Evaluating Sulfamic Acid for Microbial Remediation at Low pH
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Microbial Remediation Background
Sulfamic acid, a strong inorganic acid with the chemical formula H3NSO3, has emerged as a promising agent for microbial remediation in low pH environments. This compound, also known as amidosulfonic acid, has been traditionally used in various industrial applications, including descaling, metal cleaning, and as a component in household cleaning products. Its unique properties, particularly its stability and effectiveness in acidic conditions, have sparked interest in its potential for environmental remediation.
The exploration of sulfamic acid for microbial remediation stems from the growing need to address contamination in acidic environments, such as acid mine drainage sites, industrial waste areas, and certain agricultural soils. These low pH environments pose significant challenges for conventional remediation techniques, as many microorganisms struggle to survive and function effectively under such harsh conditions. Sulfamic acid's ability to maintain its properties in acidic settings makes it an attractive option for enhancing microbial activity in these challenging environments.
The concept of using sulfamic acid in microbial remediation is rooted in its potential to stimulate and support the growth of acid-tolerant microorganisms. These microbes play a crucial role in breaking down contaminants and restoring ecological balance in acidic ecosystems. By providing a stable and potentially nutrient-rich environment, sulfamic acid may enable the proliferation of specialized microbial communities capable of degrading pollutants that persist under low pH conditions.
Research into sulfamic acid's role in microbial remediation has gained momentum in recent years, driven by the increasing global focus on sustainable environmental management practices. Scientists and environmental engineers are exploring how this compound can be integrated into bioremediation strategies to enhance the efficiency and effectiveness of cleanup efforts in acidic sites. The potential applications range from treating acid mine drainage to remediating contaminated soils and industrial effluents.
The investigation of sulfamic acid for this purpose also aligns with the broader trend of developing innovative, eco-friendly remediation technologies. As regulatory pressures mount and public awareness of environmental issues grows, there is a pressing need for solutions that can address complex contamination scenarios while minimizing secondary environmental impacts. Sulfamic acid's biodegradability and relatively low toxicity compared to other strong acids make it an attractive candidate for environmentally conscious remediation approaches.
As research in this field progresses, scientists are focusing on understanding the mechanisms by which sulfamic acid influences microbial communities in low pH environments. This includes studying its effects on microbial metabolism, growth rates, and contaminant degradation pathways. Additionally, researchers are investigating optimal application methods, dosages, and potential synergies with other remediation techniques to maximize its effectiveness in real-world scenarios.
The exploration of sulfamic acid for microbial remediation stems from the growing need to address contamination in acidic environments, such as acid mine drainage sites, industrial waste areas, and certain agricultural soils. These low pH environments pose significant challenges for conventional remediation techniques, as many microorganisms struggle to survive and function effectively under such harsh conditions. Sulfamic acid's ability to maintain its properties in acidic settings makes it an attractive option for enhancing microbial activity in these challenging environments.
The concept of using sulfamic acid in microbial remediation is rooted in its potential to stimulate and support the growth of acid-tolerant microorganisms. These microbes play a crucial role in breaking down contaminants and restoring ecological balance in acidic ecosystems. By providing a stable and potentially nutrient-rich environment, sulfamic acid may enable the proliferation of specialized microbial communities capable of degrading pollutants that persist under low pH conditions.
Research into sulfamic acid's role in microbial remediation has gained momentum in recent years, driven by the increasing global focus on sustainable environmental management practices. Scientists and environmental engineers are exploring how this compound can be integrated into bioremediation strategies to enhance the efficiency and effectiveness of cleanup efforts in acidic sites. The potential applications range from treating acid mine drainage to remediating contaminated soils and industrial effluents.
The investigation of sulfamic acid for this purpose also aligns with the broader trend of developing innovative, eco-friendly remediation technologies. As regulatory pressures mount and public awareness of environmental issues grows, there is a pressing need for solutions that can address complex contamination scenarios while minimizing secondary environmental impacts. Sulfamic acid's biodegradability and relatively low toxicity compared to other strong acids make it an attractive candidate for environmentally conscious remediation approaches.
