Sulfamic Acid's Influence on the Efficiency of Antioxidant Delivery
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Background
Sulfamic acid, also known as amidosulfonic acid or sulfamidic acid, is a versatile compound with the chemical formula H3NSO3. It was first synthesized in 1897 by German chemist Theodor Curtius and has since found numerous applications across various industries. This strong, crystalline acid is characterized by its high solubility in water and its ability to form stable salts with many metals.
In the context of antioxidant delivery, sulfamic acid has garnered significant attention due to its unique properties and potential to enhance the efficiency of antioxidant systems. The compound's ability to act as a proton donor and its strong acidic nature play crucial roles in its interaction with antioxidants and their delivery mechanisms.
Sulfamic acid's chemical structure consists of a sulfur atom bonded to both an amino group and three oxygen atoms. This arrangement contributes to its high acidity and reactivity, making it an interesting candidate for modifying the behavior of antioxidants in various environments. The acid's pKa value of approximately 1.0 indicates its strong acidic nature, which can significantly influence the pH of solutions and, consequently, the stability and activity of antioxidants.
One of the key features of sulfamic acid that makes it relevant to antioxidant delivery is its ability to form complexes with metal ions. This property can be exploited to create more stable antioxidant formulations or to enhance the targeted delivery of antioxidants to specific sites within biological systems. Additionally, sulfamic acid's strong acidic nature can potentially alter the redox potential of antioxidant molecules, influencing their effectiveness in neutralizing free radicals and other oxidative species.
The compound's high solubility in water (14.7 g/100 mL at 0°C) facilitates its incorporation into various aqueous systems, making it suitable for a wide range of applications in antioxidant delivery. This characteristic allows for the development of diverse formulations and delivery methods, from simple solutions to more complex encapsulation systems.
Furthermore, sulfamic acid's stability at elevated temperatures and its resistance to hydrolysis make it an attractive option for applications requiring thermal processing or long-term storage. These properties ensure that antioxidant formulations containing sulfamic acid can maintain their efficacy under various environmental conditions, potentially extending the shelf life and applicability of antioxidant products.
As research in the field of antioxidant delivery continues to evolve, understanding the role of sulfamic acid in this context becomes increasingly important. Its potential to enhance the stability, bioavailability, and targeted delivery of antioxidants opens up new avenues for improving the effectiveness of antioxidant therapies in various fields, including healthcare, food preservation, and materials science.
In the context of antioxidant delivery, sulfamic acid has garnered significant attention due to its unique properties and potential to enhance the efficiency of antioxidant systems. The compound's ability to act as a proton donor and its strong acidic nature play crucial roles in its interaction with antioxidants and their delivery mechanisms.
Sulfamic acid's chemical structure consists of a sulfur atom bonded to both an amino group and three oxygen atoms. This arrangement contributes to its high acidity and reactivity, making it an interesting candidate for modifying the behavior of antioxidants in various environments. The acid's pKa value of approximately 1.0 indicates its strong acidic nature, which can significantly influence the pH of solutions and, consequently, the stability and activity of antioxidants.
One of the key features of sulfamic acid that makes it relevant to antioxidant delivery is its ability to form complexes with metal ions. This property can be exploited to create more stable antioxidant formulations or to enhance the targeted delivery of antioxidants to specific sites within biological systems. Additionally, sulfamic acid's strong acidic nature can potentially alter the redox potential of antioxidant molecules, influencing their effectiveness in neutralizing free radicals and other oxidative species.
The compound's high solubility in water (14.7 g/100 mL at 0°C) facilitates its incorporation into various aqueous systems, making it suitable for a wide range of applications in antioxidant delivery. This characteristic allows for the development of diverse formulations and delivery methods, from simple solutions to more complex encapsulation systems.
Furthermore, sulfamic acid's stability at elevated temperatures and its resistance to hydrolysis make it an attractive option for applications requiring thermal processing or long-term storage. These properties ensure that antioxidant formulations containing sulfamic acid can maintain their efficacy under various environmental conditions, potentially extending the shelf life and applicability of antioxidant products.
