How Sulfamic Acid Modulates Alkaline Catalysis Reactions
JUL 30, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid in Alkaline Catalysis: Background and Objectives
Sulfamic acid, a versatile compound with the chemical formula H3NSO3, has emerged as a significant player in the field of alkaline catalysis reactions. This sulfur-containing acid has garnered increasing attention due to its unique properties and potential to modulate various catalytic processes in alkaline environments. The exploration of sulfamic acid's role in alkaline catalysis stems from the growing need for efficient and sustainable chemical processes across industries.
The development of alkaline catalysis has been a cornerstone in numerous industrial applications, including organic synthesis, wastewater treatment, and energy production. However, traditional alkaline catalysts often face challenges such as limited selectivity, reduced efficiency in certain reaction conditions, and environmental concerns. The introduction of sulfamic acid as a modulating agent presents an opportunity to address these limitations and potentially revolutionize alkaline catalysis reactions.
The primary objective of investigating sulfamic acid's influence on alkaline catalysis is to enhance reaction efficiency, improve selectivity, and expand the scope of applications for alkaline-catalyzed processes. By understanding the mechanisms through which sulfamic acid interacts with alkaline catalysts and reaction substrates, researchers aim to develop more robust and versatile catalytic systems.
One of the key areas of interest is the ability of sulfamic acid to act as a pH buffer in alkaline solutions. This property allows for better control of reaction conditions, potentially leading to improved yields and reduced side reactions. Additionally, the strong acidic nature of sulfamic acid, combined with its stability in alkaline environments, offers unique opportunities for fine-tuning catalyst performance.
The historical context of sulfamic acid in chemistry dates back to its discovery in the early 20th century. Initially used primarily as a cleaning agent and descaler, its potential in catalysis has only recently begun to be fully explored. The evolution of analytical techniques and computational chemistry has enabled researchers to delve deeper into the molecular interactions between sulfamic acid and alkaline catalysts, paving the way for innovative applications.
As environmental concerns continue to shape industrial practices, the use of sulfamic acid in alkaline catalysis aligns with the principles of green chemistry. Its biodegradability and low toxicity make it an attractive option for developing more sustainable catalytic processes. Furthermore, the potential for sulfamic acid to enhance the efficiency of existing alkaline catalysts could lead to reduced energy consumption and waste generation in various chemical processes.
The investigation into how sulfamic acid modulates alkaline catalysis reactions represents a convergence of fundamental chemistry and practical industrial needs. By elucidating the underlying mechanisms and optimizing reaction conditions, researchers aim to unlock new possibilities in fields ranging from pharmaceutical synthesis to renewable energy technologies. This research not only promises to advance our understanding of catalytic processes but also holds the potential to drive innovation in chemical manufacturing and environmental remediation.
The development of alkaline catalysis has been a cornerstone in numerous industrial applications, including organic synthesis, wastewater treatment, and energy production. However, traditional alkaline catalysts often face challenges such as limited selectivity, reduced efficiency in certain reaction conditions, and environmental concerns. The introduction of sulfamic acid as a modulating agent presents an opportunity to address these limitations and potentially revolutionize alkaline catalysis reactions.
The primary objective of investigating sulfamic acid's influence on alkaline catalysis is to enhance reaction efficiency, improve selectivity, and expand the scope of applications for alkaline-catalyzed processes. By understanding the mechanisms through which sulfamic acid interacts with alkaline catalysts and reaction substrates, researchers aim to develop more robust and versatile catalytic systems.
One of the key areas of interest is the ability of sulfamic acid to act as a pH buffer in alkaline solutions. This property allows for better control of reaction conditions, potentially leading to improved yields and reduced side reactions. Additionally, the strong acidic nature of sulfamic acid, combined with its stability in alkaline environments, offers unique opportunities for fine-tuning catalyst performance.
The historical context of sulfamic acid in chemistry dates back to its discovery in the early 20th century. Initially used primarily as a cleaning agent and descaler, its potential in catalysis has only recently begun to be fully explored. The evolution of analytical techniques and computational chemistry has enabled researchers to delve deeper into the molecular interactions between sulfamic acid and alkaline catalysts, paving the way for innovative applications.
