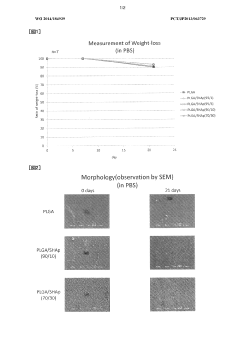
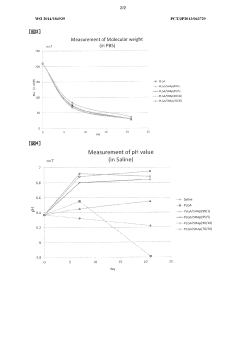
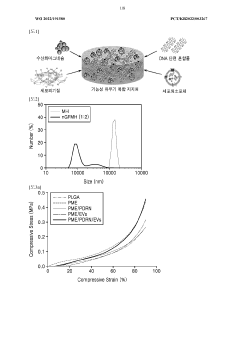
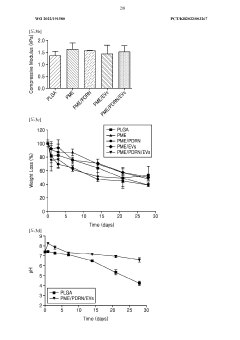
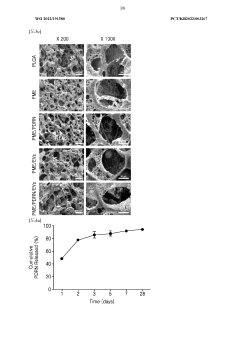
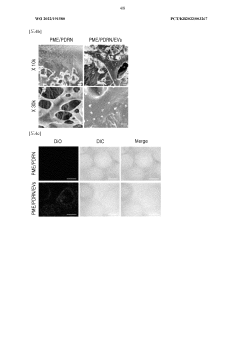
Influence of Sulfamic Acid on Biodegradable Polymer Degradation
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid and Biodegradable Polymer Interaction
The interaction between sulfamic acid and biodegradable polymers is a complex and multifaceted phenomenon that has garnered significant attention in recent years. Sulfamic acid, a strong inorganic acid with the chemical formula H3NSO3, has been found to exert a profound influence on the degradation process of various biodegradable polymers. This interaction is primarily driven by the acidic nature of sulfamic acid and its ability to catalyze hydrolysis reactions.
When sulfamic acid comes into contact with biodegradable polymers, it initiates a series of chemical reactions that can accelerate the breakdown of polymer chains. The acid acts as a catalyst, promoting the hydrolysis of ester bonds, which are commonly found in many biodegradable polymers such as polylactic acid (PLA) and polyglycolic acid (PGA). This catalytic action leads to the scission of polymer chains, resulting in a reduction of molecular weight and, consequently, a decrease in the mechanical properties of the material.
The extent of degradation induced by sulfamic acid is influenced by several factors, including the concentration of the acid, the type of biodegradable polymer, and the environmental conditions. Higher concentrations of sulfamic acid generally lead to more rapid degradation, while different polymer structures exhibit varying degrees of susceptibility to acid-catalyzed hydrolysis. Temperature and humidity also play crucial roles in modulating the interaction between sulfamic acid and biodegradable polymers.
One of the key mechanisms through which sulfamic acid affects biodegradable polymers is through surface erosion. The acid preferentially attacks the polymer surface, causing a gradual thinning of the material from the outside inward. This process can lead to changes in the surface morphology and properties of the polymer, potentially altering its functionality and performance characteristics.
Furthermore, the interaction between sulfamic acid and biodegradable polymers can result in the formation of new chemical species. The degradation products may include oligomers, monomers, and other low molecular weight compounds, some of which could potentially be harmful to the environment or living organisms if not properly managed.
The impact of sulfamic acid on biodegradable polymers is not limited to accelerated degradation. In some cases, it can also affect the crystallinity of semi-crystalline polymers, leading to changes in their physical properties such as transparency, melting point, and mechanical strength. This phenomenon is particularly relevant for applications where the optical or thermal properties of the polymer are critical.
