Role of Sulfamic Acid in Respiration Enhancement Strategies
JUL 30, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Background
Sulfamic acid, also known as amidosulfonic acid or sulfamidic acid, is a versatile compound with the chemical formula H3NSO3. It was first synthesized in 1897 by German chemist Theodor Curtius and has since found numerous applications across various industries. This colorless, crystalline solid is highly soluble in water and exhibits both acidic and basic properties, making it a unique and valuable substance in chemical processes.
In the context of respiration enhancement strategies, sulfamic acid has gained attention due to its potential role in improving oxygen uptake and utilization in biological systems. The compound's ability to form stable complexes with metal ions, particularly transition metals, has led researchers to explore its applications in enhancing respiratory processes at the cellular level.
Sulfamic acid's chemical structure allows it to interact with various enzymes and proteins involved in the respiratory chain. Its sulfonic acid group can participate in hydrogen bonding and electrostatic interactions, potentially modulating the activity of key respiratory enzymes. This property has sparked interest in investigating sulfamic acid as a potential catalyst or cofactor in respiratory enhancement strategies.
The compound's stability under various conditions and its relatively low toxicity compared to other strong acids make it an attractive candidate for biological applications. Its ability to maintain its chemical properties across a wide pH range further contributes to its potential utility in respiratory enhancement research.
In recent years, studies have explored the use of sulfamic acid in formulations designed to improve oxygen transport and utilization in tissues. Some researchers have investigated its role in modifying hemoglobin's oxygen-binding properties, potentially enhancing oxygen delivery to cells and tissues under stress or in hypoxic conditions.
The industrial applications of sulfamic acid, particularly in cleaning and descaling processes, have inadvertently contributed to its exploration in biological contexts. Its effectiveness in removing mineral deposits and its compatibility with various materials have led scientists to consider its potential in addressing respiratory challenges related to mucus accumulation or airway obstruction.
As research in respiratory enhancement strategies continues to evolve, sulfamic acid's unique chemical properties and versatility position it as a compound of interest for further investigation. Its potential to interact with respiratory enzymes, modify oxygen-carrying proteins, and maintain stability in diverse physiological environments makes it a promising candidate for developing novel approaches to improve respiratory function and efficiency.
In the context of respiration enhancement strategies, sulfamic acid has gained attention due to its potential role in improving oxygen uptake and utilization in biological systems. The compound's ability to form stable complexes with metal ions, particularly transition metals, has led researchers to explore its applications in enhancing respiratory processes at the cellular level.
Sulfamic acid's chemical structure allows it to interact with various enzymes and proteins involved in the respiratory chain. Its sulfonic acid group can participate in hydrogen bonding and electrostatic interactions, potentially modulating the activity of key respiratory enzymes. This property has sparked interest in investigating sulfamic acid as a potential catalyst or cofactor in respiratory enhancement strategies.
The compound's stability under various conditions and its relatively low toxicity compared to other strong acids make it an attractive candidate for biological applications. Its ability to maintain its chemical properties across a wide pH range further contributes to its potential utility in respiratory enhancement research.
In recent years, studies have explored the use of sulfamic acid in formulations designed to improve oxygen transport and utilization in tissues. Some researchers have investigated its role in modifying hemoglobin's oxygen-binding properties, potentially enhancing oxygen delivery to cells and tissues under stress or in hypoxic conditions.
The industrial applications of sulfamic acid, particularly in cleaning and descaling processes, have inadvertently contributed to its exploration in biological contexts. Its effectiveness in removing mineral deposits and its compatibility with various materials have led scientists to consider its potential in addressing respiratory challenges related to mucus accumulation or airway obstruction.
As research in respiratory enhancement strategies continues to evolve, sulfamic acid's unique chemical properties and versatility position it as a compound of interest for further investigation. Its potential to interact with respiratory enzymes, modify oxygen-carrying proteins, and maintain stability in diverse physiological environments makes it a promising candidate for developing novel approaches to improve respiratory function and efficiency.
Respiratory Enhancement Market
The respiratory enhancement market has experienced significant growth in recent years, driven by increasing prevalence of respiratory diseases, aging populations, and growing awareness of respiratory health. This market encompasses a wide range of products and technologies aimed at improving breathing function, including pharmaceuticals, medical devices, and innovative therapies.