As environmental concerns continue to shape industrial practices, the use of sulfamic acid in alkaline catalysis aligns with the principles of green chemistry. Its biodegradability and low toxicity make it an attractive option for developing more sustainable catalytic processes. Furthermore, the potential for sulfamic acid to enhance the efficiency of existing alkaline catalysts could lead to reduced energy consumption and waste generation in various chemical processes.
The investigation into how sulfamic acid modulates alkaline catalysis reactions represents a convergence of fundamental chemistry and practical industrial needs. By elucidating the underlying mechanisms and optimizing reaction conditions, researchers aim to unlock new possibilities in fields ranging from pharmaceutical synthesis to renewable energy technologies. This research not only promises to advance our understanding of catalytic processes but also holds the potential to drive innovation in chemical manufacturing and environmental remediation.
Industrial Applications and Market Demand
Sulfamic acid's role in modulating alkaline catalysis reactions has garnered significant attention in various industrial sectors due to its unique properties and versatile applications. The market demand for sulfamic acid in catalytic processes has been steadily increasing, driven by its effectiveness in enhancing reaction rates and selectivity in alkaline environments.
In the chemical manufacturing industry, sulfamic acid has found extensive use as a catalyst modifier in the production of specialty chemicals and pharmaceuticals. Its ability to control pH and buffer alkaline solutions has made it invaluable in processes requiring precise pH control. This has led to improved product quality and increased yields, particularly in the synthesis of complex organic compounds.
The water treatment sector has also seen a surge in demand for sulfamic acid-based catalysts. These catalysts have proven highly effective in the removal of scale and corrosion in industrial water systems, offering a more environmentally friendly alternative to traditional acid-based treatments. The growing emphasis on sustainable water management practices has further boosted the market for sulfamic acid in this application.
In the petrochemical industry, sulfamic acid has emerged as a crucial component in catalytic cracking processes. Its use in modulating alkaline catalysis reactions has resulted in enhanced conversion rates and improved selectivity in the production of high-value petrochemicals. This has led to increased efficiency and reduced energy consumption in refinery operations, driving the demand for sulfamic acid-based catalysts.
The textile industry has also benefited from sulfamic acid's catalytic properties. Its application in dyeing and finishing processes has led to improved color fastness and fabric quality. The ability of sulfamic acid to modulate alkaline catalysis reactions has enabled more uniform dye distribution and better fixation, meeting the growing demand for high-quality textiles in the global market.
The food and beverage industry has seen an uptick in the use of sulfamic acid for catalytic cleaning processes. Its effectiveness in removing mineral deposits and protein-based soils in alkaline environments has made it a preferred choice for cleaning and sanitizing equipment in food processing plants. This has led to increased demand, particularly in regions with stringent food safety regulations.
As industries continue to seek more efficient and sustainable processes, the market demand for sulfamic acid in alkaline catalysis applications is expected to grow. The ongoing research into new applications and improved formulations is likely to further expand its use across various sectors, driving innovation and creating new market opportunities.
In the chemical manufacturing industry, sulfamic acid has found extensive use as a catalyst modifier in the production of specialty chemicals and pharmaceuticals. Its ability to control pH and buffer alkaline solutions has made it invaluable in processes requiring precise pH control. This has led to improved product quality and increased yields, particularly in the synthesis of complex organic compounds.
The water treatment sector has also seen a surge in demand for sulfamic acid-based catalysts. These catalysts have proven highly effective in the removal of scale and corrosion in industrial water systems, offering a more environmentally friendly alternative to traditional acid-based treatments. The growing emphasis on sustainable water management practices has further boosted the market for sulfamic acid in this application.
In the petrochemical industry, sulfamic acid has emerged as a crucial component in catalytic cracking processes. Its use in modulating alkaline catalysis reactions has resulted in enhanced conversion rates and improved selectivity in the production of high-value petrochemicals. This has led to increased efficiency and reduced energy consumption in refinery operations, driving the demand for sulfamic acid-based catalysts.
