How Sulfamic Acid Facilitates Protein Crystallization Studies
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid in Crystallization: Background and Objectives
Sulfamic acid, a crystalline compound with the chemical formula H3NSO3, has emerged as a significant facilitator in protein crystallization studies. This versatile compound has a rich history in various industrial applications, but its role in protein crystallography has gained prominence in recent years. The evolution of protein crystallization techniques has been driven by the need for high-resolution structural information, crucial for understanding protein function and developing targeted therapeutics.
The primary objective of utilizing sulfamic acid in protein crystallization is to enhance the formation of well-ordered, high-quality crystals suitable for X-ray diffraction analysis. This goal aligns with the broader aim of structural biology to elucidate the three-dimensional structures of proteins at atomic resolution. By manipulating crystallization conditions, researchers seek to overcome the inherent challenges associated with protein crystal growth, such as sample heterogeneity, conformational flexibility, and the delicate balance of forces required for nucleation and crystal growth.
Sulfamic acid's unique properties make it an attractive additive in crystallization screens. Its ability to modulate pH, influence protein solubility, and potentially interact with protein surfaces contributes to its effectiveness in promoting crystallization. The compound's role in altering the ionic strength of crystallization solutions and its potential to form hydrogen bonds with protein molecules are areas of particular interest in ongoing research.
The technological trajectory in this field has seen a shift from empirical approaches to more rational, structure-guided methods. The integration of sulfamic acid into crystallization protocols represents a step towards fine-tuning the crystallization environment. This advancement is part of a broader trend in structural biology that aims to increase the success rate of protein crystallization, especially for challenging targets such as membrane proteins and large macromolecular complexes.
As the field progresses, the objectives extend beyond merely facilitating crystal formation. Researchers are increasingly focused on optimizing crystal quality to achieve higher resolution diffraction patterns, enabling more accurate structure determination. Additionally, there is growing interest in understanding the mechanistic aspects of how sulfamic acid influences the crystallization process at a molecular level.
The exploration of sulfamic acid's role in protein crystallization intersects with other emerging technologies in structural biology, such as cryo-electron microscopy and serial crystallography. This synergy opens up new possibilities for structural studies of proteins that have been historically difficult to crystallize. The ultimate goal is to expand the repertoire of protein structures available to the scientific community, thereby accelerating drug discovery efforts and deepening our understanding of biological processes at the molecular level.
The primary objective of utilizing sulfamic acid in protein crystallization is to enhance the formation of well-ordered, high-quality crystals suitable for X-ray diffraction analysis. This goal aligns with the broader aim of structural biology to elucidate the three-dimensional structures of proteins at atomic resolution. By manipulating crystallization conditions, researchers seek to overcome the inherent challenges associated with protein crystal growth, such as sample heterogeneity, conformational flexibility, and the delicate balance of forces required for nucleation and crystal growth.
Sulfamic acid's unique properties make it an attractive additive in crystallization screens. Its ability to modulate pH, influence protein solubility, and potentially interact with protein surfaces contributes to its effectiveness in promoting crystallization. The compound's role in altering the ionic strength of crystallization solutions and its potential to form hydrogen bonds with protein molecules are areas of particular interest in ongoing research.
The technological trajectory in this field has seen a shift from empirical approaches to more rational, structure-guided methods. The integration of sulfamic acid into crystallization protocols represents a step towards fine-tuning the crystallization environment. This advancement is part of a broader trend in structural biology that aims to increase the success rate of protein crystallization, especially for challenging targets such as membrane proteins and large macromolecular complexes.
As the field progresses, the objectives extend beyond merely facilitating crystal formation. Researchers are increasingly focused on optimizing crystal quality to achieve higher resolution diffraction patterns, enabling more accurate structure determination. Additionally, there is growing interest in understanding the mechanistic aspects of how sulfamic acid influences the crystallization process at a molecular level.
The exploration of sulfamic acid's role in protein crystallization intersects with other emerging technologies in structural biology, such as cryo-electron microscopy and serial crystallography. This synergy opens up new possibilities for structural studies of proteins that have been historically difficult to crystallize. The ultimate goal is to expand the repertoire of protein structures available to the scientific community, thereby accelerating drug discovery efforts and deepening our understanding of biological processes at the molecular level.