As research in the field of antioxidant delivery continues to evolve, understanding the role of sulfamic acid in this context becomes increasingly important. Its potential to enhance the stability, bioavailability, and targeted delivery of antioxidants opens up new avenues for improving the effectiveness of antioxidant therapies in various fields, including healthcare, food preservation, and materials science.
Antioxidant Market Analysis
The global antioxidant market has experienced significant growth in recent years, driven by increasing consumer awareness of health benefits and rising demand for natural and functional food ingredients. The market is segmented into various categories, including natural and synthetic antioxidants, with applications spanning food and beverages, pharmaceuticals, cosmetics, and industrial sectors.
In the food and beverage industry, antioxidants play a crucial role in preserving product quality and extending shelf life. The growing trend towards clean label products and natural ingredients has led to a surge in demand for natural antioxidants derived from sources such as rosemary, green tea, and grape seed extracts. This shift has prompted manufacturers to invest in research and development of novel antioxidant formulations and delivery systems.
The pharmaceutical sector represents another key market for antioxidants, particularly in the development of nutraceuticals and dietary supplements. As consumers become more health-conscious, there is an increasing demand for antioxidant-rich products that support overall well-being and address specific health concerns such as aging, cardiovascular health, and immune function.
The cosmetics industry has also embraced antioxidants as essential ingredients in skincare and anti-aging products. Antioxidants such as vitamins C and E, coenzyme Q10, and polyphenols are widely used in formulations to combat free radical damage and promote skin health. This trend is expected to continue as consumers seek effective and scientifically-backed skincare solutions.
Geographically, North America and Europe currently dominate the antioxidant market, owing to high consumer awareness and stringent regulations regarding food safety and quality. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing disposable incomes, changing dietary habits, and a growing focus on preventive healthcare.
The competitive landscape of the antioxidant market is characterized by the presence of both large multinational corporations and smaller specialized companies. Key players are focusing on product innovation, strategic partnerships, and mergers and acquisitions to strengthen their market position and expand their product portfolios.
Looking ahead, the antioxidant market is poised for continued growth, with projections indicating a compound annual growth rate of over 6% in the coming years. Factors such as the aging global population, increasing health consciousness, and growing applications in various industries are expected to drive market expansion. However, challenges such as stringent regulations, price volatility of raw materials, and the need for improved stability and bioavailability of antioxidants present opportunities for innovation and technological advancements in the field.
In the food and beverage industry, antioxidants play a crucial role in preserving product quality and extending shelf life. The growing trend towards clean label products and natural ingredients has led to a surge in demand for natural antioxidants derived from sources such as rosemary, green tea, and grape seed extracts. This shift has prompted manufacturers to invest in research and development of novel antioxidant formulations and delivery systems.
The pharmaceutical sector represents another key market for antioxidants, particularly in the development of nutraceuticals and dietary supplements. As consumers become more health-conscious, there is an increasing demand for antioxidant-rich products that support overall well-being and address specific health concerns such as aging, cardiovascular health, and immune function.
The cosmetics industry has also embraced antioxidants as essential ingredients in skincare and anti-aging products. Antioxidants such as vitamins C and E, coenzyme Q10, and polyphenols are widely used in formulations to combat free radical damage and promote skin health. This trend is expected to continue as consumers seek effective and scientifically-backed skincare solutions.
Geographically, North America and Europe currently dominate the antioxidant market, owing to high consumer awareness and stringent regulations regarding food safety and quality. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing disposable incomes, changing dietary habits, and a growing focus on preventive healthcare.
The competitive landscape of the antioxidant market is characterized by the presence of both large multinational corporations and smaller specialized companies. Key players are focusing on product innovation, strategic partnerships, and mergers and acquisitions to strengthen their market position and expand their product portfolios.
