaknesses: May require specialized equipment and higher initial capital investment.
Clariant Finance (BVI) Ltd.
Technical Solution: Clariant has developed an innovative sulfamic acid synthesis route based on the reaction of sulfur dioxide with urea. This process operates at milder conditions compared to traditional methods, reducing energy requirements and equipment corrosion [1]. The company has also implemented a novel gas absorption technology that improves the efficiency of sulfur dioxide utilization, leading to higher yields and reduced emissions [3]. Clariant's process incorporates a proprietary catalyst system that enhances reaction selectivity and reduces the formation of by-products [5]. Additionally, they have developed an advanced process control strategy using model predictive control algorithms, which optimizes process parameters in real-time and ensures consistent product quality [7].
Strengths: Lower energy consumption, reduced corrosion issues, and improved environmental profile. Weaknesses: May be more sensitive to raw material quality and require specialized gas handling equipment.
Innovative Catalysts
Process for the production of sulfuric acid
PatentActiveUS7361326B2
Innovation
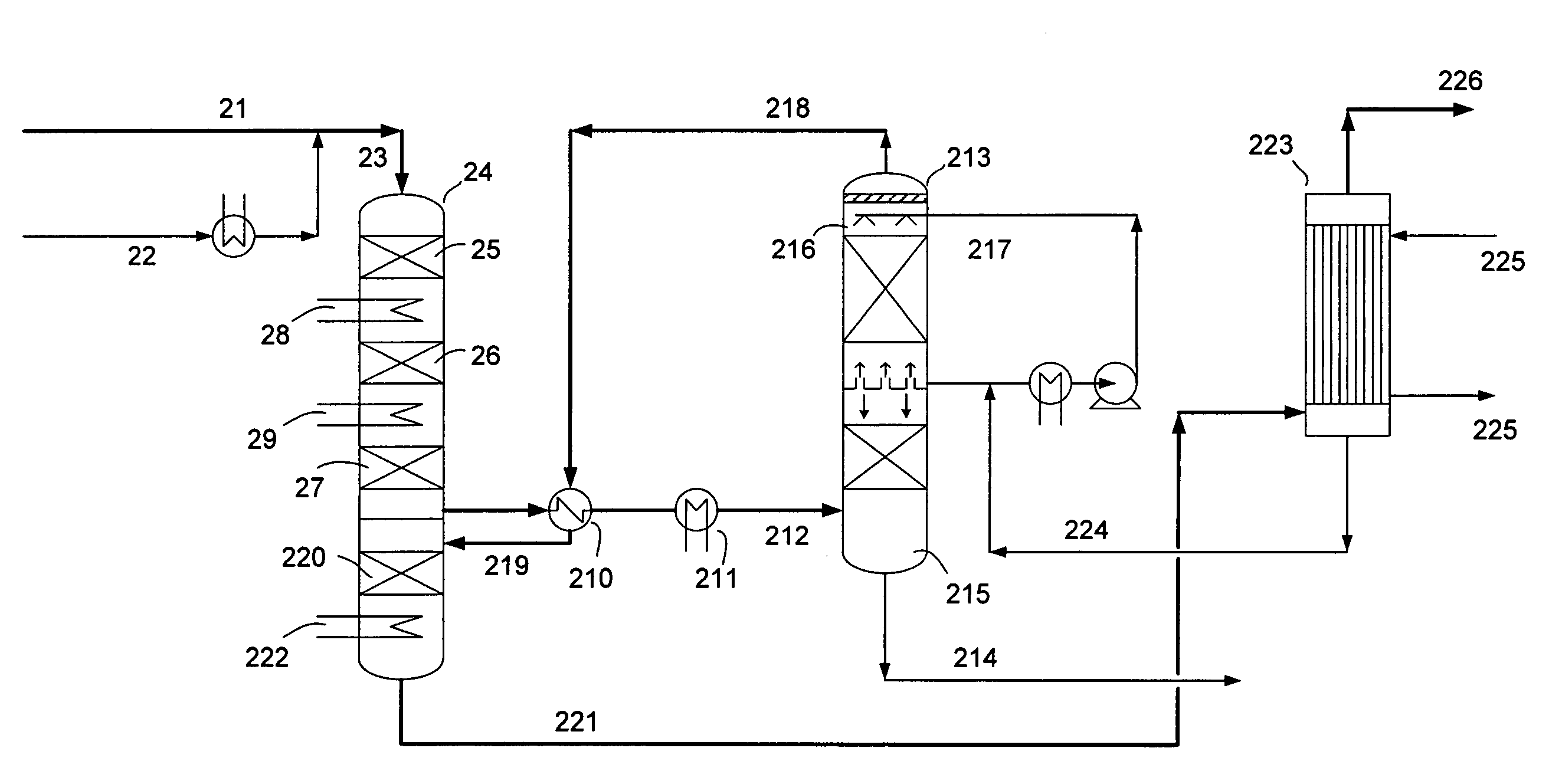
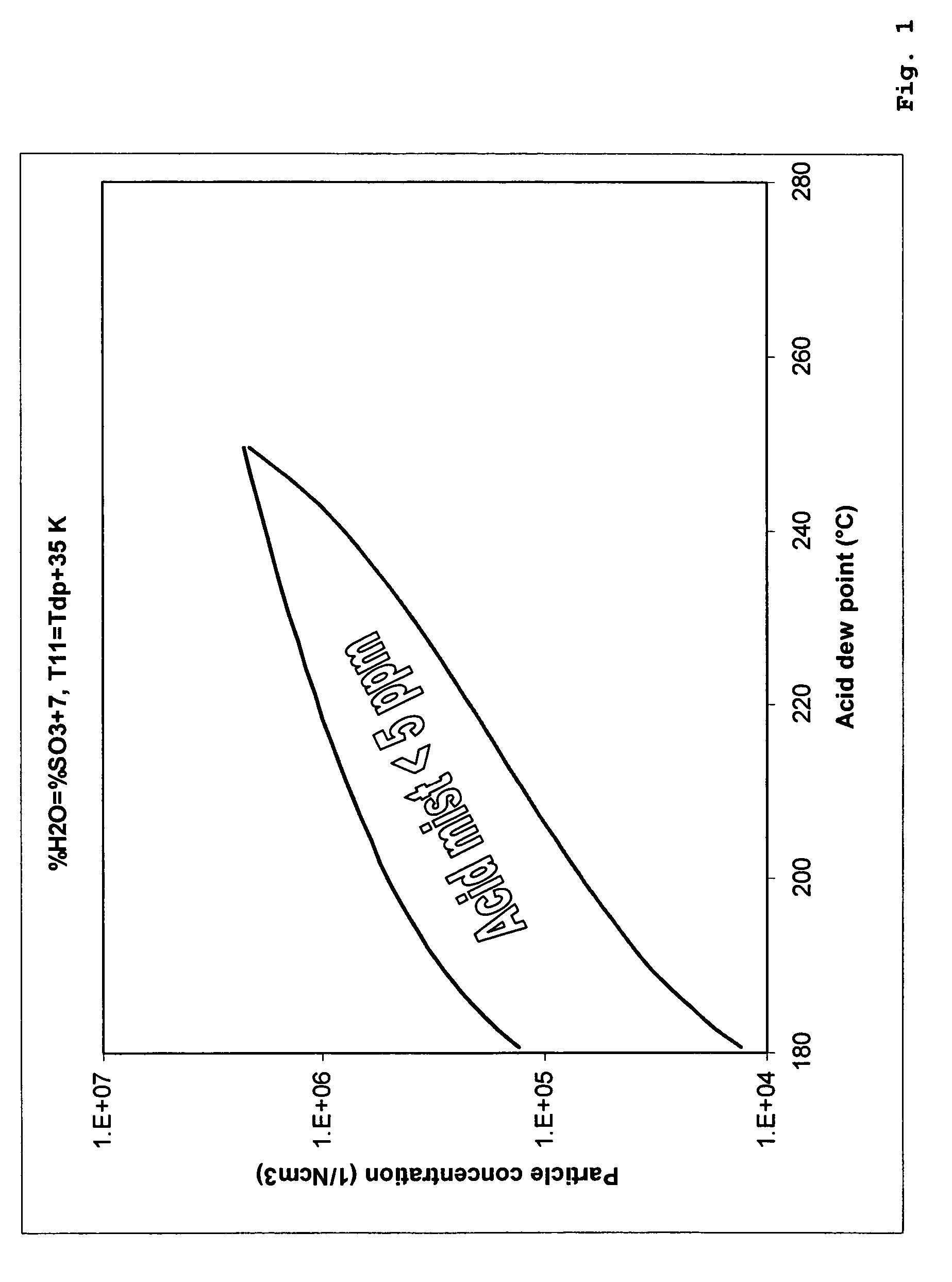
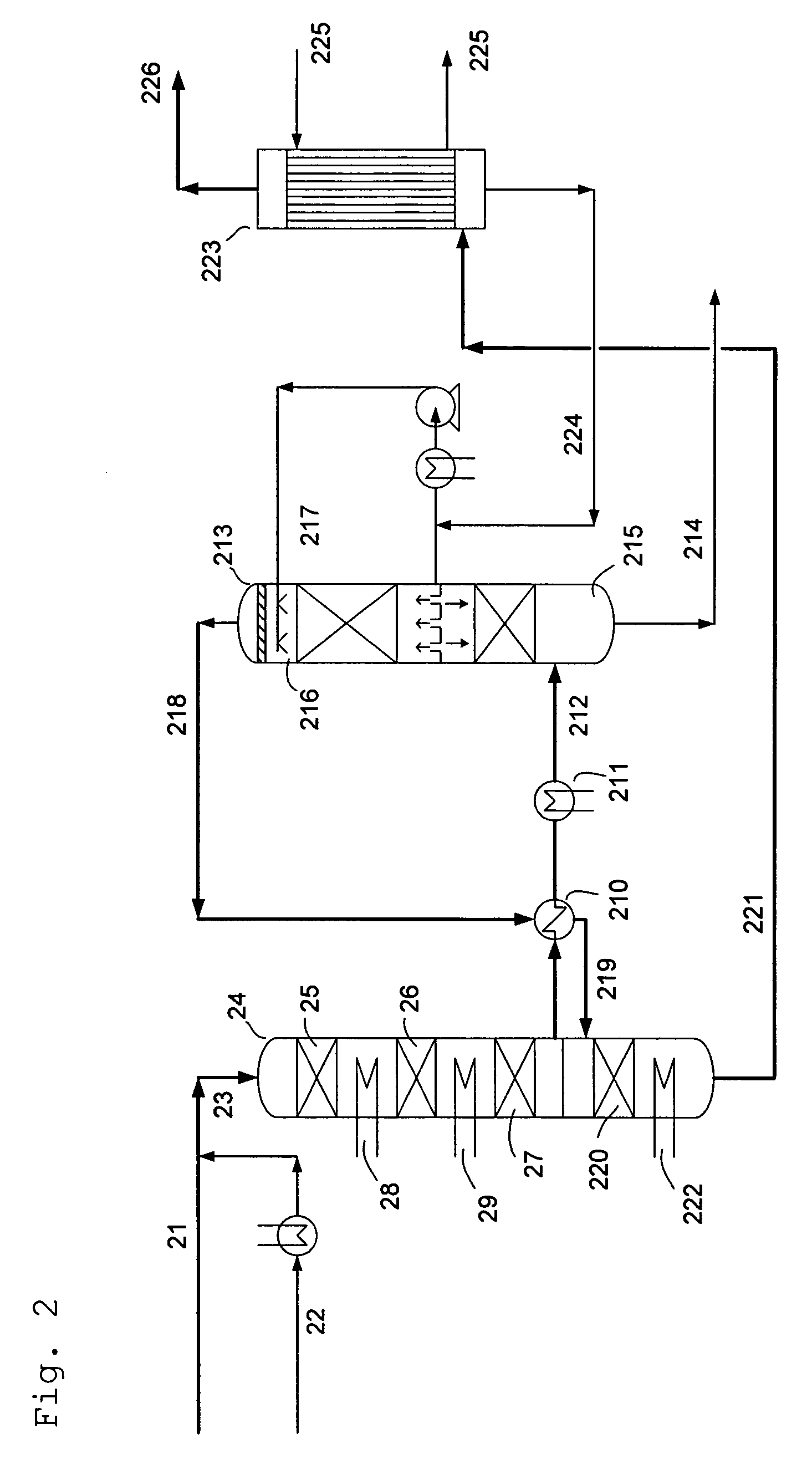
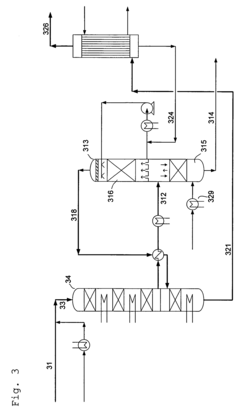
- A process involving a two-stage condensation system where SO2 is oxidized to SO3 in multiple catalytic conversion steps, with an intermediate condensation stage followed by a final wet condensation stage, using a molar excess of water to maintain a low acid dew point and incorporating solid particles as nuclei for effective acid mist control, allowing for high SO2 conversion and low acid mist emissions.