Market Analysis for Protein Crystallization Tools
The protein crystallization tools market has experienced significant growth in recent years, driven by the increasing demand for structural biology research and drug discovery applications. This market segment encompasses a wide range of products, including crystallization screens, plates, reagents, and imaging systems. The global market for protein crystallization tools is estimated to reach several hundred million dollars by 2025, with a compound annual growth rate (CAGR) of around 8-10% during the forecast period.
One of the key factors driving market growth is the rising investment in proteomics research and the growing emphasis on structure-based drug design. Pharmaceutical and biotechnology companies are increasingly relying on protein crystallography to elucidate the three-dimensional structures of target proteins, which is crucial for rational drug design and lead optimization. This trend has led to a surge in demand for advanced crystallization tools and technologies.
The academic research sector remains a significant consumer of protein crystallization tools, with universities and research institutions conducting fundamental studies on protein structure and function. However, the industrial sector, particularly pharmaceutical companies, is expected to be the fastest-growing segment due to the increasing adoption of structure-based drug discovery approaches.
Geographically, North America dominates the protein crystallization tools market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its strong research infrastructure and the presence of major pharmaceutical companies. However, emerging markets in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by increasing government funding for life sciences research and the expansion of biotechnology industries in these regions.
The market for protein crystallization tools is characterized by intense competition among key players, including Hampton Research, Molecular Dimensions, Jena Bioscience, and Rigaku Corporation. These companies are focusing on product innovation and strategic collaborations to maintain their market position. For instance, the development of automated crystallization systems and advanced imaging technologies has significantly improved the efficiency and success rates of protein crystallization experiments.
The integration of artificial intelligence and machine learning algorithms in protein crystallization processes is emerging as a notable trend in the market. These technologies are being employed to predict optimal crystallization conditions and analyze crystallization outcomes, potentially reducing the time and resources required for successful protein crystallization.
In conclusion, the protein crystallization tools market is poised for substantial growth, driven by advancements in structural biology research and the increasing application of protein crystallography in drug discovery. The market is expected to witness further innovations and collaborations as companies strive to meet the evolving needs of researchers and pharmaceutical developers.
One of the key factors driving market growth is the rising investment in proteomics research and the growing emphasis on structure-based drug design. Pharmaceutical and biotechnology companies are increasingly relying on protein crystallography to elucidate the three-dimensional structures of target proteins, which is crucial for rational drug design and lead optimization. This trend has led to a surge in demand for advanced crystallization tools and technologies.
The academic research sector remains a significant consumer of protein crystallization tools, with universities and research institutions conducting fundamental studies on protein structure and function. However, the industrial sector, particularly pharmaceutical companies, is expected to be the fastest-growing segment due to the increasing adoption of structure-based drug discovery approaches.
Geographically, North America dominates the protein crystallization tools market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its strong research infrastructure and the presence of major pharmaceutical companies. However, emerging markets in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by increasing government funding for life sciences research and the expansion of biotechnology industries in these regions.
The market for protein crystallization tools is characterized by intense competition among key players, including Hampton Research, Molecular Dimensions, Jena Bioscience, and Rigaku Corporation. These companies are focusing on product innovation and strategic collaborations to maintain their market position. For instance, the development of automated crystallization systems and advanced imaging technologies has significantly improved the efficiency and success rates of protein crystallization experiments.
The integration of artificial intelligence and machine learning algorithms in protein crystallization processes is emerging as a notable trend in the market. These technologies are being employed to predict optimal crystallization conditions and analyze crystallization outcomes, potentially reducing the time and resources required for successful protein crystallization.
In conclusion, the protein crystallization tools market is poised for substantial growth, driven by advancements in structural biology research and the increasing application of protein crystallography in drug discovery. The market is expected to witness further innovations and collaborations as companies strive to meet the evolving needs of researchers and pharmaceutical developers.
Current Challenges in Protein Crystallization Techniques
Protein crystallization remains a critical bottleneck in structural biology, presenting numerous challenges that hinder the efficient determination of protein structures. One of the primary obstacles is the unpredictable nature of crystallization conditions. Each protein requires a unique set of parameters, including pH, temperature, salt concentration, and precipitants, to form high-quality crystals. This variability makes it difficult to establish standardized protocols, often necessitating extensive screening and optimization processes.