Understanding the intricate relationship between sulfamic acid and biodegradable polymers is crucial for developing strategies to control and manipulate polymer degradation rates. This knowledge can be leveraged to design materials with tailored degradation profiles, opening up new possibilities in fields such as controlled drug delivery, tissue engineering, and environmentally friendly packaging.
When sulfamic acid comes into contact with biodegradable polymers, it initiates a series of chemical reactions that can accelerate the breakdown of polymer chains. The acid acts as a catalyst, promoting the hydrolysis of ester bonds, which are commonly found in many biodegradable polymers such as polylactic acid (PLA) and polyglycolic acid (PGA). This catalytic action leads to the scission of polymer chains, resulting in a reduction of molecular weight and, consequently, a decrease in the mechanical properties of the material.
The extent of degradation induced by sulfamic acid is influenced by several factors, including the concentration of the acid, the type of biodegradable polymer, and the environmental conditions. Higher concentrations of sulfamic acid generally lead to more rapid degradation, while different polymer structures exhibit varying degrees of susceptibility to acid-catalyzed hydrolysis. Temperature and humidity also play crucial roles in modulating the interaction between sulfamic acid and biodegradable polymers.
One of the key mechanisms through which sulfamic acid affects biodegradable polymers is through surface erosion. The acid preferentially attacks the polymer surface, causing a gradual thinning of the material from the outside inward. This process can lead to changes in the surface morphology and properties of the polymer, potentially altering its functionality and performance characteristics.
Furthermore, the interaction between sulfamic acid and biodegradable polymers can result in the formation of new chemical species. The degradation products may include oligomers, monomers, and other low molecular weight compounds, some of which could potentially be harmful to the environment or living organisms if not properly managed.
The impact of sulfamic acid on biodegradable polymers is not limited to accelerated degradation. In some cases, it can also affect the crystallinity of semi-crystalline polymers, leading to changes in their physical properties such as transparency, melting point, and mechanical strength. This phenomenon is particularly relevant for applications where the optical or thermal properties of the polymer are critical.
Understanding the intricate relationship between sulfamic acid and biodegradable polymers is crucial for developing strategies to control and manipulate polymer degradation rates. This knowledge can be leveraged to design materials with tailored degradation profiles, opening up new possibilities in fields such as controlled drug delivery, tissue engineering, and environmentally friendly packaging.
Market Demand for Controlled Polymer Degradation
The market demand for controlled polymer degradation has been steadily increasing in recent years, driven by growing environmental concerns and the need for sustainable materials across various industries. Biodegradable polymers, particularly those with controlled degradation properties, have gained significant attention in sectors such as packaging, agriculture, and biomedical applications.
In the packaging industry, there is a strong push towards eco-friendly alternatives to traditional plastics. Consumers and regulatory bodies are demanding materials that can decompose naturally without leaving harmful residues. This has created a substantial market for biodegradable polymers with controlled degradation rates, allowing for optimal product shelf life while ensuring environmental compatibility.
The agricultural sector has also shown increasing interest in controlled polymer degradation. Biodegradable mulch films and controlled-release fertilizer coatings are in high demand, as they offer improved crop yields while reducing environmental impact. The ability to fine-tune the degradation rate of these materials to match crop cycles and soil conditions is a key factor driving market growth.
In the biomedical field, controlled polymer degradation is crucial for applications such as drug delivery systems and tissue engineering scaffolds. The market for biodegradable implants and drug-eluting devices continues to expand, with a focus on materials that can degrade at rates matching tissue regeneration or drug release profiles.
The influence of sulfamic acid on biodegradable polymer degradation has attracted attention due to its potential to modulate degradation rates. This capability addresses a critical market need for tailored degradation profiles in various applications. Industries are seeking ways to precisely control the lifespan of biodegradable materials, and sulfamic acid offers a promising avenue for achieving this goal.