Concentration of sulfuric acid
PatentPendingUS20230117014A1
Innovation
- A method involving a treatment system with multiple hyperfiltration stages and specific membrane configurations that include modules with membranes suitable for passing less than 10% salt, using pumps to pressurize feed streams and generate permeate, resulting in a first fraction with a pH between 4 and 10 and a second fraction containing at least 20 weight percent sulfuric acid.
Environmental Impact
The environmental impact of sulfamic acid synthesis has become a critical concern in recent years, prompting researchers and industry professionals to explore more sustainable production methods. Traditional synthesis routes often involve the use of hazardous chemicals and generate significant amounts of waste, contributing to environmental pollution and resource depletion.
One of the primary environmental challenges associated with sulfamic acid production is the generation of sulfur dioxide (SO2) as a byproduct. SO2 is a major air pollutant that contributes to acid rain and respiratory issues in humans and animals. To address this problem, researchers have developed innovative techniques to capture and convert SO2 into useful products, such as sulfuric acid or elemental sulfur, thereby reducing emissions and improving overall process efficiency.
Water consumption and contamination are also significant environmental concerns in sulfamic acid synthesis. Conventional methods often require large volumes of water for cooling and purification processes, leading to increased water stress in manufacturing regions. Additionally, wastewater from these processes may contain high levels of sulfates and other pollutants, necessitating extensive treatment before discharge. Recent advancements in process intensification and water recycling technologies have shown promise in reducing water usage and minimizing the environmental impact of wastewater discharge.
Energy efficiency is another crucial aspect of the environmental impact of sulfamic acid production. Traditional synthesis routes typically involve high-temperature reactions and energy-intensive separation processes, contributing to increased greenhouse gas emissions. To address this issue, researchers have been exploring low-temperature catalytic processes and alternative energy sources, such as microwave-assisted synthesis, to reduce energy consumption and carbon footprint.
The use of raw materials in sulfamic acid synthesis also has significant environmental implications. Conventional methods often rely on non-renewable fossil fuel-derived feedstocks, contributing to resource depletion and climate change. Recent research has focused on developing bio-based alternatives and utilizing waste streams as raw materials, promoting a more circular economy approach to sulfamic acid production.
As environmental regulations become increasingly stringent, the sulfamic acid industry is under pressure to adopt cleaner production technologies. This has led to the development of green chemistry approaches, such as solvent-free synthesis methods and the use of environmentally benign catalysts. These innovations not only reduce the environmental impact of sulfamic acid production but also offer potential cost savings and improved product quality.
In conclusion, the environmental impact of sulfamic acid synthesis remains a significant challenge, but ongoing research and technological advancements are paving the way for more sustainable production methods. By addressing issues such as air and water pollution, energy efficiency, and raw material sourcing, the industry is moving towards a more environmentally responsible future.
One of the primary environmental challenges associated with sulfamic acid production is the generation of sulfur dioxide (SO2) as a byproduct. SO2 is a major air pollutant that contributes to acid rain and respiratory issues in humans and animals. To address this problem, researchers have developed innovative techniques to capture and convert SO2 into useful products, such as sulfuric acid or elemental sulfur, thereby reducing emissions and improving overall process efficiency.
Water consumption and contamination are also significant environmental concerns in sulfamic acid synthesis. Conventional methods often require large volumes of water for cooling and purification processes, leading to increased water stress in manufacturing regions. Additionally, wastewater from these processes may contain high levels of sulfates and other pollutants, necessitating extensive treatment before discharge. Recent advancements in process intensification and water recycling technologies have shown promise in reducing water usage and minimizing the environmental impact of wastewater discharge.
Energy efficiency is another crucial aspect of the environmental impact of sulfamic acid production. Traditional synthesis routes typically involve high-temperature reactions and energy-intensive separation processes, contributing to increased greenhouse gas emissions. To address this issue, researchers have been exploring low-temperature catalytic processes and alternative energy sources, such as microwave-assisted synthesis, to reduce energy consumption and carbon footprint.
The use of raw materials in sulfamic acid synthesis also has significant environmental implications. Conventional methods often rely on non-renewable fossil fuel-derived feedstocks, contributing to resource depletion and climate change. Recent research has focused on developing bio-based alternatives and utilizing waste streams as raw materials, promoting a more circular economy approach to sulfamic acid production.