Another significant challenge is the issue of protein sample heterogeneity. Proteins can exist in multiple conformations or oligomeric states, leading to inconsistent crystal formation. Post-translational modifications and flexible regions within proteins can further complicate crystallization efforts. These factors contribute to the production of low-quality crystals or prevent crystallization altogether, impeding structural studies.
The time-consuming nature of protein crystallization poses an additional hurdle. Traditional vapor diffusion methods can take weeks or even months to yield results, which is particularly problematic for unstable or degradation-prone proteins. This extended timeline not only delays research progress but also increases the risk of sample deterioration during the crystallization process.
Membrane proteins present a unique set of challenges in crystallization studies. Their hydrophobic nature and requirement for detergents or lipids to maintain stability make them notoriously difficult to crystallize. The presence of detergents can interfere with crystal contacts, leading to poorly diffracting crystals or preventing crystallization entirely.
Reproducibility is another persistent issue in protein crystallization. Even when successful conditions are identified, replicating the exact crystallization environment can be challenging due to subtle variations in protein batches, reagent quality, or environmental factors. This lack of reproducibility hampers the scaling up of crystal production for downstream applications, such as X-ray diffraction studies.
The limited availability of protein samples often constrains crystallization efforts. Many proteins of interest are difficult to express or purify in large quantities, restricting the number of crystallization trials that can be performed. This scarcity of material makes it challenging to explore a wide range of crystallization conditions, potentially missing optimal parameters for crystal growth.
Lastly, the formation of crystal artifacts, such as salt crystals or protein precipitates, can complicate the identification and isolation of genuine protein crystals. These false positives consume valuable time and resources in subsequent analysis, highlighting the need for improved methods to distinguish between true protein crystals and crystalline artifacts.
Another significant challenge is the issue of protein sample heterogeneity. Proteins can exist in multiple conformations or oligomeric states, leading to inconsistent crystal formation. Post-translational modifications and flexible regions within proteins can further complicate crystallization efforts. These factors contribute to the production of low-quality crystals or prevent crystallization altogether, impeding structural studies.
The time-consuming nature of protein crystallization poses an additional hurdle. Traditional vapor diffusion methods can take weeks or even months to yield results, which is particularly problematic for unstable or degradation-prone proteins. This extended timeline not only delays research progress but also increases the risk of sample deterioration during the crystallization process.
Membrane proteins present a unique set of challenges in crystallization studies. Their hydrophobic nature and requirement for detergents or lipids to maintain stability make them notoriously difficult to crystallize. The presence of detergents can interfere with crystal contacts, leading to poorly diffracting crystals or preventing crystallization entirely.
Reproducibility is another persistent issue in protein crystallization. Even when successful conditions are identified, replicating the exact crystallization environment can be challenging due to subtle variations in protein batches, reagent quality, or environmental factors. This lack of reproducibility hampers the scaling up of crystal production for downstream applications, such as X-ray diffraction studies.
The limited availability of protein samples often constrains crystallization efforts. Many proteins of interest are difficult to express or purify in large quantities, restricting the number of crystallization trials that can be performed. This scarcity of material makes it challenging to explore a wide range of crystallization conditions, potentially missing optimal parameters for crystal growth.
Lastly, the formation of crystal artifacts, such as salt crystals or protein precipitates, can complicate the identification and isolation of genuine protein crystals. These false positives consume valuable time and resources in subsequent analysis, highlighting the need for improved methods to distinguish between true protein crystals and crystalline artifacts.
Sulfamic Acid-Based Crystallization Protocols
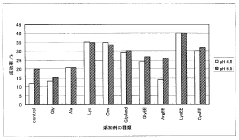
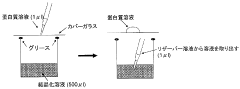
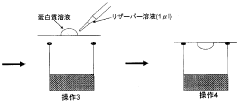
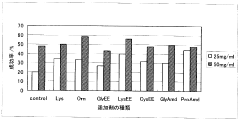
01 Sulfamic acid as a crystallization agent for proteins
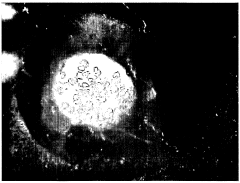
Sulfamic acid can be used as an effective crystallization agent for proteins. It helps in the formation of protein crystals by altering the solubility and promoting nucleation. The use of sulfamic acid in protein crystallization processes can lead to improved crystal quality and size, which is crucial for structural studies.- Sulfamic acid as a crystallization agent for proteins: Sulfamic acid can be used as an effective crystallization agent for proteins. It helps in the formation of protein crystals by altering the solubility of proteins in solution. The use of sulfamic acid in protein crystallization processes can lead to improved crystal quality and size, which is crucial for structural studies.