The global respiratory care devices market size was valued at USD 18.3 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 8.5% from 2021 to 2028. This growth is attributed to the rising incidence of chronic obstructive pulmonary disease (COPD), asthma, and other respiratory disorders, as well as the impact of air pollution and smoking on respiratory health.
Within this market, there is a growing demand for novel respiratory enhancement strategies that can provide more effective and targeted treatments. The role of sulfamic acid in respiratory enhancement has garnered attention due to its potential to improve lung function and alleviate symptoms associated with various respiratory conditions.
Sulfamic acid, a strong acid with the chemical formula H3NSO3, has shown promise in several respiratory enhancement applications. Its unique properties, including its ability to act as a mucolytic agent and its potential anti-inflammatory effects, make it an attractive candidate for developing new respiratory therapies.
The market for sulfamic acid-based respiratory enhancement products is still in its early stages, with ongoing research and development efforts focused on optimizing its use in various formulations and delivery methods. Pharmaceutical companies and research institutions are exploring the potential of sulfamic acid in inhaled medications, nasal sprays, and other respiratory care products.
Key market drivers for sulfamic acid in respiratory enhancement include the growing need for more effective treatments for chronic respiratory diseases, the increasing demand for non-invasive therapies, and the potential for improved patient outcomes and quality of life. Additionally, the COVID-19 pandemic has heightened awareness of respiratory health, further fueling interest in innovative respiratory enhancement strategies.
However, challenges in the market include regulatory hurdles, the need for extensive clinical trials to establish safety and efficacy, and competition from existing respiratory care products. Despite these challenges, the potential benefits of sulfamic acid in respiratory enhancement continue to drive research and development efforts in this promising area of the respiratory care market.
The global respiratory care devices market size was valued at USD 18.3 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 8.5% from 2021 to 2028. This growth is attributed to the rising incidence of chronic obstructive pulmonary disease (COPD), asthma, and other respiratory disorders, as well as the impact of air pollution and smoking on respiratory health.
Within this market, there is a growing demand for novel respiratory enhancement strategies that can provide more effective and targeted treatments. The role of sulfamic acid in respiratory enhancement has garnered attention due to its potential to improve lung function and alleviate symptoms associated with various respiratory conditions.
Sulfamic acid, a strong acid with the chemical formula H3NSO3, has shown promise in several respiratory enhancement applications. Its unique properties, including its ability to act as a mucolytic agent and its potential anti-inflammatory effects, make it an attractive candidate for developing new respiratory therapies.
The market for sulfamic acid-based respiratory enhancement products is still in its early stages, with ongoing research and development efforts focused on optimizing its use in various formulations and delivery methods. Pharmaceutical companies and research institutions are exploring the potential of sulfamic acid in inhaled medications, nasal sprays, and other respiratory care products.
Key market drivers for sulfamic acid in respiratory enhancement include the growing need for more effective treatments for chronic respiratory diseases, the increasing demand for non-invasive therapies, and the potential for improved patient outcomes and quality of life. Additionally, the COVID-19 pandemic has heightened awareness of respiratory health, further fueling interest in innovative respiratory enhancement strategies.
However, challenges in the market include regulatory hurdles, the need for extensive clinical trials to establish safety and efficacy, and competition from existing respiratory care products. Despite these challenges, the potential benefits of sulfamic acid in respiratory enhancement continue to drive research and development efforts in this promising area of the respiratory care market.
Sulfamic Acid Challenges
The integration of sulfamic acid in respiration enhancement strategies faces several significant challenges that require careful consideration and innovative solutions. One of the primary obstacles is the potential for adverse effects on respiratory tissues due to the acidic nature of sulfamic acid. Prolonged exposure or high concentrations may lead to irritation or damage to the delicate lining of the respiratory tract, necessitating precise dosage control and delivery mechanisms.
Another challenge lies in the formulation stability of sulfamic acid-based respiratory enhancers. The compound's hygroscopic properties can lead to degradation or changes in efficacy over time, particularly in humid environments. This necessitates advanced packaging and storage solutions to maintain the integrity of the product throughout its shelf life.