The textile industry has also benefited from sulfamic acid's catalytic properties. Its application in dyeing and finishing processes has led to improved color fastness and fabric quality. The ability of sulfamic acid to modulate alkaline catalysis reactions has enabled more uniform dye distribution and better fixation, meeting the growing demand for high-quality textiles in the global market.
The food and beverage industry has seen an uptick in the use of sulfamic acid for catalytic cleaning processes. Its effectiveness in removing mineral deposits and protein-based soils in alkaline environments has made it a preferred choice for cleaning and sanitizing equipment in food processing plants. This has led to increased demand, particularly in regions with stringent food safety regulations.
As industries continue to seek more efficient and sustainable processes, the market demand for sulfamic acid in alkaline catalysis applications is expected to grow. The ongoing research into new applications and improved formulations is likely to further expand its use across various sectors, driving innovation and creating new market opportunities.
Current State and Challenges in Alkaline Catalysis
Alkaline catalysis has emerged as a powerful tool in organic synthesis, offering unique advantages in various chemical transformations. However, the current state of alkaline catalysis faces several challenges that hinder its widespread application and efficiency. One of the primary issues is the limited stability of many catalysts under highly basic conditions, leading to decreased catalytic activity and shorter catalyst lifetimes. This problem is particularly pronounced in industrial-scale processes where catalyst longevity is crucial for economic viability.
Another significant challenge is the selectivity of alkaline-catalyzed reactions. While these reactions often proceed with high yields, controlling the product distribution and minimizing side reactions remain difficult tasks. This lack of selectivity can result in complex product mixtures, necessitating extensive purification steps and reducing overall process efficiency. Researchers are actively exploring strategies to enhance selectivity, including the development of novel ligand systems and the fine-tuning of reaction conditions.
The environmental impact of alkaline catalysis is also a growing concern. Many traditional alkaline catalysts rely on strong bases or metal hydroxides, which can pose disposal and waste management challenges. There is an increasing push towards developing more environmentally benign catalytic systems that maintain high activity while reducing the ecological footprint of chemical processes.
In recent years, the field has seen significant advancements in understanding the mechanistic aspects of alkaline catalysis. However, there are still gaps in our knowledge regarding the precise role of the alkaline species in catalytic cycles. This limited understanding hampers the rational design of more efficient catalytic systems and the prediction of reaction outcomes under varying conditions.
The application of alkaline catalysis in asymmetric synthesis represents another frontier with both promise and challenges. While some progress has been made in developing enantioselective alkaline-catalyzed reactions, achieving high levels of stereoselectivity consistently across a broad range of substrates remains elusive. This limitation restricts the use of alkaline catalysis in the production of pharmaceuticals and other high-value chiral compounds.
Scalability is a persistent challenge in the field of alkaline catalysis. Many reactions that show promise on a laboratory scale encounter difficulties when scaled up to industrial levels. Issues such as heat management, mass transfer limitations, and catalyst recovery become more pronounced at larger scales, necessitating innovative engineering solutions and process optimizations.
The integration of alkaline catalysis with other emerging technologies, such as flow chemistry and photocatalysis, presents both opportunities and challenges. While these combinations offer the potential for more efficient and sustainable chemical processes, they also introduce new complexities in reaction control and optimization. Researchers are actively exploring these hybrid systems to harness their synergistic benefits while addressing the associated technical hurdles.
Another significant challenge is the selectivity of alkaline-catalyzed reactions. While these reactions often proceed with high yields, controlling the product distribution and minimizing side reactions remain difficult tasks. This lack of selectivity can result in complex product mixtures, necessitating extensive purification steps and reducing overall process efficiency. Researchers are actively exploring strategies to enhance selectivity, including the development of novel ligand systems and the fine-tuning of reaction conditions.
The environmental impact of alkaline catalysis is also a growing concern. Many traditional alkaline catalysts rely on strong bases or metal hydroxides, which can pose disposal and waste management challenges. There is an increasing push towards developing more environmentally benign catalytic systems that maintain high activity while reducing the ecological footprint of chemical processes.