As research in this field progresses, scientists are focusing on understanding the mechanisms by which sulfamic acid influences microbial communities in low pH environments. This includes studying its effects on microbial metabolism, growth rates, and contaminant degradation pathways. Additionally, researchers are investigating optimal application methods, dosages, and potential synergies with other remediation techniques to maximize its effectiveness in real-world scenarios.
Market Demand Analysis
The market demand for sulfamic acid in microbial remediation at low pH environments has been steadily increasing due to its unique properties and effectiveness in addressing specific environmental challenges. Industries such as mining, wastewater treatment, and soil remediation are particularly interested in this application, driving the growth of the market.
In the mining sector, acid mine drainage (AMD) remains a significant environmental concern. The ability of sulfamic acid to facilitate microbial growth and activity at low pH levels makes it an attractive solution for bioremediation of AMD-affected sites. This has led to a growing demand from mining companies seeking sustainable and cost-effective remediation strategies.
The wastewater treatment industry is another key driver of market demand. As regulations on effluent quality become more stringent, there is an increasing need for effective treatment methods for acidic industrial wastewaters. Sulfamic acid's potential in enhancing microbial activity in these challenging environments has sparked interest among wastewater treatment facilities and environmental service providers.
Soil remediation projects, particularly those dealing with acidic soils contaminated with heavy metals or organic pollutants, represent another significant market segment. The use of sulfamic acid in promoting microbial growth and biodegradation processes in these low pH environments has gained attention from environmental consulting firms and remediation contractors.
The global bioremediation market, which encompasses the use of sulfamic acid for microbial remediation, is projected to experience substantial growth in the coming years. Factors contributing to this growth include increasing environmental awareness, stricter regulations on pollutant discharge, and the push for sustainable remediation technologies.
Geographically, North America and Europe currently dominate the market for sulfamic acid in microbial remediation applications. However, rapid industrialization and growing environmental concerns in Asia-Pacific regions, particularly in countries like China and India, are expected to drive significant market growth in these areas.
The COVID-19 pandemic has had a mixed impact on the market. While it initially caused disruptions in supply chains and project implementations, the increased focus on environmental health and sustainability in the post-pandemic recovery phase is likely to boost demand for innovative remediation solutions, including those involving sulfamic acid.
As research continues to demonstrate the efficacy of sulfamic acid in enhancing microbial remediation at low pH, it is anticipated that new applications and market opportunities will emerge. This could potentially expand the market into sectors such as agriculture, where acidic soil remediation is a growing concern, and in the treatment of acid-producing industrial processes.
In the mining sector, acid mine drainage (AMD) remains a significant environmental concern. The ability of sulfamic acid to facilitate microbial growth and activity at low pH levels makes it an attractive solution for bioremediation of AMD-affected sites. This has led to a growing demand from mining companies seeking sustainable and cost-effective remediation strategies.
The wastewater treatment industry is another key driver of market demand. As regulations on effluent quality become more stringent, there is an increasing need for effective treatment methods for acidic industrial wastewaters. Sulfamic acid's potential in enhancing microbial activity in these challenging environments has sparked interest among wastewater treatment facilities and environmental service providers.
Soil remediation projects, particularly those dealing with acidic soils contaminated with heavy metals or organic pollutants, represent another significant market segment. The use of sulfamic acid in promoting microbial growth and biodegradation processes in these low pH environments has gained attention from environmental consulting firms and remediation contractors.
The global bioremediation market, which encompasses the use of sulfamic acid for microbial remediation, is projected to experience substantial growth in the coming years. Factors contributing to this growth include increasing environmental awareness, stricter regulations on pollutant discharge, and the push for sustainable remediation technologies.