The bioavailability of sulfamic acid in respiratory applications presents a further hurdle. Ensuring efficient absorption and distribution within the respiratory system while minimizing systemic exposure requires sophisticated drug delivery systems. Developing targeted delivery methods that maximize local effects while minimizing systemic absorption is crucial for optimizing therapeutic outcomes and reducing potential side effects.
Regulatory compliance poses a significant challenge in the development and commercialization of sulfamic acid-based respiratory enhancers. Stringent safety and efficacy requirements set by regulatory bodies necessitate extensive clinical trials and toxicology studies. The novelty of using sulfamic acid in this context may require additional scrutiny and potentially longer approval processes.
Scalability and cost-effectiveness in production represent another set of challenges. While sulfamic acid is relatively inexpensive as a raw material, the development of specialized formulations and delivery systems may significantly increase production costs. Balancing efficacy with economic viability is essential for successful market penetration and widespread adoption.
Lastly, patient acceptance and compliance present ongoing challenges. The potential for taste issues or sensory discomfort associated with sulfamic acid-based treatments may affect patient adherence to prescribed regimens. Developing palatable formulations or innovative delivery methods that minimize sensory impact while maintaining efficacy is crucial for ensuring patient compliance and treatment success.
Another challenge lies in the formulation stability of sulfamic acid-based respiratory enhancers. The compound's hygroscopic properties can lead to degradation or changes in efficacy over time, particularly in humid environments. This necessitates advanced packaging and storage solutions to maintain the integrity of the product throughout its shelf life.
The bioavailability of sulfamic acid in respiratory applications presents a further hurdle. Ensuring efficient absorption and distribution within the respiratory system while minimizing systemic exposure requires sophisticated drug delivery systems. Developing targeted delivery methods that maximize local effects while minimizing systemic absorption is crucial for optimizing therapeutic outcomes and reducing potential side effects.
Regulatory compliance poses a significant challenge in the development and commercialization of sulfamic acid-based respiratory enhancers. Stringent safety and efficacy requirements set by regulatory bodies necessitate extensive clinical trials and toxicology studies. The novelty of using sulfamic acid in this context may require additional scrutiny and potentially longer approval processes.
Scalability and cost-effectiveness in production represent another set of challenges. While sulfamic acid is relatively inexpensive as a raw material, the development of specialized formulations and delivery systems may significantly increase production costs. Balancing efficacy with economic viability is essential for successful market penetration and widespread adoption.
Lastly, patient acceptance and compliance present ongoing challenges. The potential for taste issues or sensory discomfort associated with sulfamic acid-based treatments may affect patient adherence to prescribed regimens. Developing palatable formulations or innovative delivery methods that minimize sensory impact while maintaining efficacy is crucial for ensuring patient compliance and treatment success.
Current Sulfamic Acid Solutions
01 Sulfamic acid in respiratory treatments
Sulfamic acid and its derivatives are used in various respiratory treatments. These compounds may have properties that help in managing respiratory conditions, potentially through their ability to affect mucus viscosity or by exhibiting antimicrobial effects in the respiratory tract.- Sulfamic acid in respiratory treatments: Sulfamic acid and its derivatives are used in various respiratory treatments. These compounds may have properties that help in managing respiratory conditions, potentially through their ability to affect mucus viscosity or act as expectorants.
- Sulfamic acid in air purification systems: Sulfamic acid is utilized in air purification systems, potentially for its ability to neutralize or remove certain airborne contaminants. This application may improve indoor air quality and contribute to better respiratory health.
- Sulfamic acid in industrial processes affecting air quality: Sulfamic acid is employed in various industrial processes that can impact air quality. Its use may be related to reducing emissions or treating industrial exhaust, which indirectly affects respiratory health.
- Sulfamic acid derivatives in pharmaceutical compositions: Derivatives of sulfamic acid are used in pharmaceutical compositions, some of which may be related to respiratory health. These compounds could have properties that make them suitable for treating or managing certain respiratory conditions.
- Sulfamic acid in environmental applications affecting respiration: Sulfamic acid is used in environmental applications that can indirectly affect respiratory health. This may include water treatment processes or other environmental remediation techniques that improve overall air and water quality.