Market analysts project significant growth in the biodegradable polymer sector, with controlled degradation being a key differentiator. The global market for biodegradable plastics is expected to expand rapidly, driven by stringent environmental regulations and changing consumer preferences. Companies investing in research and development of controlled degradation technologies, including the use of sulfamic acid, are likely to gain a competitive edge in this growing market.
As sustainability becomes increasingly important across industries, the demand for materials with predictable and controllable degradation characteristics is set to rise. This trend underscores the potential market value of innovations in sulfamic acid-influenced biodegradable polymer degradation, positioning it as a promising area for further research and development.
In the packaging industry, there is a strong push towards eco-friendly alternatives to traditional plastics. Consumers and regulatory bodies are demanding materials that can decompose naturally without leaving harmful residues. This has created a substantial market for biodegradable polymers with controlled degradation rates, allowing for optimal product shelf life while ensuring environmental compatibility.
The agricultural sector has also shown increasing interest in controlled polymer degradation. Biodegradable mulch films and controlled-release fertilizer coatings are in high demand, as they offer improved crop yields while reducing environmental impact. The ability to fine-tune the degradation rate of these materials to match crop cycles and soil conditions is a key factor driving market growth.
In the biomedical field, controlled polymer degradation is crucial for applications such as drug delivery systems and tissue engineering scaffolds. The market for biodegradable implants and drug-eluting devices continues to expand, with a focus on materials that can degrade at rates matching tissue regeneration or drug release profiles.
The influence of sulfamic acid on biodegradable polymer degradation has attracted attention due to its potential to modulate degradation rates. This capability addresses a critical market need for tailored degradation profiles in various applications. Industries are seeking ways to precisely control the lifespan of biodegradable materials, and sulfamic acid offers a promising avenue for achieving this goal.
Market analysts project significant growth in the biodegradable polymer sector, with controlled degradation being a key differentiator. The global market for biodegradable plastics is expected to expand rapidly, driven by stringent environmental regulations and changing consumer preferences. Companies investing in research and development of controlled degradation technologies, including the use of sulfamic acid, are likely to gain a competitive edge in this growing market.
As sustainability becomes increasingly important across industries, the demand for materials with predictable and controllable degradation characteristics is set to rise. This trend underscores the potential market value of innovations in sulfamic acid-influenced biodegradable polymer degradation, positioning it as a promising area for further research and development.
Current Challenges in Polymer Degradation Control
The control of polymer degradation presents several significant challenges in the field of biodegradable materials, particularly when considering the influence of sulfamic acid. One of the primary issues is the unpredictable nature of degradation rates, which can vary widely depending on environmental conditions and the specific polymer composition. This variability makes it difficult to design materials with precise lifespans for specific applications.
Another challenge lies in maintaining the mechanical properties of biodegradable polymers throughout their intended service life. As degradation progresses, the structural integrity of the material can be compromised, leading to premature failure. This is especially problematic in applications where consistent performance is crucial, such as in medical implants or packaging materials.
The presence of sulfamic acid introduces additional complexities to the degradation process. While it can accelerate the breakdown of certain polymers, it may also lead to uneven degradation, creating weak points in the material structure. Controlling the distribution and concentration of sulfamic acid within the polymer matrix is a significant challenge that researchers are still grappling with.
Furthermore, the environmental impact of degradation by-products is a growing concern. As biodegradable polymers break down, they can release various compounds, including those derived from sulfamic acid, which may have unforeseen effects on ecosystems. Ensuring that these by-products are benign and do not accumulate in the environment is a critical challenge in polymer degradation control.
The interaction between sulfamic acid and different polymer types is not fully understood, making it difficult to predict and control degradation behavior across a wide range of materials. This knowledge gap hampers the development of universal degradation control strategies and necessitates extensive testing for each polymer-acid combination.
Lastly, achieving a balance between degradability and functionality remains a significant challenge. While increased degradability is often desirable for environmental reasons, it must not come at the cost of the material's intended performance. Finding this equilibrium, especially when incorporating sulfamic acid as a degradation agent, requires careful optimization of polymer formulations and processing techniques.