Looking ahead, the antioxidant market is poised for continued growth, with projections indicating a compound annual growth rate of over 6% in the coming years. Factors such as the aging global population, increasing health consciousness, and growing applications in various industries are expected to drive market expansion. However, challenges such as stringent regulations, price volatility of raw materials, and the need for improved stability and bioavailability of antioxidants present opportunities for innovation and technological advancements in the field.
Challenges in Delivery
The delivery of antioxidants presents several significant challenges that impact the efficiency and effectiveness of their application. One of the primary obstacles is the inherent instability of many antioxidant compounds. These molecules are highly reactive, making them susceptible to degradation during storage, processing, and delivery. Environmental factors such as light, heat, and oxygen exposure can rapidly diminish their potency, reducing their therapeutic value before they reach their intended targets.
Another major hurdle is the poor solubility of many antioxidants in aqueous solutions. This characteristic limits their bioavailability and hinders their ability to penetrate cellular membranes effectively. As a result, achieving therapeutic concentrations at the desired sites of action becomes problematic, often requiring higher doses that may lead to potential side effects or increased production costs.
The biological barriers within the human body pose additional challenges to antioxidant delivery. The gastrointestinal tract, with its varying pH levels and enzymatic activities, can degrade or inactivate antioxidants before they are absorbed. Furthermore, the blood-brain barrier presents a formidable obstacle for antioxidants targeting neurological conditions, as it restricts the passage of many compounds into the central nervous system.
Achieving controlled and sustained release of antioxidants is another significant challenge. Many antioxidants have short half-lives in the body, necessitating frequent administration to maintain therapeutic levels. Developing delivery systems that can provide a steady, prolonged release of active compounds while protecting them from premature degradation remains a complex task for researchers and formulators.
The potential for interactions between antioxidants and other components in formulations or biological systems also complicates delivery efforts. These interactions may lead to reduced efficacy, altered pharmacokinetics, or unexpected side effects. Additionally, ensuring the stability and compatibility of antioxidants within complex formulations, such as those containing multiple active ingredients or delivery enhancers, requires careful consideration and extensive testing.
In the context of Sulfamic Acid's influence on antioxidant delivery, specific challenges arise. The acidic nature of Sulfamic Acid may affect the stability and activity of certain antioxidants, potentially altering their chemical structure or reactivity. Moreover, the interaction between Sulfamic Acid and antioxidants could impact the overall pH of the delivery system, influencing solubility, absorption, and ultimately, the efficiency of antioxidant delivery.
Addressing these multifaceted challenges requires innovative approaches in formulation design, delivery technologies, and a deep understanding of the physicochemical properties of both antioxidants and delivery vehicles. As research progresses, overcoming these obstacles will be crucial for enhancing the therapeutic potential of antioxidants across various applications.
Another major hurdle is the poor solubility of many antioxidants in aqueous solutions. This characteristic limits their bioavailability and hinders their ability to penetrate cellular membranes effectively. As a result, achieving therapeutic concentrations at the desired sites of action becomes problematic, often requiring higher doses that may lead to potential side effects or increased production costs.
The biological barriers within the human body pose additional challenges to antioxidant delivery. The gastrointestinal tract, with its varying pH levels and enzymatic activities, can degrade or inactivate antioxidants before they are absorbed. Furthermore, the blood-brain barrier presents a formidable obstacle for antioxidants targeting neurological conditions, as it restricts the passage of many compounds into the central nervous system.
Achieving controlled and sustained release of antioxidants is another significant challenge. Many antioxidants have short half-lives in the body, necessitating frequent administration to maintain therapeutic levels. Developing delivery systems that can provide a steady, prolonged release of active compounds while protecting them from premature degradation remains a complex task for researchers and formulators.
The potential for interactions between antioxidants and other components in formulations or biological systems also complicates delivery efforts. These interactions may lead to reduced efficacy, altered pharmacokinetics, or unexpected side effects. Additionally, ensuring the stability and compatibility of antioxidants within complex formulations, such as those containing multiple active ingredients or delivery enhancers, requires careful consideration and extensive testing.