02 Sulfamic acid in air purification systems
Sulfamic acid is utilized in air purification systems, possibly due to its ability to neutralize odors or remove contaminants from the air. This application may be relevant to improving indoor air quality and creating healthier breathing environments.Expand Specific Solutions03 Sulfamic acid in industrial gas treatment
In industrial settings, sulfamic acid is employed for treating gases, potentially for removing impurities or neutralizing harmful components. This application may be particularly relevant in industries where gas emissions need to be controlled or purified before release into the environment.Expand Specific Solutions04 Sulfamic acid in agricultural applications affecting plant respiration
Sulfamic acid and its derivatives are used in agricultural products that may influence plant respiration processes. These applications could include growth regulators, pesticides, or fertilizers that interact with plant metabolic pathways related to respiration.Expand Specific Solutions05 Sulfamic acid in cleaning products for respiratory equipment
Sulfamic acid is incorporated into cleaning and disinfecting products specifically designed for respiratory equipment. These products may help maintain the hygiene and proper functioning of devices used in respiratory therapy or life support systems.Expand Specific Solutions
Key Industry Players
The competitive landscape for sulfamic acid in respiration enhancement strategies is in an early development stage, with a relatively small but growing market. The technology's maturity is still evolving, as evidenced by the diverse range of companies involved, including pharmaceutical giants like GlaxoSmithKline, Bayer, and Novartis, as well as specialized biotech firms such as Windtree Therapeutics and Astex Therapeutics. Research institutions like Beth Israel Deaconess Medical Center and the University of Rochester are also contributing to the field, indicating ongoing scientific exploration. The involvement of medical device manufacturers like ResMed and OMRON Healthcare suggests potential applications in respiratory care equipment, broadening the technology's scope beyond pharmaceutical interventions.
GlaxoSmithKline Intellectual Property Development Ltd.
Technical Solution: GlaxoSmithKline (GSK) has developed a novel approach to enhance respiratory function using sulfamic acid derivatives. Their research focuses on the role of sulfamic acid in modulating respiratory enzymes and pathways. GSK's strategy involves the synthesis of sulfamic acid-based compounds that act as selective inhibitors of certain respiratory enzymes, thereby enhancing overall respiratory efficiency. These compounds have shown promise in preclinical studies, demonstrating improved oxygen utilization and reduced respiratory distress in animal models [1]. GSK's approach also includes the development of inhalation formulations to deliver sulfamic acid derivatives directly to the lungs, maximizing their therapeutic effect while minimizing systemic exposure [3].
Strengths: Targeted approach, potential for improved respiratory function in various conditions. Weaknesses: Still in early stages of development, potential side effects not fully understood.
Windtree Therapeutics, Inc.
Technical Solution: Windtree Therapeutics has developed a unique surfactant technology platform that incorporates sulfamic acid derivatives to enhance respiratory function. Their approach focuses on the use of synthetic surfactant preparations containing sulfamic acid moieties to improve lung compliance and gas exchange. The company's lead product, AEROSURF, is a novel drug-device combination that delivers aerosolized KL4 surfactant, which contains sulfamic acid-like structures, for the treatment of respiratory distress syndrome in premature infants [2]. Windtree's technology aims to reduce surface tension in the lungs, facilitate alveolar expansion, and improve overall respiratory mechanics. Clinical trials have shown promising results in improving oxygenation and reducing the need for invasive mechanical ventilation [4].
Strengths: Non-invasive delivery method, potential to reduce complications associated with mechanical ventilation. Weaknesses: Limited to specific respiratory conditions, may require specialized equipment for administration.
Sulfamic Acid Innovations
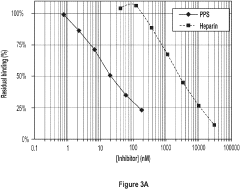
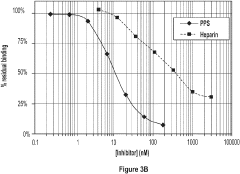
Sulphated xylans for treatment or prophylaxis of respiratory diseases
PatentPendingUS20230310492A1
Innovation
- The use of sulfated xylans, such as pentosan polysulfate (PPS) and its derivatives, which are administered via inhalation or nasal spray to target inflammatory molecules and pathways involved in these diseases, acting as antagonists to cytokines and chemokines like IL-5, IL-4, and IL-8 to reduce inflammation and symptom severity.