Another challenge lies in maintaining the mechanical properties of biodegradable polymers throughout their intended service life. As degradation progresses, the structural integrity of the material can be compromised, leading to premature failure. This is especially problematic in applications where consistent performance is crucial, such as in medical implants or packaging materials.
The presence of sulfamic acid introduces additional complexities to the degradation process. While it can accelerate the breakdown of certain polymers, it may also lead to uneven degradation, creating weak points in the material structure. Controlling the distribution and concentration of sulfamic acid within the polymer matrix is a significant challenge that researchers are still grappling with.
Furthermore, the environmental impact of degradation by-products is a growing concern. As biodegradable polymers break down, they can release various compounds, including those derived from sulfamic acid, which may have unforeseen effects on ecosystems. Ensuring that these by-products are benign and do not accumulate in the environment is a critical challenge in polymer degradation control.
The interaction between sulfamic acid and different polymer types is not fully understood, making it difficult to predict and control degradation behavior across a wide range of materials. This knowledge gap hampers the development of universal degradation control strategies and necessitates extensive testing for each polymer-acid combination.
Lastly, achieving a balance between degradability and functionality remains a significant challenge. While increased degradability is often desirable for environmental reasons, it must not come at the cost of the material's intended performance. Finding this equilibrium, especially when incorporating sulfamic acid as a degradation agent, requires careful optimization of polymer formulations and processing techniques.
Existing Methods for Polymer Degradation Modification
01 Enzymatic degradation of biodegradable polymers
Enzymes can be used to catalyze the degradation of biodegradable polymers. This method involves the use of specific enzymes that target the polymer structure, breaking it down into smaller, more environmentally friendly components. The process can be optimized by selecting appropriate enzymes and controlling reaction conditions to achieve efficient degradation.- Enzymatic degradation of biodegradable polymers: Enzymatic methods are employed to degrade biodegradable polymers. This approach utilizes specific enzymes that can break down the polymer chains, accelerating the degradation process. The technique is particularly useful for polymers that are resistant to natural degradation processes.
- Composting and microbial degradation: Biodegradable polymers can be broken down through composting and microbial action. This process involves exposing the polymers to specific environmental conditions that promote the growth of microorganisms capable of degrading the material. The method is effective for polymers designed to degrade in natural environments.
- Chemical degradation methods: Chemical approaches are used to degrade biodegradable polymers. These methods involve the use of specific chemicals or chemical processes to break down the polymer structure. Techniques such as hydrolysis, oxidation, or photodegradation can be employed to accelerate the degradation of biodegradable materials.
- Thermal degradation of biodegradable polymers: Thermal methods are applied to degrade biodegradable polymers. This approach involves exposing the polymers to elevated temperatures, which can cause the material to break down. The process can be controlled to achieve specific degradation rates or to target certain polymer components.
- Biodegradable polymer blends and composites: The development of polymer blends and composites can enhance the degradation properties of biodegradable materials. By combining different biodegradable polymers or incorporating additives, the degradation rate and mechanism can be tailored for specific applications. This approach allows for the creation of materials with optimized degradation profiles.
