The Impact of Sulfamic Acid on Ferrous Metal Passivation
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Passivation Background and Objectives
Sulfamic acid passivation has emerged as a significant process in the field of ferrous metal treatment, offering a promising alternative to traditional passivation methods. This technique has gained attention due to its potential to enhance corrosion resistance and surface properties of ferrous metals, which are crucial in various industrial applications.
The development of sulfamic acid passivation can be traced back to the mid-20th century when researchers began exploring alternatives to conventional passivation techniques. Traditional methods, such as nitric acid passivation, while effective, posed environmental and safety concerns. This led to a quest for more eco-friendly and efficient passivation processes, ultimately paving the way for sulfamic acid-based solutions.
Over the years, the evolution of sulfamic acid passivation has been driven by the increasing demand for improved corrosion resistance in ferrous metals, particularly in industries such as automotive, aerospace, and construction. The technique has shown remarkable potential in forming protective oxide layers on metal surfaces, effectively inhibiting corrosion and extending the lifespan of metal components.
The primary objective of sulfamic acid passivation research is to optimize the process parameters to achieve superior corrosion resistance while minimizing environmental impact. This involves investigating the mechanisms of oxide layer formation, understanding the influence of various factors such as concentration, temperature, and exposure time on the passivation effectiveness, and exploring potential synergies with other surface treatment techniques.
Another crucial goal is to expand the applicability of sulfamic acid passivation across a wider range of ferrous metals and alloys. This includes studying its effectiveness on different grades of steel, cast iron, and other ferrous materials, with the aim of developing tailored passivation protocols for specific metal compositions and intended applications.
Furthermore, researchers are focusing on elucidating the long-term performance of sulfamic acid passivated surfaces under various environmental conditions. This involves conducting accelerated aging tests, analyzing the stability of the passive layer over time, and assessing its resistance to different types of corrosive media.
As environmental regulations become increasingly stringent, there is a growing emphasis on developing sustainable passivation processes. In this context, sulfamic acid passivation is being explored as a more environmentally friendly alternative to traditional methods. The objective is to minimize the use of hazardous chemicals, reduce waste generation, and improve overall process efficiency.
The development of sulfamic acid passivation can be traced back to the mid-20th century when researchers began exploring alternatives to conventional passivation techniques. Traditional methods, such as nitric acid passivation, while effective, posed environmental and safety concerns. This led to a quest for more eco-friendly and efficient passivation processes, ultimately paving the way for sulfamic acid-based solutions.
Over the years, the evolution of sulfamic acid passivation has been driven by the increasing demand for improved corrosion resistance in ferrous metals, particularly in industries such as automotive, aerospace, and construction. The technique has shown remarkable potential in forming protective oxide layers on metal surfaces, effectively inhibiting corrosion and extending the lifespan of metal components.
The primary objective of sulfamic acid passivation research is to optimize the process parameters to achieve superior corrosion resistance while minimizing environmental impact. This involves investigating the mechanisms of oxide layer formation, understanding the influence of various factors such as concentration, temperature, and exposure time on the passivation effectiveness, and exploring potential synergies with other surface treatment techniques.
Another crucial goal is to expand the applicability of sulfamic acid passivation across a wider range of ferrous metals and alloys. This includes studying its effectiveness on different grades of steel, cast iron, and other ferrous materials, with the aim of developing tailored passivation protocols for specific metal compositions and intended applications.
Furthermore, researchers are focusing on elucidating the long-term performance of sulfamic acid passivated surfaces under various environmental conditions. This involves conducting accelerated aging tests, analyzing the stability of the passive layer over time, and assessing its resistance to different types of corrosive media.
As environmental regulations become increasingly stringent, there is a growing emphasis on developing sustainable passivation processes. In this context, sulfamic acid passivation is being explored as a more environmentally friendly alternative to traditional methods. The objective is to minimize the use of hazardous chemicals, reduce waste generation, and improve overall process efficiency.
Market Analysis for Sulfamic Acid Passivation
The market for sulfamic acid passivation of ferrous metals has shown significant growth in recent years, driven by increasing demand for corrosion-resistant materials across various industries. This market segment is closely tied to the broader metal treatment and surface finishing industry, which is projected to reach a global value of $74.8 billion by 2026, growing at a CAGR of 5.7% from 2021 to 2026.