Geographically, North America and Europe currently dominate the market for sulfamic acid in microbial remediation applications. However, rapid industrialization and growing environmental concerns in Asia-Pacific regions, particularly in countries like China and India, are expected to drive significant market growth in these areas.
The COVID-19 pandemic has had a mixed impact on the market. While it initially caused disruptions in supply chains and project implementations, the increased focus on environmental health and sustainability in the post-pandemic recovery phase is likely to boost demand for innovative remediation solutions, including those involving sulfamic acid.
As research continues to demonstrate the efficacy of sulfamic acid in enhancing microbial remediation at low pH, it is anticipated that new applications and market opportunities will emerge. This could potentially expand the market into sectors such as agriculture, where acidic soil remediation is a growing concern, and in the treatment of acid-producing industrial processes.
Current Challenges in Low pH Bioremediation
Bioremediation at low pH environments presents significant challenges that hinder the effectiveness of microbial remediation processes. One of the primary obstacles is the limited microbial activity and diversity in acidic conditions. Most microorganisms thrive in neutral pH ranges, and their metabolic functions are severely impaired in highly acidic environments. This restriction limits the pool of potential microbial agents suitable for bioremediation in low pH settings.
The reduced microbial activity at low pH also leads to slower degradation rates of contaminants. Enzymes responsible for breaking down pollutants often function optimally within specific pH ranges, and their efficiency decreases dramatically in acidic conditions. This results in prolonged remediation times and potentially incomplete degradation of target pollutants.
Another significant challenge is the increased mobility and bioavailability of heavy metals in acidic environments. Low pH conditions can cause the dissolution of metal-containing minerals, releasing toxic metals into the surrounding media. This not only exacerbates the contamination problem but also creates a more hostile environment for the microorganisms involved in the remediation process.
The stability and survival of introduced microbial populations in low pH environments pose additional difficulties. Many bacteria and fungi struggle to maintain cellular integrity and function in highly acidic conditions, leading to reduced population viability over time. This necessitates frequent reintroduction of microbial agents or the development of acid-tolerant strains, both of which can be costly and time-consuming.
Furthermore, the low pH can affect the bioavailability and efficacy of nutrients and electron donors necessary for microbial growth and contaminant degradation. Essential nutrients may become less accessible or form complexes that are not readily utilized by microorganisms, limiting their ability to proliferate and carry out remediation activities.
The corrosive nature of acidic environments also presents challenges for the implementation and maintenance of bioremediation systems. Equipment and materials used in the process must be resistant to acid degradation, which can significantly increase the overall cost of remediation projects.
Addressing these challenges requires innovative approaches, such as the development of acid-tolerant microbial consortia, pH adjustment strategies, and novel delivery methods for nutrients and microbial agents. The use of sulfamic acid in this context presents an intriguing avenue for research, potentially offering solutions to some of these persistent issues in low pH bioremediation.
The reduced microbial activity at low pH also leads to slower degradation rates of contaminants. Enzymes responsible for breaking down pollutants often function optimally within specific pH ranges, and their efficiency decreases dramatically in acidic conditions. This results in prolonged remediation times and potentially incomplete degradation of target pollutants.
Another significant challenge is the increased mobility and bioavailability of heavy metals in acidic environments. Low pH conditions can cause the dissolution of metal-containing minerals, releasing toxic metals into the surrounding media. This not only exacerbates the contamination problem but also creates a more hostile environment for the microorganisms involved in the remediation process.
The stability and survival of introduced microbial populations in low pH environments pose additional difficulties. Many bacteria and fungi struggle to maintain cellular integrity and function in highly acidic conditions, leading to reduced population viability over time. This necessitates frequent reintroduction of microbial agents or the development of acid-tolerant strains, both of which can be costly and time-consuming.
Furthermore, the low pH can affect the bioavailability and efficacy of nutrients and electron donors necessary for microbial growth and contaminant degradation. Essential nutrients may become less accessible or form complexes that are not readily utilized by microorganisms, limiting their ability to proliferate and carry out remediation activities.
