Sulfamic Acid in the Cross Linking of Natural Polymers
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Background and Objectives
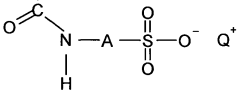
Sulfamic acid, a crystalline compound with the chemical formula H3NSO3, has emerged as a significant player in the field of natural polymer cross-linking. This versatile compound, first synthesized in the late 19th century, has found applications across various industries due to its unique properties and reactivity. In recent years, the focus on sustainable and biodegradable materials has led to increased interest in natural polymers and their modification techniques.
The evolution of sulfamic acid's role in polymer science has been marked by several key developments. Initially used primarily as a cleaning agent and descaler, its potential in polymer chemistry was recognized in the mid-20th century. Researchers began exploring its ability to interact with and modify the structure of natural polymers, opening up new possibilities for material enhancement and functionalization.
The current technological landscape demands materials with improved properties, longer shelf life, and enhanced functionality while maintaining environmental compatibility. Natural polymers, derived from renewable resources, offer a promising solution to these challenges. However, their inherent limitations, such as poor mechanical strength and high water sensitivity, often restrict their applications. This is where sulfamic acid comes into play, offering a potential solution for enhancing the properties of natural polymers through cross-linking.
The primary objective of research on sulfamic acid in the cross-linking of natural polymers is to develop innovative, sustainable, and efficient methods for improving the physicochemical properties of these materials. This includes enhancing their mechanical strength, water resistance, thermal stability, and overall durability. By achieving these improvements, researchers aim to expand the application range of natural polymers in various sectors, including packaging, biomedical engineering, and environmental remediation.
Another crucial goal is to understand the fundamental mechanisms by which sulfamic acid interacts with different natural polymers at the molecular level. This knowledge is essential for optimizing cross-linking processes and tailoring the properties of the resulting materials to specific applications. Additionally, researchers are focusing on developing environmentally friendly cross-linking methods that minimize the use of harsh chemicals and reduce the environmental impact of polymer modification processes.
The exploration of sulfamic acid in natural polymer cross-linking aligns with broader trends in materials science and sustainability. As industries seek alternatives to petroleum-based plastics, the ability to enhance the properties of natural polymers becomes increasingly valuable. This research not only contributes to the development of new materials but also supports the transition towards a more sustainable and circular economy.
The evolution of sulfamic acid's role in polymer science has been marked by several key developments. Initially used primarily as a cleaning agent and descaler, its potential in polymer chemistry was recognized in the mid-20th century. Researchers began exploring its ability to interact with and modify the structure of natural polymers, opening up new possibilities for material enhancement and functionalization.
The current technological landscape demands materials with improved properties, longer shelf life, and enhanced functionality while maintaining environmental compatibility. Natural polymers, derived from renewable resources, offer a promising solution to these challenges. However, their inherent limitations, such as poor mechanical strength and high water sensitivity, often restrict their applications. This is where sulfamic acid comes into play, offering a potential solution for enhancing the properties of natural polymers through cross-linking.
The primary objective of research on sulfamic acid in the cross-linking of natural polymers is to develop innovative, sustainable, and efficient methods for improving the physicochemical properties of these materials. This includes enhancing their mechanical strength, water resistance, thermal stability, and overall durability. By achieving these improvements, researchers aim to expand the application range of natural polymers in various sectors, including packaging, biomedical engineering, and environmental remediation.
Another crucial goal is to understand the fundamental mechanisms by which sulfamic acid interacts with different natural polymers at the molecular level. This knowledge is essential for optimizing cross-linking processes and tailoring the properties of the resulting materials to specific applications. Additionally, researchers are focusing on developing environmentally friendly cross-linking methods that minimize the use of harsh chemicals and reduce the environmental impact of polymer modification processes.
The exploration of sulfamic acid in natural polymer cross-linking aligns with broader trends in materials science and sustainability. As industries seek alternatives to petroleum-based plastics, the ability to enhance the properties of natural polymers becomes increasingly valuable. This research not only contributes to the development of new materials but also supports the transition towards a more sustainable and circular economy.
Market Analysis for Crosslinked Natural Polymers
The market for crosslinked natural polymers has been experiencing significant growth in recent years, driven by increasing demand across various industries. These polymers, enhanced through crosslinking processes, offer improved mechanical properties, thermal stability, and chemical resistance compared to their non-crosslinked counterparts. This has led to their widespread adoption in sectors such as pharmaceuticals, food and beverages, cosmetics, and agriculture.