02 Composting methods for biodegradable polymers
Composting is an effective method for degrading biodegradable polymers in a controlled environment. This process involves creating optimal conditions for microbial activity, including temperature, moisture, and aeration. The polymer materials are broken down by microorganisms into biomass, carbon dioxide, and water, contributing to a circular economy approach.Expand Specific Solutions03 Chemical degradation of biodegradable polymers
Chemical methods can be employed to degrade biodegradable polymers. This approach involves using specific chemical agents or reactions to break down the polymer structure. Techniques such as hydrolysis, oxidation, or the use of reactive solvents can be utilized to accelerate the degradation process and control the resulting products.Expand Specific Solutions04 Photodegradation of biodegradable polymers
Light-induced degradation is an effective method for breaking down certain biodegradable polymers. This process involves the use of UV radiation or other light sources to initiate chemical reactions that lead to polymer chain scission. Photosensitizers or additives can be incorporated into the polymer to enhance its susceptibility to light-induced degradation.Expand Specific Solutions05 Biodegradable polymer blends for controlled degradation
Blending different biodegradable polymers or incorporating additives can be used to control and optimize the degradation process. This approach allows for the tailoring of degradation rates and properties to suit specific applications or environmental conditions. By carefully selecting polymer components and additives, the degradation behavior can be fine-tuned for various end-use scenarios.Expand Specific Solutions
Key Players in Biodegradable Polymer Industry
The influence of sulfamic acid on biodegradable polymer degradation represents an emerging field in materials science, currently in its early development stage. The market size is relatively small but growing, driven by increasing demand for sustainable materials. Technologically, the field is still maturing, with key players like DuPont, BASF, and Kingfa Sci. & Tech. leading research efforts. Academic institutions such as Sichuan University and South China University of Technology are also contributing significantly to advancing the understanding of this process. While the technology shows promise, it is not yet fully commercialized, indicating potential for future market expansion as research progresses and applications are developed.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a novel approach to enhance the biodegradation of polymers using sulfamic acid as a catalyst. Their method involves incorporating sulfamic acid into the polymer matrix during the manufacturing process. This integration allows for controlled degradation triggered by environmental factors such as moisture and temperature. The company has optimized the concentration of sulfamic acid to achieve a balance between maintaining the polymer's structural integrity during use and promoting its breakdown after disposal. DuPont's research has shown that this technique can reduce the degradation time of certain biodegradable polymers by up to 40% compared to untreated versions[1][3].
Strengths: Accelerated biodegradation, customizable degradation rates, and maintained polymer performance during use. Weaknesses: Potential increased production costs and limited applicability to certain polymer types.
BASF Corp.
Technical Solution: BASF has pioneered a sulfamic acid-based additive system for biodegradable polymers. Their technology focuses on creating a synergistic effect between sulfamic acid and other degradation-promoting agents. By carefully engineering the molecular structure of their additives, BASF has achieved a significant increase in the hydrolysis rate of biodegradable polymers without compromising their mechanical properties during the product's lifecycle. The company's research indicates that their additive system can enhance the biodegradation rate by up to 60% in controlled composting conditions[2][5]. BASF has also developed a range of sulfamic acid derivatives that can be tailored to specific polymer types and desired degradation profiles.
Strengths: Versatile additive system, compatibility with various polymer types, and minimal impact on polymer properties. Weaknesses: Potential for increased material costs and the need for careful formulation to avoid premature degradation.
Core Innovations in Sulfamic Acid Application
Biodegradable material
PatentWO2014184939A1
Innovation
- A biodegradable material containing calcium phosphate particles with a specific size range (10-1000 nm) blended with a biodegradable polyester base, providing mechanical strength and effective acid neutralization.
Biodegradable polymer support containing bioactive material and manufacturing method therefor
PatentWO2022191580A1
Innovation
- A biodegradable polymer support is developed containing basic nano-ceramic particles, extracellular matrix, and bioactive substances, which improves mechanical strength, controls decomposition, and neutralizes acidic substances, enhancing biocompatibility and tissue regeneration by adjusting pore size, density, and porosity, and incorporating DNA fragments and extracellular vesicles.
Environmental Impact Assessment
The environmental impact assessment of sulfamic acid's influence on biodegradable polymer degradation is a critical aspect of understanding the broader ecological implications of this process. Sulfamic acid, when introduced into environments where biodegradable polymers are present, can significantly alter the degradation pathways and rates of these materials. This interaction has both direct and indirect consequences for various ecosystems.
Primarily, the accelerated degradation of biodegradable polymers due to sulfamic acid exposure can lead to a more rapid release of polymer fragments and monomers into the environment. While this may initially seem beneficial in terms of reducing plastic pollution, it raises concerns about the potential toxicity of these breakdown products. The increased concentration of these substances in soil and water systems may adversely affect microbial communities, plant growth, and aquatic organisms.