The corrosive nature of acidic environments also presents challenges for the implementation and maintenance of bioremediation systems. Equipment and materials used in the process must be resistant to acid degradation, which can significantly increase the overall cost of remediation projects.
Addressing these challenges requires innovative approaches, such as the development of acid-tolerant microbial consortia, pH adjustment strategies, and novel delivery methods for nutrients and microbial agents. The use of sulfamic acid in this context presents an intriguing avenue for research, potentially offering solutions to some of these persistent issues in low pH bioremediation.
Existing Low pH Remediation Solutions
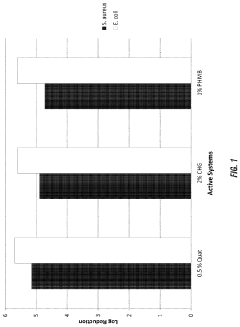
01 Microbial degradation of sulfamic acid
Certain microorganisms can be used for the biodegradation of sulfamic acid in contaminated environments. These microbes have enzymes capable of breaking down sulfamic acid into less harmful compounds, making them useful for bioremediation purposes.- Microbial degradation of sulfamic acid: Certain microorganisms can be used to degrade sulfamic acid in contaminated environments. These microbes metabolize sulfamic acid as a source of nitrogen and sulfur, effectively breaking it down into less harmful compounds. This biological approach offers an eco-friendly method for remediating sulfamic acid contamination in soil and water.
- Sulfamic acid as a biocide in microbial control: Sulfamic acid can be utilized as an antimicrobial agent to control unwanted microbial growth in various applications. Its biocidal properties make it effective in treating water systems, industrial processes, and cleaning formulations. The use of sulfamic acid in this context helps prevent microbial contamination and biofilm formation.
- Sulfamic acid in soil remediation techniques: Sulfamic acid can be employed in soil remediation processes to address specific contaminants. It may be used to adjust soil pH, enhance the solubility of certain pollutants, or as part of a chemical treatment process. This application of sulfamic acid aids in the cleanup of contaminated soils and improves overall soil quality.
- Sulfamic acid derivatives for environmental applications: Modified forms of sulfamic acid or its derivatives can be developed for specialized environmental applications. These compounds may offer improved performance in microbial remediation, reduced environmental impact, or enhanced specificity for certain contaminants. Research in this area aims to create more effective and sustainable remediation solutions.
- Combination of sulfamic acid with other remediation methods: Sulfamic acid can be used in conjunction with other remediation techniques to enhance overall effectiveness. This may include combining it with physical treatments, other chemical agents, or biological processes. Such integrated approaches can address a wider range of contaminants and improve the efficiency of remediation efforts.