In the pharmaceutical industry, crosslinked natural polymers are extensively used in drug delivery systems, wound dressings, and tissue engineering applications. The controlled release properties and biocompatibility of these materials make them particularly attractive for developing advanced drug formulations. The food and beverage sector utilizes crosslinked natural polymers as thickeners, stabilizers, and emulsifiers, capitalizing on their enhanced functional properties.
The cosmetics industry has also embraced crosslinked natural polymers for their ability to improve the texture, stability, and efficacy of various personal care products. These materials are increasingly being used in skincare formulations, hair care products, and color cosmetics. In agriculture, crosslinked natural polymers find applications in soil conditioning, controlled release fertilizers, and seed coatings, contributing to improved crop yields and sustainable farming practices.
The global market for crosslinked natural polymers is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by the increasing preference for eco-friendly and sustainable materials across industries, as well as ongoing research and development efforts to expand the application scope of these polymers.
Regionally, North America and Europe currently dominate the market for crosslinked natural polymers, owing to their advanced healthcare and personal care industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by the expanding pharmaceutical and food industries in countries like China and India.
Key market trends include the development of novel crosslinking techniques to enhance polymer properties, the exploration of new natural polymer sources, and the integration of crosslinked natural polymers into advanced materials and composites. The increasing focus on biodegradable and biocompatible materials is expected to further boost the demand for these polymers in the coming years.
In the pharmaceutical industry, crosslinked natural polymers are extensively used in drug delivery systems, wound dressings, and tissue engineering applications. The controlled release properties and biocompatibility of these materials make them particularly attractive for developing advanced drug formulations. The food and beverage sector utilizes crosslinked natural polymers as thickeners, stabilizers, and emulsifiers, capitalizing on their enhanced functional properties.
The cosmetics industry has also embraced crosslinked natural polymers for their ability to improve the texture, stability, and efficacy of various personal care products. These materials are increasingly being used in skincare formulations, hair care products, and color cosmetics. In agriculture, crosslinked natural polymers find applications in soil conditioning, controlled release fertilizers, and seed coatings, contributing to improved crop yields and sustainable farming practices.
The global market for crosslinked natural polymers is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by the increasing preference for eco-friendly and sustainable materials across industries, as well as ongoing research and development efforts to expand the application scope of these polymers.
Regionally, North America and Europe currently dominate the market for crosslinked natural polymers, owing to their advanced healthcare and personal care industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by the expanding pharmaceutical and food industries in countries like China and India.
Key market trends include the development of novel crosslinking techniques to enhance polymer properties, the exploration of new natural polymer sources, and the integration of crosslinked natural polymers into advanced materials and composites. The increasing focus on biodegradable and biocompatible materials is expected to further boost the demand for these polymers in the coming years.
Current Challenges in Natural Polymer Crosslinking
Natural polymer crosslinking, while promising for various applications, faces several significant challenges that hinder its widespread adoption and effectiveness. One of the primary obstacles is the lack of precise control over the crosslinking process. The heterogeneous nature of natural polymers, such as proteins and polysaccharides, makes it difficult to achieve uniform and reproducible crosslinking results. This variability can lead to inconsistent mechanical properties and performance in the final products.
Another major challenge is the potential for undesired side reactions during the crosslinking process. Many crosslinking agents are highly reactive and can interact with functional groups other than those intended for crosslinking. This can result in the formation of unwanted byproducts, alterations in the polymer structure, or even degradation of the natural polymer. Such side reactions can compromise the integrity and functionality of the crosslinked material.
The biocompatibility and toxicity of crosslinking agents pose additional concerns, especially in biomedical applications. Many traditional crosslinking agents, such as glutaraldehyde, are known to be cytotoxic and can elicit adverse biological responses. Finding alternative crosslinking agents that are both effective and biocompatible remains an ongoing challenge in the field.
Furthermore, the preservation of the natural polymer's inherent properties during the crosslinking process is crucial yet challenging. Excessive crosslinking can lead to a loss of flexibility, biodegradability, or other desirable characteristics of the original polymer. Striking the right balance between enhancing mechanical properties through crosslinking and maintaining the polymer's native features is a delicate task that requires careful optimization.