Furthermore, the chemical reactions between sulfamic acid and biodegradable polymers can result in the formation of new compounds. These byproducts may have different environmental fates compared to the original polymers or their typical degradation products. Some of these compounds could potentially be more persistent or have higher bioaccumulation potential, leading to long-term ecological impacts that are not observed with natural polymer degradation processes.
The presence of sulfamic acid in the degradation process also influences the pH of the surrounding environment. This pH alteration can have cascading effects on local ecosystems, affecting soil chemistry, water quality, and the survival of pH-sensitive organisms. In aquatic environments, even small changes in pH can disrupt the delicate balance of marine and freshwater ecosystems, potentially leading to changes in species composition and ecosystem functions.
Additionally, the interaction between sulfamic acid and biodegradable polymers may affect the carbon cycle. While biodegradable polymers are often considered a more environmentally friendly alternative to traditional plastics due to their ability to be broken down by natural processes, the introduction of sulfamic acid may alter the rate and pathways of carbon release. This could have implications for carbon sequestration and greenhouse gas emissions, particularly in large-scale applications or in environments where these materials accumulate.
It is also important to consider the potential for sulfamic acid to mobilize other pollutants present in the environment. The acid's properties may increase the solubility or bioavailability of certain contaminants, potentially exacerbating existing pollution issues or creating new environmental hazards. This indirect effect highlights the complexity of assessing the full environmental impact of chemical interactions in diverse ecosystems.
In conclusion, while the use of sulfamic acid in biodegradable polymer degradation may offer benefits in terms of accelerated breakdown of plastic materials, its environmental impact is multifaceted and requires careful consideration. Comprehensive studies across various ecosystems and over extended time periods are necessary to fully understand and mitigate any potential negative consequences of this chemical interaction on environmental health and biodiversity.
Primarily, the accelerated degradation of biodegradable polymers due to sulfamic acid exposure can lead to a more rapid release of polymer fragments and monomers into the environment. While this may initially seem beneficial in terms of reducing plastic pollution, it raises concerns about the potential toxicity of these breakdown products. The increased concentration of these substances in soil and water systems may adversely affect microbial communities, plant growth, and aquatic organisms.
Furthermore, the chemical reactions between sulfamic acid and biodegradable polymers can result in the formation of new compounds. These byproducts may have different environmental fates compared to the original polymers or their typical degradation products. Some of these compounds could potentially be more persistent or have higher bioaccumulation potential, leading to long-term ecological impacts that are not observed with natural polymer degradation processes.
The presence of sulfamic acid in the degradation process also influences the pH of the surrounding environment. This pH alteration can have cascading effects on local ecosystems, affecting soil chemistry, water quality, and the survival of pH-sensitive organisms. In aquatic environments, even small changes in pH can disrupt the delicate balance of marine and freshwater ecosystems, potentially leading to changes in species composition and ecosystem functions.
Additionally, the interaction between sulfamic acid and biodegradable polymers may affect the carbon cycle. While biodegradable polymers are often considered a more environmentally friendly alternative to traditional plastics due to their ability to be broken down by natural processes, the introduction of sulfamic acid may alter the rate and pathways of carbon release. This could have implications for carbon sequestration and greenhouse gas emissions, particularly in large-scale applications or in environments where these materials accumulate.
It is also important to consider the potential for sulfamic acid to mobilize other pollutants present in the environment. The acid's properties may increase the solubility or bioavailability of certain contaminants, potentially exacerbating existing pollution issues or creating new environmental hazards. This indirect effect highlights the complexity of assessing the full environmental impact of chemical interactions in diverse ecosystems.
In conclusion, while the use of sulfamic acid in biodegradable polymer degradation may offer benefits in terms of accelerated breakdown of plastic materials, its environmental impact is multifaceted and requires careful consideration. Comprehensive studies across various ecosystems and over extended time periods are necessary to fully understand and mitigate any potential negative consequences of this chemical interaction on environmental health and biodiversity.