02 Sulfamic acid as a microbial growth inhibitor
Sulfamic acid can be utilized as an antimicrobial agent to inhibit the growth of certain microorganisms. This property can be exploited in various applications where microbial control is necessary, such as in industrial processes or water treatment systems.Expand Specific Solutions03 Sulfamic acid in soil remediation
Sulfamic acid can be used in soil remediation processes to treat contaminated soils. It can help in the removal of certain pollutants and can also be used to adjust soil pH, which can promote the growth of beneficial microorganisms for further bioremediation.Expand Specific Solutions04 Sulfamic acid derivatives for enhanced microbial remediation
Modified forms of sulfamic acid or its derivatives can be developed to enhance microbial remediation processes. These compounds may have improved properties such as increased biodegradability or better targeting of specific contaminants.Expand Specific Solutions05 Combination of sulfamic acid with other remediation techniques
Sulfamic acid can be used in combination with other remediation techniques such as physical or chemical treatments to enhance overall effectiveness. This integrated approach can address a wider range of contaminants and improve the efficiency of the remediation process.Expand Specific Solutions
Key Players in Bioremediation Industry
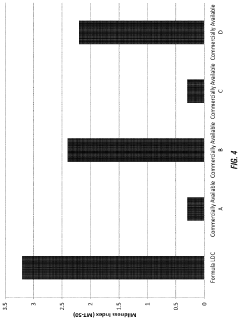
The evaluation of sulfamic acid for microbial remediation at low pH presents a competitive landscape in an emerging field. The market is in its early growth stage, with increasing interest from both industrial and academic sectors. Key players like Ecolab USA, Inc., JohnsonDiversey Co. Ltd., and Kurita Water Industries Ltd. are leveraging their expertise in water treatment and cleaning solutions to explore this niche. Research institutions such as the Indian Institutes of Technology and Zhejiang University are contributing to technological advancements. The market size is relatively small but growing, driven by environmental concerns and stringent regulations. While the technology is still developing, companies like Novozymes A/S and Evoqua Water Technologies LLC are making strides in improving the efficiency and applicability of sulfamic acid-based microbial remediation techniques.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed a sulfamic acid-based microbial remediation system for low pH environments. Their approach involves using sulfamic acid as a primary component in a formulation designed to target and eliminate microbial contamination in acidic conditions. The system incorporates buffering agents to maintain optimal pH levels for microbial activity while leveraging sulfamic acid's antimicrobial properties. Ecolab's technology also includes surfactants to enhance penetration and contact with microbial cells, improving the overall efficacy of the treatment[1][3]. The company has conducted extensive field trials in industrial settings, demonstrating a significant reduction in microbial populations at pH levels as low as 2.5[5].
Strengths: Highly effective in low pH environments, versatile application across industries, and environmentally friendly. Weaknesses: May require frequent reapplication in dynamic systems and potential for acid-resistant microbial strains to develop over time.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute has pioneered a novel approach to sulfamic acid-based microbial remediation at low pH, focusing on enhancing the acid's effectiveness through nanotechnology. Their method involves encapsulating sulfamic acid within biodegradable nanoparticles, allowing for controlled release in acidic environments. This approach significantly increases the surface area contact between the acid and microbial cells, improving its antimicrobial efficacy. The nanoparticles are designed to degrade slowly in low pH conditions, providing a sustained release of sulfamic acid over time[2]. Additionally, the Institute has developed a complementary biofilm disruption technology that works synergistically with the sulfamic acid nanoparticles, targeting microbial communities in hard-to-reach areas[4].
Strengths: Enhanced efficacy through nanotechnology, prolonged activity in acidic conditions, and ability to penetrate biofilms. Weaknesses: Potentially higher production costs and the need for specialized equipment for nanoparticle synthesis.
Sulfamic Acid Remediation Innovations
Self-sustained microbial detoxification of soluble sulfate from environmental effluent
PatentInactiveUS20120312743A1
Innovation
- A self-sustained bioremediation system using immobilized lactic acid bacteria to produce lactic acid, which serves as a substrate for sulfate-reducing bacteria, eliminating the need for commercial lactic acid and reducing costs.
Antimicrobial compositions containing cationic active ingredients
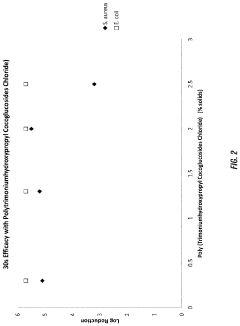
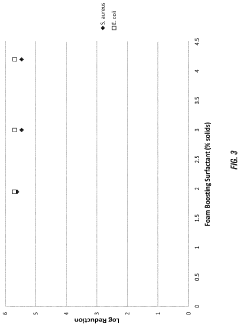
PatentActiveUS20230157937A1
Innovation
- A composition comprising a cationic active ingredient, a foam boosting surfactant, a novel foam boosting copolymer, and a chelating agent, which is free of Triclosan, anionic surfactants, and C1 to C4 alcohols, offering rapid cidal efficacy and stable copious foam with reduced skin irritancy.