In the context of Sulfamic Acid's influence on antioxidant delivery, specific challenges arise. The acidic nature of Sulfamic Acid may affect the stability and activity of certain antioxidants, potentially altering their chemical structure or reactivity. Moreover, the interaction between Sulfamic Acid and antioxidants could impact the overall pH of the delivery system, influencing solubility, absorption, and ultimately, the efficiency of antioxidant delivery.
Addressing these multifaceted challenges requires innovative approaches in formulation design, delivery technologies, and a deep understanding of the physicochemical properties of both antioxidants and delivery vehicles. As research progresses, overcoming these obstacles will be crucial for enhancing the therapeutic potential of antioxidants across various applications.
Current Delivery Methods
01 Sulfamic acid in cleaning applications
Sulfamic acid is widely used in cleaning formulations due to its high efficiency in removing scale, rust, and other deposits. It is particularly effective in descaling operations for industrial equipment and household appliances. The acid's ability to dissolve mineral deposits without damaging underlying surfaces makes it a preferred choice in many cleaning products.- Sulfamic acid in cleaning compositions: Sulfamic acid is utilized in various cleaning compositions to enhance their efficiency. It acts as a powerful descaling agent, effectively removing mineral deposits and limescale. These compositions are particularly useful in household and industrial cleaning applications, providing improved cleaning performance and efficiency.
- Sulfamic acid in water treatment: Sulfamic acid is employed in water treatment processes to improve efficiency. It is used for pH adjustment, scale prevention, and as a disinfectant. The acid's properties make it effective in treating industrial wastewater, cooling systems, and municipal water supplies, contributing to better water quality and system performance.
- Sulfamic acid in chemical synthesis: Sulfamic acid serves as an efficient reagent in various chemical synthesis processes. It is used as a catalyst, sulfonating agent, and in the preparation of other chemical compounds. Its application in organic synthesis leads to improved reaction yields and selectivity, making it a valuable tool in the chemical industry.
- Sulfamic acid in agriculture: Sulfamic acid finds applications in agriculture to enhance efficiency. It is used in fertilizer formulations, soil treatment, and as a herbicide. The acid helps in nutrient uptake, pH adjustment of soil, and weed control, contributing to improved crop yields and agricultural productivity.
- Sulfamic acid in metal processing: Sulfamic acid is utilized in metal processing industries to improve efficiency. It is employed in metal cleaning, pickling, and surface treatment processes. The acid effectively removes rust, scale, and other contaminants from metal surfaces, enhancing the quality of metal products and improving overall process efficiency.
02 Sulfamic acid in water treatment
In water treatment processes, sulfamic acid demonstrates high efficiency in controlling pH levels and removing scale buildup in pipes and cooling systems. It is used in boiler water treatment to prevent corrosion and scale formation. The acid's effectiveness in dissolving calcium and magnesium deposits contributes to improved system efficiency and longevity.Expand Specific Solutions03 Sulfamic acid in metal processing
Sulfamic acid shows high efficiency in metal processing applications, particularly in pickling and surface treatment of metals. It is effective in removing oxide layers and preparing metal surfaces for further processing or coating. The acid's ability to dissolve metal oxides without excessive attack on the base metal makes it valuable in the metallurgical industry.Expand Specific Solutions04 Sulfamic acid in agriculture
In agricultural applications, sulfamic acid demonstrates efficiency as a soil acidifier and fertilizer additive. It helps in adjusting soil pH and improving nutrient uptake by plants. The acid's stability and slow-release properties make it useful in formulating long-lasting fertilizers and soil amendments for various crops.Expand Specific Solutions05 Sulfamic acid in chemical synthesis
Sulfamic acid shows high efficiency as a reagent in various chemical synthesis processes. It is used in the production of dyes, pharmaceuticals, and other organic compounds. The acid's unique properties, such as its ability to act as both a nucleophile and an electrophile, make it versatile in organic synthesis reactions and industrial chemical processes.Expand Specific Solutions
Industry Leaders
The competitive landscape for research on sulfamic acid's influence on antioxidant delivery efficiency is in an early development stage, with a growing market potential as antioxidants gain importance in various industries. The technology is still emerging, with academic institutions and research organizations leading the way. Key players include Nanjing University, Cornerstone Pharmaceuticals, and Harvard College, who are exploring novel approaches to enhance antioxidant delivery. Pharmaceutical companies like L'Oréal and BioNTech are also showing interest, recognizing the potential applications in skincare and drug development. As the field matures, collaborations between academia and industry are likely to accelerate progress and commercialization efforts.