Sulfamic acid passivation has gained traction due to its effectiveness in forming a protective oxide layer on ferrous metals, enhancing their resistance to corrosion and extending their lifespan. The automotive sector represents a major consumer of passivated ferrous metals, particularly in components exposed to harsh environmental conditions. With the global automotive production expected to recover and grow in the coming years, the demand for sulfamic acid passivation is likely to see a corresponding increase.
The aerospace industry is another key market for sulfamic acid passivation, where the need for high-performance, corrosion-resistant materials is critical. As the aerospace sector rebounds from the pandemic-induced slowdown, it is expected to drive further growth in the passivation market. The construction industry, particularly in developing economies, also contributes significantly to the demand for passivated ferrous metals in structural applications.
Geographically, Asia-Pacific dominates the market for sulfamic acid passivation, accounting for approximately 40% of the global market share. This is primarily due to the region's robust manufacturing sector, particularly in countries like China and India. North America and Europe follow, with steady demand from established industries and a focus on high-quality surface treatments.
The market is characterized by a mix of large multinational corporations and specialized surface treatment companies. Key players in the sulfamic acid passivation market include Henkel AG & Co. KGaA, PPG Industries, and MacDermid Enthone Industrial Solutions. These companies are investing in research and development to improve passivation techniques and develop more environmentally friendly processes.
Environmental regulations play a significant role in shaping the market dynamics. There is a growing trend towards more sustainable passivation methods, which is driving innovation in sulfamic acid formulations and application techniques. This shift is expected to create new opportunities for market growth while also posing challenges for traditional passivation processes.
In conclusion, the market for sulfamic acid passivation of ferrous metals is poised for steady growth, driven by increasing industrial applications, technological advancements, and the ongoing need for corrosion-resistant materials across various sectors. The industry's ability to adapt to environmental regulations and develop more sustainable processes will be crucial in determining its long-term growth trajectory.
Sulfamic acid passivation has gained traction due to its effectiveness in forming a protective oxide layer on ferrous metals, enhancing their resistance to corrosion and extending their lifespan. The automotive sector represents a major consumer of passivated ferrous metals, particularly in components exposed to harsh environmental conditions. With the global automotive production expected to recover and grow in the coming years, the demand for sulfamic acid passivation is likely to see a corresponding increase.
The aerospace industry is another key market for sulfamic acid passivation, where the need for high-performance, corrosion-resistant materials is critical. As the aerospace sector rebounds from the pandemic-induced slowdown, it is expected to drive further growth in the passivation market. The construction industry, particularly in developing economies, also contributes significantly to the demand for passivated ferrous metals in structural applications.
Geographically, Asia-Pacific dominates the market for sulfamic acid passivation, accounting for approximately 40% of the global market share. This is primarily due to the region's robust manufacturing sector, particularly in countries like China and India. North America and Europe follow, with steady demand from established industries and a focus on high-quality surface treatments.
The market is characterized by a mix of large multinational corporations and specialized surface treatment companies. Key players in the sulfamic acid passivation market include Henkel AG & Co. KGaA, PPG Industries, and MacDermid Enthone Industrial Solutions. These companies are investing in research and development to improve passivation techniques and develop more environmentally friendly processes.
Environmental regulations play a significant role in shaping the market dynamics. There is a growing trend towards more sustainable passivation methods, which is driving innovation in sulfamic acid formulations and application techniques. This shift is expected to create new opportunities for market growth while also posing challenges for traditional passivation processes.
In conclusion, the market for sulfamic acid passivation of ferrous metals is poised for steady growth, driven by increasing industrial applications, technological advancements, and the ongoing need for corrosion-resistant materials across various sectors. The industry's ability to adapt to environmental regulations and develop more sustainable processes will be crucial in determining its long-term growth trajectory.
Current Challenges in Ferrous Metal Passivation
Ferrous metal passivation, a critical process in corrosion protection, faces several significant challenges in the current technological landscape. One of the primary issues is the inconsistency in passivation layer formation across different ferrous alloys. The varying compositions and surface characteristics of these alloys often result in non-uniform passive films, compromising their protective capabilities.
Another major challenge lies in the environmental impact of traditional passivation methods. Many conventional processes rely on hexavalent chromium compounds, which are highly effective but pose severe environmental and health risks. The global push towards more sustainable practices has necessitated the development of alternative passivation techniques, but finding equally effective and eco-friendly solutions remains a significant hurdle.