Environmental Impact Assessment
The environmental impact assessment of using sulfamic acid for microbial remediation at low pH is a critical aspect that requires thorough evaluation. Sulfamic acid, while effective in certain remediation processes, can have significant implications for the surrounding ecosystem.
One of the primary concerns is the potential for soil acidification. The introduction of sulfamic acid into the environment can lower the pH of soil and water bodies, potentially disrupting the natural balance of local ecosystems. This acidification may lead to changes in soil chemistry, affecting nutrient availability and microbial communities essential for soil health.
Aquatic ecosystems are particularly vulnerable to the effects of sulfamic acid. The increased acidity can impact water quality, potentially harming fish, amphibians, and other aquatic organisms. Changes in pH can also alter the solubility and bioavailability of metals, potentially leading to increased toxicity in aquatic environments.
The use of sulfamic acid may also affect plant life in the treated areas. Some plant species may be sensitive to changes in soil pH, leading to reduced growth or even mortality. This could result in shifts in local vegetation composition, potentially impacting biodiversity and ecosystem functions.
Groundwater contamination is another significant concern. If not properly managed, sulfamic acid could leach into groundwater systems, potentially affecting drinking water sources and underground ecosystems. The mobility of sulfamic acid in soil and its potential for long-term persistence in the environment must be carefully assessed.
Air quality may also be impacted, particularly if sulfamic acid is applied through spraying or other methods that could generate aerosols. Inhalation of sulfamic acid particles could pose health risks to both humans and wildlife in the vicinity of the treatment area.
The assessment should also consider the potential for indirect ecological effects. Changes in microbial communities due to pH alterations could have cascading effects on nutrient cycling, decomposition processes, and overall ecosystem functioning.
Mitigation strategies should be an integral part of the environmental impact assessment. These may include buffer zones around sensitive areas, controlled application methods, and monitoring programs to track long-term environmental changes. Additionally, the assessment should explore alternative remediation techniques that may have less severe environmental impacts.
In conclusion, while sulfamic acid may offer benefits for microbial remediation at low pH, its use must be carefully balanced against potential environmental risks. A comprehensive environmental impact assessment is crucial to ensure that the remediation process does not cause unintended harm to the ecosystem it aims to restore.
One of the primary concerns is the potential for soil acidification. The introduction of sulfamic acid into the environment can lower the pH of soil and water bodies, potentially disrupting the natural balance of local ecosystems. This acidification may lead to changes in soil chemistry, affecting nutrient availability and microbial communities essential for soil health.
Aquatic ecosystems are particularly vulnerable to the effects of sulfamic acid. The increased acidity can impact water quality, potentially harming fish, amphibians, and other aquatic organisms. Changes in pH can also alter the solubility and bioavailability of metals, potentially leading to increased toxicity in aquatic environments.
The use of sulfamic acid may also affect plant life in the treated areas. Some plant species may be sensitive to changes in soil pH, leading to reduced growth or even mortality. This could result in shifts in local vegetation composition, potentially impacting biodiversity and ecosystem functions.
Groundwater contamination is another significant concern. If not properly managed, sulfamic acid could leach into groundwater systems, potentially affecting drinking water sources and underground ecosystems. The mobility of sulfamic acid in soil and its potential for long-term persistence in the environment must be carefully assessed.
Air quality may also be impacted, particularly if sulfamic acid is applied through spraying or other methods that could generate aerosols. Inhalation of sulfamic acid particles could pose health risks to both humans and wildlife in the vicinity of the treatment area.
The assessment should also consider the potential for indirect ecological effects. Changes in microbial communities due to pH alterations could have cascading effects on nutrient cycling, decomposition processes, and overall ecosystem functioning.