President & Fellows of Harvard College
Technical Solution: Harvard College has developed a novel approach to enhance antioxidant delivery efficiency using sulfamic acid. Their research focuses on the synergistic effects of sulfamic acid and antioxidants in nanoparticle formulations. The team has engineered a pH-responsive nanocarrier system that incorporates sulfamic acid to improve the stability and bioavailability of antioxidants[1]. This system allows for controlled release of antioxidants in specific physiological environments, potentially increasing their therapeutic efficacy. The nanocarriers are designed to protect antioxidants from degradation during circulation and facilitate their targeted delivery to cells and tissues[3]. Harvard's research also explores the potential of sulfamic acid to enhance the permeability of antioxidants across biological barriers, thereby improving their absorption and distribution[5].
Strengths: Advanced nanocarrier technology, pH-responsive delivery system, potential for improved antioxidant stability and bioavailability. Weaknesses: Complexity of formulation may increase production costs, potential regulatory challenges for novel delivery systems.
L'Oréal SA
Technical Solution: L'Oréal has developed a cutting-edge approach to enhance antioxidant delivery in skincare products using sulfamic acid. Their research focuses on creating stable emulsions that incorporate sulfamic acid to improve the penetration and efficacy of antioxidants in topical formulations. The company has patented a technology that uses sulfamic acid as a pH adjuster and stabilizer in antioxidant-rich creams and serums[2]. This innovation allows for better preservation of antioxidant potency during product shelf life and enhances skin penetration upon application. L'Oréal's formulations typically combine sulfamic acid with a blend of antioxidants such as vitamin C, vitamin E, and ferulic acid to create synergistic effects[4]. The company has also explored the use of sulfamic acid in microencapsulation techniques to protect sensitive antioxidants from degradation and control their release over time[6].
Strengths: Expertise in skincare formulations, patented technology for antioxidant stabilization, established market presence. Weaknesses: Limited to topical applications, potential skin sensitivity issues for some users.
Key Innovations
Isozyme of autoclavable superoxide dismutase (SOD) derived from curcuma longa l
PatentWO2005017134A2
Innovation
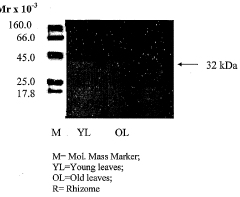
- A novel, purified isoenzyme of SOD extracted from Curcuma longa L leaves and rhizomes, which is heat stable, autoclavable, and microwavable, maintaining activity from 0°C to 100°C without external stabilizers, and possessing anti-inflammatory, anti-bacterial, and anti-fungal properties.
Compositions for the systemic treatment of pathological conditions resulting from oxidative stress and/or redox imbalance
PatentWO2014116097A2
Innovation
- A pharmaceutical composition featuring sulfur compounds, thiosulfate compounds, thionite compounds, or thionate compounds, combined with an enteric vehicle to ensure delivery to the lower gastrointestinal tract, thereby maintaining therapeutic efficacy while avoiding stomach degradation.
Regulatory Considerations
The regulatory landscape surrounding the use of sulfamic acid in antioxidant delivery systems is complex and multifaceted. Various regulatory bodies, including the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe, have established guidelines and regulations that govern the use of sulfamic acid in pharmaceutical and food applications.