The scalability of natural polymer crosslinking processes presents another significant hurdle. Many crosslinking techniques that work well at the laboratory scale face difficulties when scaled up for industrial production. Issues such as heat and mass transfer limitations, uneven distribution of crosslinking agents, and longer processing times can arise, affecting the quality and consistency of the final product.
Lastly, the environmental impact of crosslinking processes is an increasing concern. Many traditional crosslinking methods involve the use of harsh chemicals or energy-intensive procedures. Developing more sustainable and eco-friendly crosslinking approaches that minimize environmental footprint while maintaining efficiency is a pressing challenge in the field of natural polymer modification.
Another major challenge is the potential for undesired side reactions during the crosslinking process. Many crosslinking agents are highly reactive and can interact with functional groups other than those intended for crosslinking. This can result in the formation of unwanted byproducts, alterations in the polymer structure, or even degradation of the natural polymer. Such side reactions can compromise the integrity and functionality of the crosslinked material.
The biocompatibility and toxicity of crosslinking agents pose additional concerns, especially in biomedical applications. Many traditional crosslinking agents, such as glutaraldehyde, are known to be cytotoxic and can elicit adverse biological responses. Finding alternative crosslinking agents that are both effective and biocompatible remains an ongoing challenge in the field.
Furthermore, the preservation of the natural polymer's inherent properties during the crosslinking process is crucial yet challenging. Excessive crosslinking can lead to a loss of flexibility, biodegradability, or other desirable characteristics of the original polymer. Striking the right balance between enhancing mechanical properties through crosslinking and maintaining the polymer's native features is a delicate task that requires careful optimization.
The scalability of natural polymer crosslinking processes presents another significant hurdle. Many crosslinking techniques that work well at the laboratory scale face difficulties when scaled up for industrial production. Issues such as heat and mass transfer limitations, uneven distribution of crosslinking agents, and longer processing times can arise, affecting the quality and consistency of the final product.
Lastly, the environmental impact of crosslinking processes is an increasing concern. Many traditional crosslinking methods involve the use of harsh chemicals or energy-intensive procedures. Developing more sustainable and eco-friendly crosslinking approaches that minimize environmental footprint while maintaining efficiency is a pressing challenge in the field of natural polymer modification.
Existing Sulfamic Acid Crosslinking Solutions
01 Sulfamic acid as a cross-linking agent
Sulfamic acid can be used as a cross-linking agent in various polymer systems. It forms covalent bonds between polymer chains, enhancing the mechanical properties and chemical resistance of the resulting material. This cross-linking method is particularly useful in applications requiring improved thermal stability and durability.- Sulfamic acid as a cross-linking agent: Sulfamic acid is used as a cross-linking agent in various polymer systems. It can form covalent bonds between polymer chains, improving the mechanical properties and chemical resistance of the resulting material. This cross-linking process is often employed in the production of resins, adhesives, and coatings.
- Cross-linking in hydrogel formation: Sulfamic acid cross-linking is utilized in the formation of hydrogels. These hydrogels have applications in biomedical fields, such as drug delivery systems and tissue engineering. The cross-linking process helps control the swelling behavior and mechanical properties of the hydrogels.
- Cross-linking in textile industry: Sulfamic acid cross-linking is employed in the textile industry to improve the properties of fabrics. This process can enhance wrinkle resistance, dimensional stability, and durability of textiles. It is particularly useful in the treatment of cellulose-based fibers.
- Cross-linking in water treatment: Sulfamic acid cross-linking is used in water treatment applications. It can be employed to create cross-linked polymers that act as flocculants or coagulants, aiding in the removal of contaminants from water. This process is valuable in both industrial and municipal water treatment systems.
- Cross-linking in adhesive formulations: Sulfamic acid cross-linking is utilized in the development of high-performance adhesives. The cross-linking process can improve the bond strength, heat resistance, and chemical resistance of adhesives. This technology finds applications in various industries, including automotive, construction, and electronics.