Mitigation strategies should be an integral part of the environmental impact assessment. These may include buffer zones around sensitive areas, controlled application methods, and monitoring programs to track long-term environmental changes. Additionally, the assessment should explore alternative remediation techniques that may have less severe environmental impacts.
In conclusion, while sulfamic acid may offer benefits for microbial remediation at low pH, its use must be carefully balanced against potential environmental risks. A comprehensive environmental impact assessment is crucial to ensure that the remediation process does not cause unintended harm to the ecosystem it aims to restore.
Regulatory Framework for Bioremediation Agents
The regulatory framework for bioremediation agents plays a crucial role in the evaluation and implementation of sulfamic acid for microbial remediation at low pH. This framework encompasses various national and international regulations, guidelines, and standards that govern the use of biological agents in environmental remediation processes.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing bioremediation activities. The EPA's Toxic Substances Control Act (TSCA) and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) provide the legal basis for regulating bioremediation agents. These regulations ensure that the use of sulfamic acid and associated microbial agents does not pose unacceptable risks to human health or the environment.
The European Union has established the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of sulfamic acid in bioremediation processes. REACH requires manufacturers and importers to assess and manage the risks associated with substances they produce or import, including those used in environmental remediation.
International organizations, such as the Organization for Economic Co-operation and Development (OECD), have developed guidelines for the testing and assessment of chemicals used in bioremediation. These guidelines provide a standardized approach for evaluating the safety and efficacy of sulfamic acid and other bioremediation agents across different countries.
Specific to low pH environments, regulatory frameworks often require additional considerations due to the potential for increased mobility and bioavailability of contaminants. Agencies may mandate more stringent monitoring and reporting requirements for bioremediation projects involving acidic conditions to ensure the effectiveness of the treatment and prevent unintended environmental consequences.
Regulatory approval processes for using sulfamic acid in microbial remediation typically involve submitting detailed documentation on the agent's composition, mode of action, potential environmental impacts, and proposed application methods. Authorities may require laboratory and field studies demonstrating the agent's efficacy and safety under various environmental conditions, including low pH scenarios.
It is important to note that regulatory frameworks for bioremediation agents are continually evolving as new scientific knowledge emerges and environmental concerns shift. Researchers and practitioners working with sulfamic acid for microbial remediation must stay informed about the latest regulatory developments and adapt their approaches accordingly to ensure compliance and maximize the potential for successful remediation outcomes.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing bioremediation activities. The EPA's Toxic Substances Control Act (TSCA) and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) provide the legal basis for regulating bioremediation agents. These regulations ensure that the use of sulfamic acid and associated microbial agents does not pose unacceptable risks to human health or the environment.
The European Union has established the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of sulfamic acid in bioremediation processes. REACH requires manufacturers and importers to assess and manage the risks associated with substances they produce or import, including those used in environmental remediation.
International organizations, such as the Organization for Economic Co-operation and Development (OECD), have developed guidelines for the testing and assessment of chemicals used in bioremediation. These guidelines provide a standardized approach for evaluating the safety and efficacy of sulfamic acid and other bioremediation agents across different countries.
Specific to low pH environments, regulatory frameworks often require additional considerations due to the potential for increased mobility and bioavailability of contaminants. Agencies may mandate more stringent monitoring and reporting requirements for bioremediation projects involving acidic conditions to ensure the effectiveness of the treatment and prevent unintended environmental consequences.
Regulatory approval processes for using sulfamic acid in microbial remediation typically involve submitting detailed documentation on the agent's composition, mode of action, potential environmental impacts, and proposed application methods. Authorities may require laboratory and field studies demonstrating the agent's efficacy and safety under various environmental conditions, including low pH scenarios.
It is important to note that regulatory frameworks for bioremediation agents are continually evolving as new scientific knowledge emerges and environmental concerns shift. Researchers and practitioners working with sulfamic acid for microbial remediation must stay informed about the latest regulatory developments and adapt their approaches accordingly to ensure compliance and maximize the potential for successful remediation outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!