In the United States, sulfamic acid is generally recognized as safe (GRAS) by the FDA for use as a food additive. However, its application in antioxidant delivery systems may require additional scrutiny and approval processes. The FDA's Center for Drug Evaluation and Research (CDER) oversees the regulation of drug products, including those involving novel delivery systems. Manufacturers seeking to incorporate sulfamic acid into antioxidant delivery mechanisms must demonstrate the safety and efficacy of their formulations through rigorous clinical trials and toxicology studies.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which affects the use of sulfamic acid in various applications. Under REACH, manufacturers and importers must register substances like sulfamic acid and provide detailed safety information. The European Food Safety Authority (EFSA) also plays a crucial role in evaluating the safety of food additives, including sulfamic acid, when used in food-related applications.
Regulatory considerations extend beyond safety assessments to include quality control measures and manufacturing standards. Good Manufacturing Practice (GMP) guidelines, enforced by regulatory agencies worldwide, ensure that pharmaceutical products, including those utilizing sulfamic acid in antioxidant delivery systems, are consistently produced and controlled according to quality standards. Manufacturers must implement robust quality management systems and maintain detailed documentation of their production processes.
Environmental regulations also impact the use of sulfamic acid in antioxidant delivery systems. The Environmental Protection Agency (EPA) in the United States and the European Environment Agency (EEA) in Europe have established guidelines for the handling, storage, and disposal of chemicals, including sulfamic acid. Compliance with these regulations is essential to prevent environmental contamination and ensure worker safety.
As research continues to explore the potential of sulfamic acid in enhancing antioxidant delivery efficiency, regulatory frameworks may evolve to address new findings and applications. Companies developing innovative antioxidant delivery systems incorporating sulfamic acid should maintain open communication with regulatory authorities and stay informed about emerging guidelines and requirements. This proactive approach can help streamline the approval process and ensure compliance with evolving regulatory standards.
In the United States, sulfamic acid is generally recognized as safe (GRAS) by the FDA for use as a food additive. However, its application in antioxidant delivery systems may require additional scrutiny and approval processes. The FDA's Center for Drug Evaluation and Research (CDER) oversees the regulation of drug products, including those involving novel delivery systems. Manufacturers seeking to incorporate sulfamic acid into antioxidant delivery mechanisms must demonstrate the safety and efficacy of their formulations through rigorous clinical trials and toxicology studies.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which affects the use of sulfamic acid in various applications. Under REACH, manufacturers and importers must register substances like sulfamic acid and provide detailed safety information. The European Food Safety Authority (EFSA) also plays a crucial role in evaluating the safety of food additives, including sulfamic acid, when used in food-related applications.
Regulatory considerations extend beyond safety assessments to include quality control measures and manufacturing standards. Good Manufacturing Practice (GMP) guidelines, enforced by regulatory agencies worldwide, ensure that pharmaceutical products, including those utilizing sulfamic acid in antioxidant delivery systems, are consistently produced and controlled according to quality standards. Manufacturers must implement robust quality management systems and maintain detailed documentation of their production processes.
Environmental regulations also impact the use of sulfamic acid in antioxidant delivery systems. The Environmental Protection Agency (EPA) in the United States and the European Environment Agency (EEA) in Europe have established guidelines for the handling, storage, and disposal of chemicals, including sulfamic acid. Compliance with these regulations is essential to prevent environmental contamination and ensure worker safety.
As research continues to explore the potential of sulfamic acid in enhancing antioxidant delivery efficiency, regulatory frameworks may evolve to address new findings and applications. Companies developing innovative antioxidant delivery systems incorporating sulfamic acid should maintain open communication with regulatory authorities and stay informed about emerging guidelines and requirements. This proactive approach can help streamline the approval process and ensure compliance with evolving regulatory standards.
Environmental Impact
The environmental impact of sulfamic acid's influence on antioxidant delivery efficiency is a crucial aspect to consider in the development and application of this technology. Sulfamic acid, while effective in enhancing antioxidant delivery, may have both positive and negative effects on the environment.