02 Cross-linking in hydrogel formation
Sulfamic acid cross-linking is employed in the synthesis of hydrogels. The process involves the reaction of sulfamic acid with functional groups on polymer chains, creating a three-dimensional network structure. These hydrogels find applications in biomedical fields, drug delivery systems, and water treatment technologies.Expand Specific Solutions03 Cross-linking in textile and fiber industries
Sulfamic acid cross-linking is utilized in textile and fiber industries to improve the properties of fabrics and fibers. This process enhances wrinkle resistance, dimensional stability, and durability of textiles. It also contributes to the development of flame-retardant and water-repellent finishes for various fabric types.Expand Specific Solutions04 Cross-linking in adhesive formulations
Sulfamic acid is used as a cross-linking agent in adhesive formulations to improve bond strength and durability. The cross-linking reaction enhances the adhesive's resistance to heat, moisture, and chemicals. This application is particularly valuable in industrial and construction adhesives where high-performance bonding is required.Expand Specific Solutions05 Cross-linking in coating technologies
Sulfamic acid cross-linking is employed in coating technologies to enhance the performance of protective and decorative coatings. The cross-linking process improves the coating's resistance to abrasion, chemicals, and weathering. This technique is widely used in automotive, marine, and industrial coatings to extend the lifespan and maintain the appearance of coated surfaces.Expand Specific Solutions
Key Players in Polymer Crosslinking Industry
The research on sulfamic acid in cross-linking natural polymers is in an emerging stage, with growing market potential due to increasing demand for sustainable materials. The technology's maturity is still developing, as evidenced by ongoing research efforts from diverse organizations. Key players include academic institutions like Technion Research & Development Foundation, Centre National de la Recherche Scientifique, and The Scripps Research Institute, alongside industry leaders such as DuPont de Nemours, L'Oréal SA, and Clariant International AG. This mix of academic and industrial involvement suggests a collaborative approach to advancing the technology, with potential for commercialization as research progresses. The competitive landscape is characterized by a balance between fundamental research and practical applications, indicating opportunities for innovation and market growth in the coming years.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed innovative approaches for the cross-linking of natural polymers using sulfamic acid. Their research focuses on enhancing the mechanical and thermal properties of biopolymers through environmentally friendly cross-linking methods. The CNRS team has successfully demonstrated the use of sulfamic acid as an efficient cross-linking agent for various natural polymers, including cellulose, chitosan, and alginate[1]. Their method involves the formation of sulfate ester linkages between polymer chains, resulting in improved stability and functionality of the materials[2]. The process is carried out under mild conditions, typically at temperatures below 100°C and atmospheric pressure, making it energy-efficient and suitable for industrial scale-up[3].
Strengths: Eco-friendly approach, improved material properties, and potential for industrial applications. Weaknesses: May require optimization for specific polymer types and end-use requirements.
L'Oréal SA
Technical Solution: L'Oréal has invested in research on sulfamic acid-based cross-linking of natural polymers for cosmetic applications. Their focus has been on developing novel hair care and skin care formulations using cross-linked biopolymers. L'Oréal's approach involves the use of sulfamic acid as a mild cross-linking agent for proteins and polysaccharides commonly used in cosmetic products, such as keratin, collagen, and hyaluronic acid[8]. The company has patented several methods for creating cross-linked polymer networks that provide improved moisture retention, film-forming properties, and long-lasting effects in cosmetic formulations[9]. L'Oréal's research has also explored the combination of sulfamic acid with other cross-linking agents to achieve synergistic effects and tailor the properties of the resulting materials for specific cosmetic applications[10].
Strengths: Specialized applications in the cosmetics industry, potential for novel product formulations, and improved performance of natural ingredients. Weaknesses: Limited to cosmetic applications and may face regulatory challenges in some markets.
Core Innovations in Sulfamic Acid Application
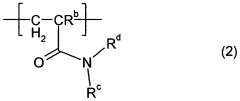
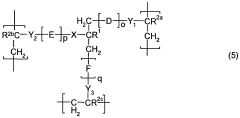
Polymers based on sulfonic acids, amides and special cross-linking agents
PatentActiveEP2683751A1
Innovation
- Development of polymers comprising specific monomers with sulfonic acid groups, such as 2-acrylamido-2-methylpropanesulfonic acid or its sulfonates, combined with open-chain amides and trifunctional crosslinkers, which enhance skin feel, thickening properties, and stability in saline compositions.