In recent years, the field has seen significant advancements in understanding the mechanistic aspects of alkaline catalysis. However, there are still gaps in our knowledge regarding the precise role of the alkaline species in catalytic cycles. This limited understanding hampers the rational design of more efficient catalytic systems and the prediction of reaction outcomes under varying conditions.
The application of alkaline catalysis in asymmetric synthesis represents another frontier with both promise and challenges. While some progress has been made in developing enantioselective alkaline-catalyzed reactions, achieving high levels of stereoselectivity consistently across a broad range of substrates remains elusive. This limitation restricts the use of alkaline catalysis in the production of pharmaceuticals and other high-value chiral compounds.
Scalability is a persistent challenge in the field of alkaline catalysis. Many reactions that show promise on a laboratory scale encounter difficulties when scaled up to industrial levels. Issues such as heat management, mass transfer limitations, and catalyst recovery become more pronounced at larger scales, necessitating innovative engineering solutions and process optimizations.
The integration of alkaline catalysis with other emerging technologies, such as flow chemistry and photocatalysis, presents both opportunities and challenges. While these combinations offer the potential for more efficient and sustainable chemical processes, they also introduce new complexities in reaction control and optimization. Researchers are actively exploring these hybrid systems to harness their synergistic benefits while addressing the associated technical hurdles.
Existing Mechanisms of Sulfamic Acid Modulation
01 Synthesis and purification of sulfamic acid derivatives
Various methods for synthesizing and purifying sulfamic acid derivatives are described. These processes involve different reaction conditions, catalysts, and purification techniques to obtain high-quality sulfamic acid compounds with specific properties for industrial applications.- Synthesis and purification of sulfamic acid: Various methods for synthesizing and purifying sulfamic acid are described. These processes involve different chemical reactions and purification techniques to produce high-quality sulfamic acid for industrial applications.
- Sulfamic acid in cleaning compositions: Sulfamic acid is utilized as a key ingredient in cleaning formulations. It acts as a descaling agent and pH regulator in various cleaning products, enhancing their effectiveness in removing mineral deposits and other tough stains.
- Sulfamic acid in agricultural applications: The use of sulfamic acid in agricultural products is explored. It is incorporated into fertilizers and pesticides to improve their efficacy and provide additional benefits to crop growth and protection.
- Sulfamic acid in water treatment: Sulfamic acid is employed in water treatment processes. It is used for pH adjustment, scale removal, and as a disinfectant in various water treatment applications, including industrial and municipal water systems.
- Sulfamic acid derivatives and complexes: Research on sulfamic acid derivatives and complexes is conducted to develop new compounds with enhanced properties. These modified forms of sulfamic acid are explored for potential applications in various industries, including pharmaceuticals and materials science.
02 Use of sulfamic acid in cleaning compositions
Sulfamic acid and its derivatives are incorporated into cleaning formulations for various purposes. These compositions are designed to effectively remove scale, rust, and other deposits from surfaces, particularly in industrial and household applications.Expand Specific Solutions03 Sulfamic acid in water treatment processes
Sulfamic acid is utilized in water treatment applications for pH adjustment, scale prevention, and corrosion control. Various methods and systems are described for incorporating sulfamic acid into water treatment processes to improve efficiency and effectiveness.Expand Specific Solutions04 Sulfamic acid modulation in agricultural applications
The use of sulfamic acid and its derivatives in agricultural formulations is explored. These compounds are employed for soil treatment, plant growth regulation, and pest control, with various methods of application and formulation described to enhance their effectiveness.Expand Specific Solutions05 Sulfamic acid in electroplating and metal surface treatment
Sulfamic acid is used in electroplating baths and metal surface treatment processes. Various formulations and methods are described for improving the quality of metal coatings, enhancing corrosion resistance, and optimizing the electroplating process using sulfamic acid-based solutions.Expand Specific Solutions
Key Players in Sulfamic Acid and Alkaline Catalysis Research
The field of sulfamic acid modulation in alkaline catalysis reactions is in a nascent stage of development, with significant potential for growth. The market size is relatively small but expanding as researchers explore novel applications in chemical synthesis and industrial processes. Technologically, the area is still evolving, with varying levels of maturity across different applications. Key players like BASF Corp., Henkel AG & Co. KGaA, and Sumitomo Chemical Co., Ltd. are at the forefront of research and development, leveraging their expertise in chemical engineering and catalysis. Academic institutions such as Tokyo Institute of Technology and Fuzhou University are contributing fundamental research, while companies like UOP LLC and Sinopec Research Institute of Petroleum Processing are focusing on practical industrial applications.