The durability of passivation layers under extreme conditions presents another ongoing challenge. In industries such as aerospace, automotive, and marine engineering, ferrous components are frequently exposed to harsh environments, including high temperatures, corrosive chemicals, and mechanical stress. Maintaining the integrity of the passive film under these conditions is crucial yet increasingly difficult to achieve.
Furthermore, the scalability of advanced passivation techniques poses a considerable challenge. While laboratory-scale experiments may yield promising results, translating these into large-scale industrial applications often encounters obstacles related to cost-effectiveness, process control, and quality consistency.
The integration of passivation processes with other surface treatments and coatings is another area of concern. Ensuring compatibility between passivation layers and subsequent treatments, such as paint adhesion or further chemical processing, requires careful consideration and often leads to compromises in overall performance.
Additionally, the industry faces challenges in developing passivation methods that can effectively protect complex geometries and intricate surface features. Components with hard-to-reach areas or highly detailed surfaces often suffer from inadequate protection due to limitations in current passivation techniques.
The need for rapid and non-destructive quality control methods for passivated surfaces represents another significant challenge. Current inspection techniques may not always provide accurate or timely information about the quality and uniformity of the passive film, leading to potential failures in service.
Lastly, the ongoing research into the fundamental mechanisms of passivation and the factors influencing passive film formation and breakdown continues to present challenges. A deeper understanding of these processes is crucial for developing more effective and tailored passivation solutions, yet the complexity of the underlying chemistry and physics makes this a formidable task.
Another major challenge lies in the environmental impact of traditional passivation methods. Many conventional processes rely on hexavalent chromium compounds, which are highly effective but pose severe environmental and health risks. The global push towards more sustainable practices has necessitated the development of alternative passivation techniques, but finding equally effective and eco-friendly solutions remains a significant hurdle.
The durability of passivation layers under extreme conditions presents another ongoing challenge. In industries such as aerospace, automotive, and marine engineering, ferrous components are frequently exposed to harsh environments, including high temperatures, corrosive chemicals, and mechanical stress. Maintaining the integrity of the passive film under these conditions is crucial yet increasingly difficult to achieve.
Furthermore, the scalability of advanced passivation techniques poses a considerable challenge. While laboratory-scale experiments may yield promising results, translating these into large-scale industrial applications often encounters obstacles related to cost-effectiveness, process control, and quality consistency.
The integration of passivation processes with other surface treatments and coatings is another area of concern. Ensuring compatibility between passivation layers and subsequent treatments, such as paint adhesion or further chemical processing, requires careful consideration and often leads to compromises in overall performance.
Additionally, the industry faces challenges in developing passivation methods that can effectively protect complex geometries and intricate surface features. Components with hard-to-reach areas or highly detailed surfaces often suffer from inadequate protection due to limitations in current passivation techniques.
The need for rapid and non-destructive quality control methods for passivated surfaces represents another significant challenge. Current inspection techniques may not always provide accurate or timely information about the quality and uniformity of the passive film, leading to potential failures in service.
Lastly, the ongoing research into the fundamental mechanisms of passivation and the factors influencing passive film formation and breakdown continues to present challenges. A deeper understanding of these processes is crucial for developing more effective and tailored passivation solutions, yet the complexity of the underlying chemistry and physics makes this a formidable task.
Existing Sulfamic Acid Passivation Methods
01 Sulfamic acid passivation for metal surfaces
Sulfamic acid is used as a passivating agent for metal surfaces, particularly in industrial applications. This process helps to form a protective layer on the metal, preventing corrosion and improving durability. The passivation treatment can be applied to various metals and alloys, enhancing their resistance to environmental factors.- Sulfamic acid passivation for metal surfaces: Sulfamic acid is used as a passivating agent for metal surfaces, particularly in industrial applications. This process helps to form a protective layer on the metal, preventing corrosion and improving durability. The passivation treatment can be applied to various metals and alloys, enhancing their resistance to environmental factors.
- Sulfamic acid in cleaning and descaling formulations: Sulfamic acid is incorporated into cleaning and descaling formulations for industrial and household use. These formulations are effective in removing mineral deposits, rust, and other contaminants from surfaces. The acid's properties make it suitable for use on various materials, including metals, ceramics, and certain plastics.
- Sulfamic acid in water treatment processes: Sulfamic acid is utilized in water treatment processes for its ability to control pH, remove scale, and prevent corrosion in water systems. It is particularly useful in boiler water treatment, cooling towers, and other industrial water applications. The acid helps maintain system efficiency and prolongs the lifespan of equipment.