Polymers based on sulfonic acids, amides and special cross-linking agents
PatentWO2012119746A1
Innovation
- Development of polymers comprising specific monomers with sulfonic acid groups, such as 2-acrylamido-2-methylpropanesulfonic acid or its sulfonates, combined with open-chain amides and trifunctional crosslinkers, which provide a balanced composition of structural units to enhance skin feel, thickening properties, and salt stability.
Environmental Impact Assessment
The use of sulfamic acid in the cross-linking of natural polymers has significant environmental implications that warrant careful consideration. This process, while offering potential benefits in various applications, also poses certain risks to ecosystems and human health. The environmental impact assessment of this technology reveals both positive and negative aspects that need to be balanced for sustainable implementation.
One of the primary environmental concerns is the potential release of sulfamic acid or its derivatives into aquatic ecosystems. Sulfamic acid, being highly soluble in water, can easily disperse in water bodies if not properly contained or treated. This dispersion may lead to localized acidification of water, potentially affecting pH-sensitive aquatic organisms and disrupting ecosystem balance. Furthermore, the increased acidity can mobilize heavy metals in sediments, exacerbating water pollution issues.
However, the cross-linking process itself may offer some environmental benefits. By enhancing the stability and durability of natural polymers, it can potentially reduce the need for synthetic, petroleum-based materials in certain applications. This substitution could lead to a decrease in the carbon footprint associated with polymer production and use. Additionally, the improved properties of cross-linked natural polymers may extend the lifespan of products, reducing waste generation and the demand for raw materials.
The production and handling of sulfamic acid also present environmental challenges. Its manufacture involves energy-intensive processes and potentially hazardous chemicals, contributing to industrial emissions and the risk of accidental releases. Proper safety measures and waste management protocols are crucial to mitigate these risks and minimize environmental contamination.
On the positive side, the use of natural polymers as base materials aligns with the principles of green chemistry and sustainability. These polymers are often derived from renewable resources, reducing dependence on fossil fuels. Moreover, many natural polymers are biodegradable, which can help address issues related to plastic pollution and waste accumulation in the environment.
The environmental impact of this technology also extends to land use and biodiversity. Increased demand for natural polymers could drive changes in agricultural practices, potentially leading to land-use changes and impacts on local ecosystems. However, if managed sustainably, it could also promote the cultivation of diverse crop species, contributing to agricultural biodiversity.
In terms of human health, the environmental persistence of cross-linked polymers and potential leaching of sulfamic acid or its byproducts into food or water supplies need to be carefully evaluated. Long-term exposure studies and risk assessments are essential to ensure the safety of these materials in various applications, particularly those involving direct human contact or consumption.
One of the primary environmental concerns is the potential release of sulfamic acid or its derivatives into aquatic ecosystems. Sulfamic acid, being highly soluble in water, can easily disperse in water bodies if not properly contained or treated. This dispersion may lead to localized acidification of water, potentially affecting pH-sensitive aquatic organisms and disrupting ecosystem balance. Furthermore, the increased acidity can mobilize heavy metals in sediments, exacerbating water pollution issues.
However, the cross-linking process itself may offer some environmental benefits. By enhancing the stability and durability of natural polymers, it can potentially reduce the need for synthetic, petroleum-based materials in certain applications. This substitution could lead to a decrease in the carbon footprint associated with polymer production and use. Additionally, the improved properties of cross-linked natural polymers may extend the lifespan of products, reducing waste generation and the demand for raw materials.
The production and handling of sulfamic acid also present environmental challenges. Its manufacture involves energy-intensive processes and potentially hazardous chemicals, contributing to industrial emissions and the risk of accidental releases. Proper safety measures and waste management protocols are crucial to mitigate these risks and minimize environmental contamination.
On the positive side, the use of natural polymers as base materials aligns with the principles of green chemistry and sustainability. These polymers are often derived from renewable resources, reducing dependence on fossil fuels. Moreover, many natural polymers are biodegradable, which can help address issues related to plastic pollution and waste accumulation in the environment.
The environmental impact of this technology also extends to land use and biodiversity. Increased demand for natural polymers could drive changes in agricultural practices, potentially leading to land-use changes and impacts on local ecosystems. However, if managed sustainably, it could also promote the cultivation of diverse crop species, contributing to agricultural biodiversity.