BASF Corp.
Technical Solution: BASF has developed a novel approach to modulate alkaline catalysis reactions using sulfamic acid. Their method involves incorporating sulfamic acid into the catalyst system to control pH levels and reaction rates. This technique allows for precise tuning of the catalytic activity, resulting in improved selectivity and yield of desired products. BASF's research has shown that sulfamic acid can act as a buffer, maintaining optimal pH conditions throughout the reaction process[1]. Additionally, they have discovered that sulfamic acid can form complexes with metal catalysts, enhancing their stability and performance in alkaline environments[3].
Strengths: Improved control over reaction conditions, enhanced catalyst stability, and increased product selectivity. Weaknesses: Potential increased complexity in catalyst preparation and possible limitations in certain reaction types.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to modulating alkaline catalysis reactions using sulfamic acid in their refining processes. Their method involves the strategic addition of sulfamic acid to alkaline catalysts used in hydrocracking and hydrodesulfurization reactions. Sinopec's research has demonstrated that sulfamic acid can effectively neutralize excess alkalinity, preventing unwanted side reactions and improving product quality. They have also discovered that sulfamic acid can enhance the dispersion of active metal sites on catalyst supports, leading to increased catalytic activity[6]. Furthermore, Sinopec has implemented a continuous monitoring and dosing system for sulfamic acid, allowing real-time adjustment of catalyst alkalinity based on feedstock composition and reaction conditions[8].
Strengths: Improved product quality, enhanced catalyst dispersion, and real-time alkalinity control. Weaknesses: Potential increased operational complexity and the need for specialized monitoring equipment.
Innovative Approaches in Sulfamic Acid-Alkaline Interactions
Sulfuric acid catalyzed alkylation process
PatentWO2023039356A1
Innovation
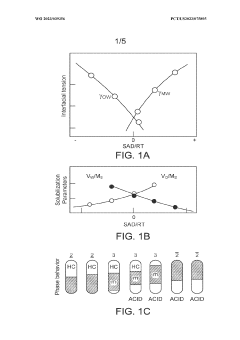
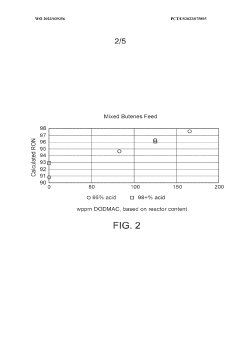
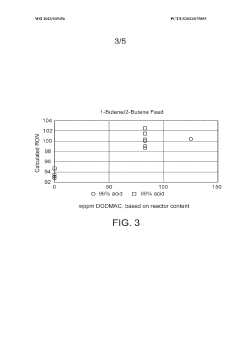
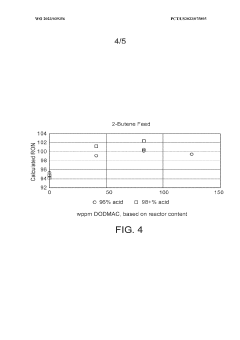
- The process involves selecting a C3-C5 olefin composition and optimizing the concentration of sulfuric acid and surfactants, such as dioctadecyl-dimethyl-ammonium chloride, to form a Winsor Type III phase system, which enhances mass transfer and reaction efficiency, allowing for higher Research Octane Number (RON) and yield by creating a bi-continuous micro-emulsion system that facilitates micellar catalysis.