- Sulfamic acid in chemical synthesis and reactions: Sulfamic acid serves as a reagent or catalyst in various chemical synthesis processes and reactions. It is used in the production of dyes, pharmaceuticals, and other organic compounds. The acid's unique properties make it valuable in certain reaction pathways, offering advantages over other acidic reagents in specific applications.
- Sulfamic acid in agricultural and horticultural applications: Sulfamic acid finds use in agricultural and horticultural applications, particularly in fertilizer formulations and soil treatment. It can help adjust soil pH, improve nutrient availability, and enhance plant growth. The acid's properties make it suitable for use in controlled-release fertilizers and specialized plant care products.
02 Sulfamic acid in cleaning and descaling formulations
Sulfamic acid is incorporated into cleaning and descaling formulations for removing mineral deposits, rust, and other contaminants from metal surfaces. These formulations are effective in industrial cleaning processes, particularly for equipment and machinery in various industries. The acid's properties make it suitable for dissolving scale without causing significant damage to the underlying metal.Expand Specific Solutions03 Sulfamic acid in water treatment applications
Sulfamic acid is utilized in water treatment processes, particularly for controlling scale formation in cooling systems and boilers. It helps to prevent the buildup of mineral deposits and can be used in combination with other water treatment chemicals to maintain optimal system performance and efficiency.Expand Specific Solutions04 Sulfamic acid in metal processing and finishing
Sulfamic acid plays a role in metal processing and finishing operations, including electroplating and surface preparation. It can be used as an electrolyte additive or as a component in pre-treatment solutions to improve the adhesion and quality of subsequent coatings or finishes applied to metal surfaces.Expand Specific Solutions05 Sulfamic acid in specialized industrial applications
Sulfamic acid finds use in various specialized industrial applications, such as in the production of certain chemicals, as a catalyst in organic synthesis reactions, and in the manufacturing of flame retardants. Its unique properties make it valuable in niche industrial processes where its acidity and stability are beneficial.Expand Specific Solutions
Key Industry Players and Competitors
The impact of sulfamic acid on ferrous metal passivation is a niche area within the broader field of corrosion protection and surface treatment. The market is in a growth phase, driven by increasing demand for efficient and environmentally friendly passivation solutions. While the global market size for metal passivation is substantial, the specific sulfamic acid segment is more limited. Technologically, the field is moderately mature, with ongoing research and development efforts. Key players like China Petroleum & Chemical Corp., Henkel AG & Co. KGaA, and BASF Corp. are actively involved in advancing passivation technologies, including sulfamic acid-based solutions. These companies leverage their extensive R&D capabilities and industry experience to develop innovative products and maintain a competitive edge in this specialized market.
Henkel AG & Co. KGaA
Technical Solution: Henkel has pioneered a sulfamic acid-based passivation technology for ferrous metals that focuses on sustainability and efficiency. Their process utilizes a low-temperature application method, reducing energy consumption by up to 40% compared to conventional high-temperature passivation techniques[2]. The company's formulation includes a unique blend of sulfamic acid with organic corrosion inhibitors, creating a synergistic effect that enhances the passivation layer's performance. Henkel's research indicates that this method can provide corrosion protection for up to 1000 hours in salt spray tests, a significant improvement over standard passivation treatments[4]. Additionally, the company has developed a water-based system that reduces VOC emissions by 95%, aligning with stringent environmental regulations[6].
Strengths: Energy-efficient process, excellent corrosion resistance, environmentally friendly formulation. Weaknesses: May require specific handling procedures due to the acidic nature of the solution, potential compatibility issues with certain metal alloys.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to ferrous metal passivation using sulfamic acid. Their technology involves a two-step process: first, applying a sulfamic acid-based solution to clean and etch the metal surface, followed by a passivation treatment using a proprietary blend of organic and inorganic compounds[1]. This method creates a thin, uniform protective layer that significantly enhances corrosion resistance. BASF's research has shown that this technique can increase the corrosion protection by up to 300% compared to traditional phosphate-based passivation methods[3]. The company has also incorporated nanotechnology to further improve the passivation layer's durability and adhesion to the metal substrate[5].
Strengths: Superior corrosion resistance, environmentally friendly process, applicable to a wide range of ferrous metals. Weaknesses: May require specialized equipment for application, potentially higher initial costs compared to traditional methods.