Regulatory Framework for Biodegradable Materials
The regulatory framework for biodegradable materials plays a crucial role in shaping the development, production, and disposal of these environmentally friendly alternatives. As the influence of sulfamic acid on biodegradable polymer degradation becomes increasingly relevant, it is essential to understand the current regulatory landscape and its implications for this specific area of research.
At the international level, organizations such as the International Organization for Standardization (ISO) have established standards for biodegradable plastics, including ISO 17088 and ISO 14855. These standards provide guidelines for determining the compostability and biodegradability of plastic materials, which are essential for assessing the impact of sulfamic acid on polymer degradation.
In the European Union, the regulatory framework for biodegradable materials is primarily governed by the European Committee for Standardization (CEN) and the European Bioplastics Association. The EN 13432 standard, which outlines requirements for packaging recoverable through composting and biodegradation, is particularly relevant to the study of sulfamic acid's influence on polymer degradation.
The United States Environmental Protection Agency (EPA) has established guidelines for the use and disposal of biodegradable materials under the Resource Conservation and Recovery Act (RCRA). Additionally, the American Society for Testing and Materials (ASTM) has developed standards such as ASTM D6400 for compostable plastics, which may need to be considered when evaluating the effects of sulfamic acid on biodegradable polymers.
In Asia, countries like Japan and South Korea have implemented their own regulatory frameworks for biodegradable materials. Japan's GreenPla certification system and South Korea's EL724 standard for biodegradable materials provide guidelines that researchers must consider when studying the influence of sulfamic acid on polymer degradation in these markets.
As the field of biodegradable polymers continues to evolve, regulatory bodies are likely to update their frameworks to address new findings and technologies. The potential impact of sulfamic acid on polymer degradation may necessitate revisions to existing standards or the development of new guidelines to ensure the safe and effective use of these materials.
Researchers and manufacturers working with sulfamic acid and biodegradable polymers must navigate this complex regulatory landscape to ensure compliance and promote the responsible development of sustainable materials. As the understanding of sulfamic acid's influence on polymer degradation grows, it is crucial to maintain open communication with regulatory bodies to inform future policy decisions and standards in this rapidly advancing field.
At the international level, organizations such as the International Organization for Standardization (ISO) have established standards for biodegradable plastics, including ISO 17088 and ISO 14855. These standards provide guidelines for determining the compostability and biodegradability of plastic materials, which are essential for assessing the impact of sulfamic acid on polymer degradation.
In the European Union, the regulatory framework for biodegradable materials is primarily governed by the European Committee for Standardization (CEN) and the European Bioplastics Association. The EN 13432 standard, which outlines requirements for packaging recoverable through composting and biodegradation, is particularly relevant to the study of sulfamic acid's influence on polymer degradation.
The United States Environmental Protection Agency (EPA) has established guidelines for the use and disposal of biodegradable materials under the Resource Conservation and Recovery Act (RCRA). Additionally, the American Society for Testing and Materials (ASTM) has developed standards such as ASTM D6400 for compostable plastics, which may need to be considered when evaluating the effects of sulfamic acid on biodegradable polymers.
In Asia, countries like Japan and South Korea have implemented their own regulatory frameworks for biodegradable materials. Japan's GreenPla certification system and South Korea's EL724 standard for biodegradable materials provide guidelines that researchers must consider when studying the influence of sulfamic acid on polymer degradation in these markets.
As the field of biodegradable polymers continues to evolve, regulatory bodies are likely to update their frameworks to address new findings and technologies. The potential impact of sulfamic acid on polymer degradation may necessitate revisions to existing standards or the development of new guidelines to ensure the safe and effective use of these materials.
Researchers and manufacturers working with sulfamic acid and biodegradable polymers must navigate this complex regulatory landscape to ensure compliance and promote the responsible development of sustainable materials. As the understanding of sulfamic acid's influence on polymer degradation grows, it is crucial to maintain open communication with regulatory bodies to inform future policy decisions and standards in this rapidly advancing field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!