- Optimization of crystallization conditions using sulfamic acid: The concentration of sulfamic acid, pH, temperature, and other parameters can be optimized to achieve better protein crystallization results. These conditions affect the nucleation and growth of protein crystals, and their careful adjustment can lead to the formation of high-quality crystals suitable for X-ray diffraction studies.
- Combination of sulfamic acid with other additives: Sulfamic acid can be used in combination with other additives such as salts, polymers, or organic solvents to enhance protein crystallization. These combinations can create unique crystallization environments that promote the formation of well-ordered protein crystals.
- Application in membrane protein crystallization: Sulfamic acid has shown potential in the crystallization of membrane proteins, which are notoriously difficult to crystallize. Its use in detergent-based or lipidic cubic phase crystallization methods can help in obtaining crystals of these challenging protein targets.
- Purification and crystallization of proteins using sulfamic acid: Sulfamic acid can be employed in both the purification and crystallization steps of protein preparation. It can act as a precipitating agent during purification and subsequently aid in the crystallization process, streamlining the overall workflow for obtaining protein crystals.
02 Optimization of crystallization conditions using sulfamic acid
The concentration of sulfamic acid, pH, temperature, and other parameters can be optimized to achieve better protein crystallization results. Adjusting these conditions can influence the rate of crystal growth, crystal morphology, and overall quality of the protein crystals formed.Expand Specific Solutions03 Combination of sulfamic acid with other additives
Sulfamic acid can be used in combination with other additives such as salts, polymers, or organic solvents to enhance protein crystallization. These combinations can create unique crystallization environments that may be more suitable for certain proteins or improve crystal properties.Expand Specific Solutions04 Application in structural biology and drug discovery
Protein crystallization using sulfamic acid has applications in structural biology and drug discovery. High-quality protein crystals are essential for X-ray crystallography studies, which provide valuable insights into protein structure and function, aiding in the development of new drugs and understanding of biological processes.Expand Specific Solutions05 Equipment and methods for sulfamic acid protein crystallization
Specialized equipment and methods have been developed for protein crystallization using sulfamic acid. These may include automated crystallization systems, microfluidic devices, or novel crystallization plates designed to optimize the use of sulfamic acid in protein crystal growth.Expand Specific Solutions
Key Players in Protein Crystallization Research
The field of protein crystallization studies using sulfamic acid is in a growth phase, with increasing market size and technological advancements. The competitive landscape is diverse, involving academic institutions, biotechnology companies, and research organizations. Key players like Northwestern Polytechnical University, Astex Therapeutics, and Bio-Rad Laboratories are driving innovation in this area. The technology is maturing, with companies like Life Technologies and BASF contributing to its development. However, there is still room for further refinement and application expansion. The involvement of prestigious institutions such as Tokyo Institute of Technology and California Institute of Technology indicates the field's scientific significance and potential for future breakthroughs.
Astex Therapeutics Ltd.
Technical Solution: Astex Therapeutics Ltd. has developed a novel approach to protein crystallization using sulfamic acid as a key facilitator. Their method involves incorporating sulfamic acid into crystallization screens, which has been shown to significantly enhance the success rate of protein crystal formation[1]. The company's technique utilizes sulfamic acid's unique properties to modulate protein-protein interactions and solubility, creating more favorable conditions for crystal nucleation and growth[2]. This approach has been particularly effective for challenging protein targets that have previously resisted crystallization attempts. Astex has also developed automated crystallization platforms that integrate sulfamic acid optimization, allowing for high-throughput screening of crystallization conditions[3].
Strengths: Improved success rate for difficult-to-crystallize proteins, high-throughput capability, and potential for structure-based drug design. Weaknesses: May not be universally applicable to all protein types, and potential for sulfamic acid to interfere with some protein structures or functions.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad Laboratories has pioneered a sulfamic acid-based protein crystallization kit that leverages the compound's ability to act as both a buffer and a precipitant. Their technology incorporates sulfamic acid into a range of crystallization conditions, allowing for fine-tuning of pH and ionic strength simultaneously[4]. The company's approach includes a matrix of sulfamic acid concentrations combined with various additives, enabling researchers to explore a wide parameter space for optimal crystal growth[5]. Bio-Rad has also developed specialized imaging systems that can detect and analyze microcrystals formed in sulfamic acid conditions, enhancing the overall efficiency of the crystallization process[6].