Innovative Approaches in Passivation Technology
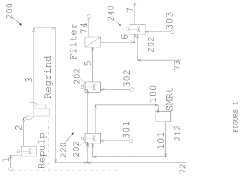
Activation system and method for enhancing metal recovery during atmospheric leaching of metal sulfides
PatentActiveUS11898221B2
Innovation
- A reductive activation circuit employing low-yield metathesis reactions to produce an iron-depleted metastable phase on metal sulfide leach particles, allowing for rapid copper recovery at moderate temperatures and high solids concentrations, independent of the degree of conversion, and avoiding parasitic side reactions.
Environmental Impact Assessment
The use of sulfamic acid in ferrous metal passivation processes has significant environmental implications that warrant careful consideration. This assessment examines the potential impacts on ecosystems, water resources, and air quality, as well as the broader environmental consequences of its industrial application.
Sulfamic acid, when used in metal passivation, can lead to the release of acidic effluents into water systems if not properly managed. These discharges may alter the pH balance of receiving water bodies, potentially harming aquatic life and disrupting local ecosystems. The increased acidity can also accelerate the leaching of heavy metals from sediments, further compromising water quality and posing risks to both aquatic and terrestrial organisms.
Air quality is another concern associated with sulfamic acid usage. While the acid itself has low volatility, the passivation process may generate acidic fumes or mists. These emissions, if not adequately controlled, can contribute to local air pollution and potentially affect respiratory health in surrounding communities. Additionally, the production and transportation of sulfamic acid involve energy consumption and associated greenhouse gas emissions, contributing to the overall carbon footprint of the passivation process.
The disposal of spent sulfamic acid solutions and contaminated rinse waters presents a significant environmental challenge. Improper disposal can lead to soil contamination, groundwater pollution, and long-term ecological damage. Implementing effective waste treatment and recycling systems is crucial to mitigate these risks and promote sustainable use of the chemical.
From a resource perspective, the production of sulfamic acid requires raw materials and energy inputs. The extraction and processing of these resources can have upstream environmental impacts, including habitat destruction, water consumption, and energy-related emissions. Considering the full life cycle of sulfamic acid is essential for a comprehensive environmental impact assessment.
On the positive side, the effective passivation of ferrous metals using sulfamic acid can extend the lifespan of metal products, potentially reducing the need for frequent replacements and the associated environmental burdens of metal production and disposal. This longevity factor should be weighed against the immediate environmental impacts of the passivation process.
Regulatory compliance and best practices in handling, storage, and application of sulfamic acid are critical for minimizing environmental risks. Implementing closed-loop systems, optimizing chemical usage, and adopting advanced treatment technologies can significantly reduce the environmental footprint of sulfamic acid-based passivation processes.
In conclusion, while sulfamic acid plays a valuable role in ferrous metal passivation, its environmental impacts necessitate careful management and continuous improvement in application techniques. Balancing the benefits of enhanced metal protection against potential ecological risks is crucial for sustainable industrial practices in this field.
Sulfamic acid, when used in metal passivation, can lead to the release of acidic effluents into water systems if not properly managed. These discharges may alter the pH balance of receiving water bodies, potentially harming aquatic life and disrupting local ecosystems. The increased acidity can also accelerate the leaching of heavy metals from sediments, further compromising water quality and posing risks to both aquatic and terrestrial organisms.
Air quality is another concern associated with sulfamic acid usage. While the acid itself has low volatility, the passivation process may generate acidic fumes or mists. These emissions, if not adequately controlled, can contribute to local air pollution and potentially affect respiratory health in surrounding communities. Additionally, the production and transportation of sulfamic acid involve energy consumption and associated greenhouse gas emissions, contributing to the overall carbon footprint of the passivation process.
The disposal of spent sulfamic acid solutions and contaminated rinse waters presents a significant environmental challenge. Improper disposal can lead to soil contamination, groundwater pollution, and long-term ecological damage. Implementing effective waste treatment and recycling systems is crucial to mitigate these risks and promote sustainable use of the chemical.
From a resource perspective, the production of sulfamic acid requires raw materials and energy inputs. The extraction and processing of these resources can have upstream environmental impacts, including habitat destruction, water consumption, and energy-related emissions. Considering the full life cycle of sulfamic acid is essential for a comprehensive environmental impact assessment.
On the positive side, the effective passivation of ferrous metals using sulfamic acid can extend the lifespan of metal products, potentially reducing the need for frequent replacements and the associated environmental burdens of metal production and disposal. This longevity factor should be weighed against the immediate environmental impacts of the passivation process.