One of the primary environmental concerns is the potential for sulfamic acid to contribute to soil and water acidification. When released into the environment, sulfamic acid can lower the pH of soil and water bodies, potentially affecting aquatic ecosystems and plant growth. This acidification may lead to changes in microbial communities and nutrient availability in affected areas.
However, the use of sulfamic acid in antioxidant delivery systems may also have some positive environmental implications. By improving the efficiency of antioxidant delivery, it could potentially reduce the overall amount of antioxidants needed in various applications. This reduction in chemical usage could lead to decreased environmental contamination and a lower ecological footprint for industries relying on antioxidants.
The biodegradability of sulfamic acid is another important factor to consider. While it can break down in the environment, the rate of degradation may vary depending on environmental conditions. Proper waste management and treatment protocols are essential to minimize any potential long-term environmental accumulation.
In terms of aquatic toxicity, studies have shown that sulfamic acid can have adverse effects on certain aquatic organisms at high concentrations. However, at the levels typically used in antioxidant delivery systems, the risk to aquatic life is generally considered low. Nevertheless, continuous monitoring and assessment of aquatic ecosystems near production or application sites are necessary to ensure environmental safety.
The production process of sulfamic acid itself also has environmental implications. Manufacturing sulfamic acid involves the use of various chemicals and energy-intensive processes. Efforts to improve production efficiency and adopt cleaner technologies can help mitigate the environmental impact associated with its manufacture.
When considering the broader environmental impact, it's important to weigh the potential benefits of improved antioxidant delivery against the environmental risks associated with sulfamic acid use. This balance includes factors such as reduced oxidative damage in various applications, which could lead to extended product lifespans and decreased waste generation.
In conclusion, while sulfamic acid offers significant benefits in enhancing antioxidant delivery efficiency, its environmental impact must be carefully managed. Ongoing research into more environmentally friendly alternatives or methods to mitigate its negative effects is crucial for sustainable implementation of this technology.
One of the primary environmental concerns is the potential for sulfamic acid to contribute to soil and water acidification. When released into the environment, sulfamic acid can lower the pH of soil and water bodies, potentially affecting aquatic ecosystems and plant growth. This acidification may lead to changes in microbial communities and nutrient availability in affected areas.
However, the use of sulfamic acid in antioxidant delivery systems may also have some positive environmental implications. By improving the efficiency of antioxidant delivery, it could potentially reduce the overall amount of antioxidants needed in various applications. This reduction in chemical usage could lead to decreased environmental contamination and a lower ecological footprint for industries relying on antioxidants.
The biodegradability of sulfamic acid is another important factor to consider. While it can break down in the environment, the rate of degradation may vary depending on environmental conditions. Proper waste management and treatment protocols are essential to minimize any potential long-term environmental accumulation.
In terms of aquatic toxicity, studies have shown that sulfamic acid can have adverse effects on certain aquatic organisms at high concentrations. However, at the levels typically used in antioxidant delivery systems, the risk to aquatic life is generally considered low. Nevertheless, continuous monitoring and assessment of aquatic ecosystems near production or application sites are necessary to ensure environmental safety.
The production process of sulfamic acid itself also has environmental implications. Manufacturing sulfamic acid involves the use of various chemicals and energy-intensive processes. Efforts to improve production efficiency and adopt cleaner technologies can help mitigate the environmental impact associated with its manufacture.
When considering the broader environmental impact, it's important to weigh the potential benefits of improved antioxidant delivery against the environmental risks associated with sulfamic acid use. This balance includes factors such as reduced oxidative damage in various applications, which could lead to extended product lifespans and decreased waste generation.
In conclusion, while sulfamic acid offers significant benefits in enhancing antioxidant delivery efficiency, its environmental impact must be carefully managed. Ongoing research into more environmentally friendly alternatives or methods to mitigate its negative effects is crucial for sustainable implementation of this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!