Environmental Impact and Green Chemistry Considerations
The use of sulfamic acid in alkaline catalysis reactions presents significant environmental and green chemistry considerations. This approach aligns with the principles of sustainable chemistry by potentially reducing the environmental impact of industrial processes. Sulfamic acid, being a solid at room temperature, offers safer handling and storage compared to traditional liquid acids, minimizing the risk of spills and associated environmental contamination.
In terms of green chemistry, the modulation of alkaline catalysis reactions by sulfamic acid can lead to improved atom economy and reduced waste generation. By enhancing reaction efficiency, this method may decrease the overall amount of reagents required, thereby reducing the environmental footprint of chemical processes. Additionally, the potential for lower reaction temperatures and milder conditions could result in energy savings, contributing to the overall sustainability of industrial operations.
The biodegradability of sulfamic acid is an important factor to consider. While it can decompose into sulfate and ammonium ions in the environment, the rate and impact of this decomposition process require careful assessment. Proper waste management strategies must be implemented to prevent potential eutrophication or acidification of aquatic ecosystems due to sulfate release.
From a life cycle perspective, the production of sulfamic acid itself should be evaluated. If its synthesis involves more environmentally friendly processes compared to alternative catalysts or reagents, this could further justify its use in alkaline catalysis reactions. However, a comprehensive life cycle analysis would be necessary to fully understand the environmental implications across the entire supply chain.
The potential for recycling or regenerating sulfamic acid in industrial processes is another crucial aspect of its environmental profile. If effective recovery methods can be developed, it would significantly reduce waste and resource consumption, aligning with circular economy principles. This could involve techniques such as crystallization or membrane separation to isolate and reuse the acid.
Lastly, the impact on worker safety and occupational health should not be overlooked. While sulfamic acid is generally considered less hazardous than strong mineral acids, proper handling procedures and personal protective equipment are still essential. The reduced volatility of sulfamic acid compared to liquid acids may lead to improved air quality in industrial settings, potentially reducing respiratory risks for workers.
In conclusion, the use of sulfamic acid in modulating alkaline catalysis reactions shows promise from an environmental and green chemistry standpoint. However, a holistic approach considering all aspects of its production, use, and disposal is necessary to fully realize its potential as a sustainable alternative in industrial chemistry.
In terms of green chemistry, the modulation of alkaline catalysis reactions by sulfamic acid can lead to improved atom economy and reduced waste generation. By enhancing reaction efficiency, this method may decrease the overall amount of reagents required, thereby reducing the environmental footprint of chemical processes. Additionally, the potential for lower reaction temperatures and milder conditions could result in energy savings, contributing to the overall sustainability of industrial operations.
The biodegradability of sulfamic acid is an important factor to consider. While it can decompose into sulfate and ammonium ions in the environment, the rate and impact of this decomposition process require careful assessment. Proper waste management strategies must be implemented to prevent potential eutrophication or acidification of aquatic ecosystems due to sulfate release.
From a life cycle perspective, the production of sulfamic acid itself should be evaluated. If its synthesis involves more environmentally friendly processes compared to alternative catalysts or reagents, this could further justify its use in alkaline catalysis reactions. However, a comprehensive life cycle analysis would be necessary to fully understand the environmental implications across the entire supply chain.
The potential for recycling or regenerating sulfamic acid in industrial processes is another crucial aspect of its environmental profile. If effective recovery methods can be developed, it would significantly reduce waste and resource consumption, aligning with circular economy principles. This could involve techniques such as crystallization or membrane separation to isolate and reuse the acid.
Lastly, the impact on worker safety and occupational health should not be overlooked. While sulfamic acid is generally considered less hazardous than strong mineral acids, proper handling procedures and personal protective equipment are still essential. The reduced volatility of sulfamic acid compared to liquid acids may lead to improved air quality in industrial settings, potentially reducing respiratory risks for workers.
In conclusion, the use of sulfamic acid in modulating alkaline catalysis reactions shows promise from an environmental and green chemistry standpoint. However, a holistic approach considering all aspects of its production, use, and disposal is necessary to fully realize its potential as a sustainable alternative in industrial chemistry.