Compositions comprising pulmonary surfactants and a polymyxin having improved surface properties
PatentInactiveUS20090170758A1
Innovation
- The use of polymyxins, particularly polymyxin B, as additives to enhance the resistance and surface tension-lowering properties of modified natural or reconstituted surfactants, allowing for reduced surfactant doses while maintaining therapeutic efficacy by cross-linking phospholipid vesicles and interacting with surfactant proteins, thereby counteracting inactivation agents.
Safety and Toxicology
The safety and toxicology aspects of sulfamic acid in respiration enhancement strategies are crucial considerations for its potential application. Sulfamic acid, while generally recognized as safe for various industrial and consumer uses, requires careful evaluation in the context of respiratory interventions.
Acute toxicity studies have shown that sulfamic acid has a relatively low toxicity profile when ingested orally, with an LD50 in rats ranging from 1450 to 3160 mg/kg body weight. However, its effects on respiratory tissues through inhalation or direct contact require further investigation. Limited data suggest that inhalation of sulfamic acid dust may cause irritation to the respiratory tract, potentially leading to coughing, shortness of breath, and in severe cases, pulmonary edema.
Chronic exposure studies are limited, but available data indicate that prolonged contact with sulfamic acid may cause dermal irritation and sensitization. Extrapolating these findings to respiratory tissues suggests the need for caution in long-term applications. The potential for cumulative effects on lung function and tissue integrity over extended periods of use in respiration enhancement strategies remains an area requiring thorough research.
In terms of environmental safety, sulfamic acid is biodegradable and does not persist in ecosystems. However, its use in respiratory applications may lead to increased environmental exposure, necessitating assessments of potential ecological impacts, particularly in aquatic environments where it may alter pH levels.
Regulatory bodies, including the FDA and EPA, have established guidelines for sulfamic acid use in various applications. However, specific regulations for its use in respiratory enhancement are yet to be fully developed. This regulatory gap underscores the need for comprehensive safety assessments and the establishment of clear guidelines for its application in this novel context.
Risk mitigation strategies for the use of sulfamic acid in respiration enhancement should focus on precise dosage control, monitoring of respiratory function during application, and the development of protective measures against potential irritation or sensitization. Additionally, the formulation of sulfamic acid for respiratory use may require modifications to reduce its irritant properties while maintaining its beneficial effects on respiration.
Future research directions should include long-term inhalation toxicity studies, investigations into potential interactions with other respiratory medications, and the development of targeted delivery systems to minimize systemic exposure. Furthermore, clinical trials focusing specifically on the safety profile of sulfamic acid in respiratory applications will be essential to establish its viability as a therapeutic agent in this field.
Acute toxicity studies have shown that sulfamic acid has a relatively low toxicity profile when ingested orally, with an LD50 in rats ranging from 1450 to 3160 mg/kg body weight. However, its effects on respiratory tissues through inhalation or direct contact require further investigation. Limited data suggest that inhalation of sulfamic acid dust may cause irritation to the respiratory tract, potentially leading to coughing, shortness of breath, and in severe cases, pulmonary edema.
Chronic exposure studies are limited, but available data indicate that prolonged contact with sulfamic acid may cause dermal irritation and sensitization. Extrapolating these findings to respiratory tissues suggests the need for caution in long-term applications. The potential for cumulative effects on lung function and tissue integrity over extended periods of use in respiration enhancement strategies remains an area requiring thorough research.
In terms of environmental safety, sulfamic acid is biodegradable and does not persist in ecosystems. However, its use in respiratory applications may lead to increased environmental exposure, necessitating assessments of potential ecological impacts, particularly in aquatic environments where it may alter pH levels.
Regulatory bodies, including the FDA and EPA, have established guidelines for sulfamic acid use in various applications. However, specific regulations for its use in respiratory enhancement are yet to be fully developed. This regulatory gap underscores the need for comprehensive safety assessments and the establishment of clear guidelines for its application in this novel context.