As environmental regulations become increasingly stringent, the sulfamic acid industry is under pressure to adopt cleaner production technologies. This has led to the development of green chemistry approaches, such as solvent-free synthesis methods and the use of environmentally benign catalysts. These innovations not only reduce the environmental impact of sulfamic acid production but also offer potential cost savings and improved product quality.
In conclusion, the environmental impact of sulfamic acid synthesis remains a significant challenge, but ongoing research and technological advancements are paving the way for more sustainable production methods. By addressing issues such as air and water pollution, energy efficiency, and raw material sourcing, the industry is moving towards a more environmentally responsible future.
Safety Regulations
Safety regulations play a crucial role in the advancement of sulfamic acid synthesis routes and techniques. As the production and handling of sulfamic acid involve potentially hazardous materials and processes, stringent safety measures are essential to protect workers, the environment, and the surrounding communities.
The primary safety concerns in sulfamic acid synthesis include the handling of corrosive substances, potential for exothermic reactions, and the generation of toxic fumes. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established comprehensive guidelines for the safe production and use of sulfamic acid.
One of the key safety regulations focuses on personal protective equipment (PPE). Workers involved in sulfamic acid synthesis must wear appropriate PPE, including chemical-resistant gloves, safety goggles, face shields, and protective clothing. Respiratory protection may also be required in certain processes to prevent inhalation of harmful vapors or dust particles.
Proper storage and handling procedures are another critical aspect of safety regulations. Sulfamic acid must be stored in cool, dry areas away from incompatible materials such as strong oxidizing agents and alkalis. Containers should be tightly sealed and properly labeled to prevent accidental exposure or mishandling.
Emergency response protocols are an integral part of safety regulations in sulfamic acid production facilities. These include the installation of emergency showers and eyewash stations, as well as the development of comprehensive spill response plans. Regular safety drills and training sessions are mandated to ensure that all personnel are prepared to handle potential emergencies effectively.
Environmental protection measures are also a significant component of safety regulations. Proper waste disposal methods must be implemented to prevent contamination of soil and water sources. This includes the treatment of wastewater and the safe disposal of any byproducts or residues from the synthesis process.
As synthesis techniques evolve, safety regulations are continuously updated to address new challenges and risks. For instance, the development of novel catalysts or reaction conditions may introduce unforeseen hazards that require additional safety measures. Regulatory bodies work closely with industry experts to assess these emerging risks and develop appropriate guidelines.
Compliance with safety regulations is not only a legal requirement but also a critical factor in the overall efficiency and sustainability of sulfamic acid production. Companies that prioritize safety often see improvements in product quality, reduced downtime, and enhanced worker productivity. Moreover, adherence to safety regulations helps build trust with stakeholders and maintains a positive corporate image.
The primary safety concerns in sulfamic acid synthesis include the handling of corrosive substances, potential for exothermic reactions, and the generation of toxic fumes. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established comprehensive guidelines for the safe production and use of sulfamic acid.
One of the key safety regulations focuses on personal protective equipment (PPE). Workers involved in sulfamic acid synthesis must wear appropriate PPE, including chemical-resistant gloves, safety goggles, face shields, and protective clothing. Respiratory protection may also be required in certain processes to prevent inhalation of harmful vapors or dust particles.
Proper storage and handling procedures are another critical aspect of safety regulations. Sulfamic acid must be stored in cool, dry areas away from incompatible materials such as strong oxidizing agents and alkalis. Containers should be tightly sealed and properly labeled to prevent accidental exposure or mishandling.
Emergency response protocols are an integral part of safety regulations in sulfamic acid production facilities. These include the installation of emergency showers and eyewash stations, as well as the development of comprehensive spill response plans. Regular safety drills and training sessions are mandated to ensure that all personnel are prepared to handle potential emergencies effectively.
Environmental protection measures are also a significant component of safety regulations. Proper waste disposal methods must be implemented to prevent contamination of soil and water sources. This includes the treatment of wastewater and the safe disposal of any byproducts or residues from the synthesis process.
As synthesis techniques evolve, safety regulations are continuously updated to address new challenges and risks. For instance, the development of novel catalysts or reaction conditions may introduce unforeseen hazards that require additional safety measures. Regulatory bodies work closely with industry experts to assess these emerging risks and develop appropriate guidelines.
Compliance with safety regulations is not only a legal requirement but also a critical factor in the overall efficiency and sustainability of sulfamic acid production. Companies that prioritize safety often see improvements in product quality, reduced downtime, and enhanced worker productivity. Moreover, adherence to safety regulations helps build trust with stakeholders and maintains a positive corporate image.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!