Regulatory Framework for Industrial Catalytic Processes
The regulatory framework for industrial catalytic processes involving sulfamic acid and alkaline catalysis reactions is a complex and evolving landscape. Governments and international bodies have established stringent guidelines to ensure the safe and environmentally responsible use of these chemical processes in industrial settings.
At the core of this regulatory framework are environmental protection agencies, such as the U.S. Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA). These organizations set standards for emissions, waste management, and chemical handling related to catalytic processes. For sulfamic acid and alkaline catalysis reactions, specific regulations focus on pH control, neutralization procedures, and the proper disposal of reaction by-products.
Occupational health and safety regulations also play a crucial role in governing these industrial processes. Agencies like the Occupational Safety and Health Administration (OSHA) in the United States mandate strict safety protocols for workers handling sulfamic acid and alkaline catalysts. These include requirements for personal protective equipment, proper ventilation systems, and emergency response procedures.
The transportation and storage of sulfamic acid and alkaline catalysts are subject to rigorous regulations as well. International agreements, such as the United Nations Recommendations on the Transport of Dangerous Goods, provide guidelines for the safe movement of these chemicals across borders. National regulations, like the U.S. Department of Transportation's Hazardous Materials Regulations, further specify packaging, labeling, and documentation requirements.
In recent years, there has been an increased focus on sustainable chemistry practices within the regulatory framework. This has led to the development of green chemistry initiatives that encourage the use of less hazardous alternatives and the optimization of catalytic processes to reduce waste and energy consumption. Regulatory bodies are increasingly incorporating these principles into their guidelines for industrial catalytic processes.
Compliance with these regulations often requires extensive documentation and reporting. Companies must maintain detailed records of their catalytic processes, including reaction conditions, chemical inventories, and waste management practices. Regular audits and inspections by regulatory agencies ensure adherence to these standards and help identify areas for improvement in safety and environmental performance.
As research continues to advance our understanding of how sulfamic acid modulates alkaline catalysis reactions, regulatory frameworks are expected to evolve. This may include updates to existing regulations or the introduction of new guidelines to address emerging concerns or capitalize on new opportunities for process optimization and environmental protection.
At the core of this regulatory framework are environmental protection agencies, such as the U.S. Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA). These organizations set standards for emissions, waste management, and chemical handling related to catalytic processes. For sulfamic acid and alkaline catalysis reactions, specific regulations focus on pH control, neutralization procedures, and the proper disposal of reaction by-products.
Occupational health and safety regulations also play a crucial role in governing these industrial processes. Agencies like the Occupational Safety and Health Administration (OSHA) in the United States mandate strict safety protocols for workers handling sulfamic acid and alkaline catalysts. These include requirements for personal protective equipment, proper ventilation systems, and emergency response procedures.
The transportation and storage of sulfamic acid and alkaline catalysts are subject to rigorous regulations as well. International agreements, such as the United Nations Recommendations on the Transport of Dangerous Goods, provide guidelines for the safe movement of these chemicals across borders. National regulations, like the U.S. Department of Transportation's Hazardous Materials Regulations, further specify packaging, labeling, and documentation requirements.
In recent years, there has been an increased focus on sustainable chemistry practices within the regulatory framework. This has led to the development of green chemistry initiatives that encourage the use of less hazardous alternatives and the optimization of catalytic processes to reduce waste and energy consumption. Regulatory bodies are increasingly incorporating these principles into their guidelines for industrial catalytic processes.
Compliance with these regulations often requires extensive documentation and reporting. Companies must maintain detailed records of their catalytic processes, including reaction conditions, chemical inventories, and waste management practices. Regular audits and inspections by regulatory agencies ensure adherence to these standards and help identify areas for improvement in safety and environmental performance.
As research continues to advance our understanding of how sulfamic acid modulates alkaline catalysis reactions, regulatory frameworks are expected to evolve. This may include updates to existing regulations or the introduction of new guidelines to address emerging concerns or capitalize on new opportunities for process optimization and environmental protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!