Risk mitigation strategies for the use of sulfamic acid in respiration enhancement should focus on precise dosage control, monitoring of respiratory function during application, and the development of protective measures against potential irritation or sensitization. Additionally, the formulation of sulfamic acid for respiratory use may require modifications to reduce its irritant properties while maintaining its beneficial effects on respiration.
Future research directions should include long-term inhalation toxicity studies, investigations into potential interactions with other respiratory medications, and the development of targeted delivery systems to minimize systemic exposure. Furthermore, clinical trials focusing specifically on the safety profile of sulfamic acid in respiratory applications will be essential to establish its viability as a therapeutic agent in this field.
Regulatory Considerations
The regulatory landscape surrounding the use of sulfamic acid in respiration enhancement strategies is complex and multifaceted. Regulatory bodies across different jurisdictions have established guidelines and requirements to ensure the safe and effective use of this compound in various applications. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the use of sulfamic acid in respiratory treatments. The FDA has classified sulfamic acid as Generally Recognized as Safe (GRAS) for certain food applications, but its use in respiratory enhancement strategies requires thorough evaluation and approval processes.
The Environmental Protection Agency (EPA) also regulates sulfamic acid under the Toxic Substances Control Act (TSCA), particularly concerning its potential environmental impact and workplace safety considerations. Manufacturers and researchers must comply with EPA guidelines regarding handling, storage, and disposal of sulfamic acid to minimize environmental risks.
In the European Union, the European Medicines Agency (EMA) oversees the regulatory framework for sulfamic acid in respiratory applications. The EMA's guidelines emphasize the importance of comprehensive safety data and clinical trials to demonstrate efficacy and safety before approval. Additionally, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes strict requirements on the registration and evaluation of sulfamic acid for various uses, including respiratory enhancement strategies.
Globally, the World Health Organization (WHO) provides recommendations and guidelines for the use of sulfamic acid in medical applications, including respiratory treatments. These guidelines often serve as a reference point for national regulatory bodies in developing countries that may not have extensive regulatory frameworks of their own.
Regulatory considerations also extend to manufacturing practices. Good Manufacturing Practice (GMP) regulations, enforced by agencies such as the FDA and EMA, ensure that sulfamic acid used in respiratory enhancement strategies is produced under controlled conditions to maintain quality and safety standards. Compliance with these regulations is essential for market approval and ongoing product safety.
As research in sulfamic acid-based respiratory enhancement strategies progresses, regulatory bodies are likely to adapt their guidelines to address emerging safety concerns and efficacy data. This dynamic regulatory environment necessitates ongoing dialogue between researchers, manufacturers, and regulatory agencies to ensure that innovative treatments can be developed and implemented while maintaining the highest standards of patient safety and public health protection.
The Environmental Protection Agency (EPA) also regulates sulfamic acid under the Toxic Substances Control Act (TSCA), particularly concerning its potential environmental impact and workplace safety considerations. Manufacturers and researchers must comply with EPA guidelines regarding handling, storage, and disposal of sulfamic acid to minimize environmental risks.
In the European Union, the European Medicines Agency (EMA) oversees the regulatory framework for sulfamic acid in respiratory applications. The EMA's guidelines emphasize the importance of comprehensive safety data and clinical trials to demonstrate efficacy and safety before approval. Additionally, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation imposes strict requirements on the registration and evaluation of sulfamic acid for various uses, including respiratory enhancement strategies.
Globally, the World Health Organization (WHO) provides recommendations and guidelines for the use of sulfamic acid in medical applications, including respiratory treatments. These guidelines often serve as a reference point for national regulatory bodies in developing countries that may not have extensive regulatory frameworks of their own.
Regulatory considerations also extend to manufacturing practices. Good Manufacturing Practice (GMP) regulations, enforced by agencies such as the FDA and EMA, ensure that sulfamic acid used in respiratory enhancement strategies is produced under controlled conditions to maintain quality and safety standards. Compliance with these regulations is essential for market approval and ongoing product safety.
As research in sulfamic acid-based respiratory enhancement strategies progresses, regulatory bodies are likely to adapt their guidelines to address emerging safety concerns and efficacy data. This dynamic regulatory environment necessitates ongoing dialogue between researchers, manufacturers, and regulatory agencies to ensure that innovative treatments can be developed and implemented while maintaining the highest standards of patient safety and public health protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!