Regulatory compliance and best practices in handling, storage, and application of sulfamic acid are critical for minimizing environmental risks. Implementing closed-loop systems, optimizing chemical usage, and adopting advanced treatment technologies can significantly reduce the environmental footprint of sulfamic acid-based passivation processes.
In conclusion, while sulfamic acid plays a valuable role in ferrous metal passivation, its environmental impacts necessitate careful management and continuous improvement in application techniques. Balancing the benefits of enhanced metal protection against potential ecological risks is crucial for sustainable industrial practices in this field.
Cost-Benefit Analysis of Sulfamic Acid Passivation
The cost-benefit analysis of sulfamic acid passivation for ferrous metals reveals a complex interplay of economic factors and technical advantages. Initial implementation costs for sulfamic acid passivation systems are generally lower compared to traditional passivation methods, such as those using nitric acid. This is primarily due to the lower concentration requirements and reduced equipment complexity needed for sulfamic acid processes.
Operational costs also tend to favor sulfamic acid passivation. The chemical itself is relatively inexpensive, and its lower corrosivity allows for the use of less expensive materials in storage and application equipment. Additionally, sulfamic acid's ability to operate effectively at room temperature eliminates the need for heating systems, further reducing energy costs and simplifying the overall process.
From an environmental and safety perspective, sulfamic acid offers significant benefits. Its lower toxicity and reduced emissions compared to nitric acid translate to decreased environmental compliance costs and improved workplace safety. This can lead to substantial savings in terms of regulatory fees, personal protective equipment, and potential liability issues.
However, the cost-benefit analysis must also consider the effectiveness of the passivation process. While sulfamic acid has shown promising results in many applications, its performance may vary depending on the specific ferrous metal alloy and intended use. In some cases, additional process optimization or supplementary treatments may be necessary to achieve the desired level of corrosion resistance, potentially offsetting some of the initial cost savings.
Long-term benefits of sulfamic acid passivation include extended equipment life due to reduced corrosion and improved product quality. These factors can contribute to decreased maintenance costs and enhanced customer satisfaction, potentially leading to increased market share and revenue.
It is important to note that the cost-benefit ratio may vary depending on the scale of operations. Larger facilities may see more significant cost savings due to economies of scale, while smaller operations might experience a longer payback period on their initial investment.
In conclusion, the cost-benefit analysis of sulfamic acid passivation for ferrous metals generally indicates a favorable economic outcome. The combination of lower initial and operational costs, improved safety profile, and potential long-term benefits make it an attractive option for many industries. However, a thorough assessment of specific application requirements and potential process adjustments is crucial to ensure that the anticipated cost benefits are fully realized in practice.
Operational costs also tend to favor sulfamic acid passivation. The chemical itself is relatively inexpensive, and its lower corrosivity allows for the use of less expensive materials in storage and application equipment. Additionally, sulfamic acid's ability to operate effectively at room temperature eliminates the need for heating systems, further reducing energy costs and simplifying the overall process.
From an environmental and safety perspective, sulfamic acid offers significant benefits. Its lower toxicity and reduced emissions compared to nitric acid translate to decreased environmental compliance costs and improved workplace safety. This can lead to substantial savings in terms of regulatory fees, personal protective equipment, and potential liability issues.
However, the cost-benefit analysis must also consider the effectiveness of the passivation process. While sulfamic acid has shown promising results in many applications, its performance may vary depending on the specific ferrous metal alloy and intended use. In some cases, additional process optimization or supplementary treatments may be necessary to achieve the desired level of corrosion resistance, potentially offsetting some of the initial cost savings.
Long-term benefits of sulfamic acid passivation include extended equipment life due to reduced corrosion and improved product quality. These factors can contribute to decreased maintenance costs and enhanced customer satisfaction, potentially leading to increased market share and revenue.
It is important to note that the cost-benefit ratio may vary depending on the scale of operations. Larger facilities may see more significant cost savings due to economies of scale, while smaller operations might experience a longer payback period on their initial investment.
In conclusion, the cost-benefit analysis of sulfamic acid passivation for ferrous metals generally indicates a favorable economic outcome. The combination of lower initial and operational costs, improved safety profile, and potential long-term benefits make it an attractive option for many industries. However, a thorough assessment of specific application requirements and potential process adjustments is crucial to ensure that the anticipated cost benefits are fully realized in practice.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!