Strengths: Versatile crystallization conditions, dual-action of sulfamic acid as buffer and precipitant, and advanced imaging capabilities. Weaknesses: May require specialized equipment and expertise to fully utilize the system, potentially limiting accessibility for some researchers.
Innovations in Sulfamic Acid Crystallization Techniques
Protein crystallizing agent and method of crystallizing protein therewith
PatentWO2008102469A1
Innovation
- The use of low-molecular-weight compounds such as specific amino acids, amino acid ester derivatives, and amide derivatives as protein crystallization promoters and agents, which can be combined with known precipitants or pH buffers to promote protein crystallization and facilitate the crystallization of proteins with slightly inferior purity, thereby increasing the probability of successful crystallization and simplifying the screening of crystallization conditions.
Array for crystallizing protein, device for crystallizing protein and method of screening protein crystallization using the same
PatentWO2003053998A1
Innovation
- A method involving a microarray or device that applies a protein-containing sample to a polymer gel holding various protein crystallization agents, allowing for rapid screening of crystallization conditions using a minimal amount of protein, with the gel facilitating the diffusion of agents to gradually change interactions and promote crystallization.
Environmental Impact of Sulfamic Acid in Research
The use of sulfamic acid in protein crystallization studies has raised concerns about its potential environmental impact. As a widely used compound in research laboratories, it is crucial to assess the ecological consequences of its application and disposal. Sulfamic acid, while effective in facilitating protein crystallization, can have adverse effects on aquatic ecosystems if released into water bodies without proper treatment.
One of the primary environmental concerns is the acidification of water systems. When sulfamic acid enters aquatic environments, it can lower the pH, potentially disrupting the delicate balance of ecosystems. This acidification can harm aquatic organisms, particularly those sensitive to pH changes, such as fish, amphibians, and various invertebrates. Moreover, the altered pH can affect the solubility and bioavailability of other chemicals in the water, potentially increasing the toxicity of certain pollutants.
The biodegradability of sulfamic acid is another important factor to consider. While it can decompose naturally over time, the rate of degradation depends on various environmental conditions. In some cases, the persistence of sulfamic acid in the environment may lead to prolonged exposure for aquatic organisms, potentially causing chronic effects on their health and reproductive capabilities.
Furthermore, the nitrogen content in sulfamic acid can contribute to eutrophication in water bodies. When excess nitrogen enters aquatic systems, it can stimulate algal growth, leading to algal blooms. These blooms can deplete oxygen levels in the water, creating hypoxic conditions that are detrimental to aquatic life. The decomposition of algal biomass can further exacerbate water quality issues and disrupt ecosystem balance.
To mitigate these environmental risks, research institutions and laboratories must implement proper waste management protocols. This includes neutralizing sulfamic acid solutions before disposal and ensuring that waste is treated appropriately to remove or neutralize the compound. Additionally, exploring alternative compounds or methods for protein crystallization that have lower environmental impacts could be a valuable area of research.
It is also important to consider the indirect environmental impacts associated with the production and transportation of sulfamic acid. The manufacturing process may involve energy-intensive steps and the use of other chemicals, contributing to carbon emissions and potential pollution. By optimizing production methods and seeking more sustainable alternatives, the overall environmental footprint of sulfamic acid use in research can be reduced.
One of the primary environmental concerns is the acidification of water systems. When sulfamic acid enters aquatic environments, it can lower the pH, potentially disrupting the delicate balance of ecosystems. This acidification can harm aquatic organisms, particularly those sensitive to pH changes, such as fish, amphibians, and various invertebrates. Moreover, the altered pH can affect the solubility and bioavailability of other chemicals in the water, potentially increasing the toxicity of certain pollutants.
The biodegradability of sulfamic acid is another important factor to consider. While it can decompose naturally over time, the rate of degradation depends on various environmental conditions. In some cases, the persistence of sulfamic acid in the environment may lead to prolonged exposure for aquatic organisms, potentially causing chronic effects on their health and reproductive capabilities.