In terms of human health, the environmental persistence of cross-linked polymers and potential leaching of sulfamic acid or its byproducts into food or water supplies need to be carefully evaluated. Long-term exposure studies and risk assessments are essential to ensure the safety of these materials in various applications, particularly those involving direct human contact or consumption.
Regulatory Framework for Polymer Crosslinking Agents
The regulatory framework for polymer crosslinking agents, particularly in the context of sulfamic acid use in natural polymer crosslinking, is a complex and evolving landscape. Governments and regulatory bodies worldwide have established guidelines and regulations to ensure the safety and efficacy of these agents in various applications, including food, pharmaceuticals, and industrial processes.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating crosslinking agents used in food and pharmaceutical applications. The FDA has established specific guidelines for the use of sulfamic acid and other crosslinking agents in food packaging materials and drug delivery systems. These regulations are outlined in the Code of Federal Regulations (CFR), particularly in Title 21, which deals with food and drugs.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the manufacture, import, and use of chemical substances, including crosslinking agents. Under REACH, manufacturers and importers must register substances produced or imported in quantities over one tonne per year with the European Chemicals Agency (ECHA). This regulation ensures that the risks associated with these substances are properly assessed and managed.
In Asia, countries like Japan and China have their own regulatory frameworks for polymer crosslinking agents. The Japanese Ministry of Health, Labour and Welfare (MHLW) has established guidelines for the use of these agents in food contact materials, while China's National Medical Products Administration (NMPA) regulates their use in pharmaceutical applications.
Environmental considerations are also a significant aspect of the regulatory framework for polymer crosslinking agents. Many countries have implemented regulations to control the environmental impact of these substances. For instance, the U.S. Environmental Protection Agency (EPA) regulates the release of sulfamic acid and other crosslinking agents into the environment under the Clean Water Act and the Toxic Substances Control Act (TSCA).
Occupational health and safety regulations are another critical component of the regulatory framework. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) have established guidelines for the safe handling and use of crosslinking agents in industrial settings. These regulations typically include requirements for personal protective equipment, exposure limits, and proper storage and handling procedures.
As research on sulfamic acid in the crosslinking of natural polymers progresses, regulatory bodies are likely to update their frameworks to address new findings and potential applications. This may include revisions to existing regulations or the development of new guidelines specific to natural polymer crosslinking. Researchers and manufacturers working in this field must stay informed about these regulatory developments to ensure compliance and promote the safe and effective use of sulfamic acid and other crosslinking agents in natural polymer applications.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating crosslinking agents used in food and pharmaceutical applications. The FDA has established specific guidelines for the use of sulfamic acid and other crosslinking agents in food packaging materials and drug delivery systems. These regulations are outlined in the Code of Federal Regulations (CFR), particularly in Title 21, which deals with food and drugs.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the manufacture, import, and use of chemical substances, including crosslinking agents. Under REACH, manufacturers and importers must register substances produced or imported in quantities over one tonne per year with the European Chemicals Agency (ECHA). This regulation ensures that the risks associated with these substances are properly assessed and managed.
In Asia, countries like Japan and China have their own regulatory frameworks for polymer crosslinking agents. The Japanese Ministry of Health, Labour and Welfare (MHLW) has established guidelines for the use of these agents in food contact materials, while China's National Medical Products Administration (NMPA) regulates their use in pharmaceutical applications.
Environmental considerations are also a significant aspect of the regulatory framework for polymer crosslinking agents. Many countries have implemented regulations to control the environmental impact of these substances. For instance, the U.S. Environmental Protection Agency (EPA) regulates the release of sulfamic acid and other crosslinking agents into the environment under the Clean Water Act and the Toxic Substances Control Act (TSCA).
Occupational health and safety regulations are another critical component of the regulatory framework. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) have established guidelines for the safe handling and use of crosslinking agents in industrial settings. These regulations typically include requirements for personal protective equipment, exposure limits, and proper storage and handling procedures.
As research on sulfamic acid in the crosslinking of natural polymers progresses, regulatory bodies are likely to update their frameworks to address new findings and potential applications. This may include revisions to existing regulations or the development of new guidelines specific to natural polymer crosslinking. Researchers and manufacturers working in this field must stay informed about these regulatory developments to ensure compliance and promote the safe and effective use of sulfamic acid and other crosslinking agents in natural polymer applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!