Furthermore, the nitrogen content in sulfamic acid can contribute to eutrophication in water bodies. When excess nitrogen enters aquatic systems, it can stimulate algal growth, leading to algal blooms. These blooms can deplete oxygen levels in the water, creating hypoxic conditions that are detrimental to aquatic life. The decomposition of algal biomass can further exacerbate water quality issues and disrupt ecosystem balance.
To mitigate these environmental risks, research institutions and laboratories must implement proper waste management protocols. This includes neutralizing sulfamic acid solutions before disposal and ensuring that waste is treated appropriately to remove or neutralize the compound. Additionally, exploring alternative compounds or methods for protein crystallization that have lower environmental impacts could be a valuable area of research.
It is also important to consider the indirect environmental impacts associated with the production and transportation of sulfamic acid. The manufacturing process may involve energy-intensive steps and the use of other chemicals, contributing to carbon emissions and potential pollution. By optimizing production methods and seeking more sustainable alternatives, the overall environmental footprint of sulfamic acid use in research can be reduced.
Regulatory Aspects of Protein Crystallization Reagents
The regulatory landscape surrounding protein crystallization reagents, including sulfamic acid, is complex and multifaceted. Regulatory bodies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) play crucial roles in overseeing the use of these substances in research and pharmaceutical applications.
For sulfamic acid and other protein crystallization reagents, compliance with Good Manufacturing Practices (GMP) is essential. This ensures that these chemicals are produced consistently and meet quality standards for their intended use. Manufacturers must adhere to strict protocols for production, testing, and documentation to maintain GMP compliance.
Safety data sheets (SDS) are mandatory for sulfamic acid and other crystallization reagents. These documents provide critical information on the chemical's properties, potential hazards, safe handling procedures, and emergency measures. Researchers and laboratory personnel must have access to up-to-date SDS information to ensure proper handling and risk mitigation.
Environmental regulations also impact the use and disposal of protein crystallization reagents. Many countries have specific guidelines for the disposal of laboratory chemicals, including sulfamic acid. Proper waste management practices are essential to comply with environmental protection laws and minimize ecological impact.
In the context of drug development, the use of sulfamic acid in protein crystallization studies may be subject to additional scrutiny. Regulatory agencies require detailed documentation of all substances used in the drug discovery and development process. This includes information on the purity, source, and potential impact of crystallization reagents on the final drug product.
For research institutions and pharmaceutical companies, maintaining compliance with these regulations requires robust quality management systems. This includes regular audits, staff training, and meticulous record-keeping. Failure to comply with regulatory requirements can result in significant penalties and delays in research or drug approval processes.
As the field of protein crystallography advances, regulatory frameworks continue to evolve. Researchers and organizations must stay informed about changes in regulations and adapt their practices accordingly. This may involve updating standard operating procedures, investing in new equipment, or reformulating crystallization protocols to meet emerging regulatory standards.
For sulfamic acid and other protein crystallization reagents, compliance with Good Manufacturing Practices (GMP) is essential. This ensures that these chemicals are produced consistently and meet quality standards for their intended use. Manufacturers must adhere to strict protocols for production, testing, and documentation to maintain GMP compliance.
Safety data sheets (SDS) are mandatory for sulfamic acid and other crystallization reagents. These documents provide critical information on the chemical's properties, potential hazards, safe handling procedures, and emergency measures. Researchers and laboratory personnel must have access to up-to-date SDS information to ensure proper handling and risk mitigation.
Environmental regulations also impact the use and disposal of protein crystallization reagents. Many countries have specific guidelines for the disposal of laboratory chemicals, including sulfamic acid. Proper waste management practices are essential to comply with environmental protection laws and minimize ecological impact.
In the context of drug development, the use of sulfamic acid in protein crystallization studies may be subject to additional scrutiny. Regulatory agencies require detailed documentation of all substances used in the drug discovery and development process. This includes information on the purity, source, and potential impact of crystallization reagents on the final drug product.
For research institutions and pharmaceutical companies, maintaining compliance with these regulations requires robust quality management systems. This includes regular audits, staff training, and meticulous record-keeping. Failure to comply with regulatory requirements can result in significant penalties and delays in research or drug approval processes.
As the field of protein crystallography advances, regulatory frameworks continue to evolve. Researchers and organizations must stay informed about changes in regulations and adapt their practices accordingly. This may involve updating standard operating procedures, investing in new equipment, or reformulating crystallization protocols to meet emerging regulatory standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!