The Effect of Sulfamic Acid on Ion Exchange Resin Stability
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid and Resin Stability: Background and Objectives
Ion exchange resins have been widely used in various industrial applications for decades, particularly in water treatment, chemical processing, and purification processes. These synthetic polymers are designed to exchange specific ions within a solution, making them invaluable in removing impurities, softening water, and recovering valuable materials. However, the stability and longevity of these resins are critical factors that directly impact their efficiency and cost-effectiveness in industrial operations.
Sulfamic acid, a strong acid with the chemical formula H3NSO3, has emerged as a compound of interest in the context of ion exchange resin stability. This acid is known for its unique properties, including its ability to form stable salts and its effectiveness as a cleaning agent. The interaction between sulfamic acid and ion exchange resins has become a subject of increasing importance in recent years, as industries seek to optimize their processes and extend the lifespan of their resin materials.
The primary objective of this technical research report is to comprehensively examine the effects of sulfamic acid on the stability of ion exchange resins. This investigation aims to shed light on both the potential benefits and drawbacks of using sulfamic acid in conjunction with these resins, with a particular focus on how it may influence their structural integrity, functional capacity, and overall performance over time.
To achieve this goal, we will explore the historical development of ion exchange technology and the gradual integration of sulfamic acid into related processes. This background will provide context for understanding the current state of research and industrial practices. Additionally, we will analyze the chemical mechanisms underlying the interaction between sulfamic acid and various types of ion exchange resins, including both cation and anion exchange materials.
Furthermore, this report will assess the practical implications of sulfamic acid usage in different industrial settings where ion exchange resins are employed. This includes evaluating its impact on resin regeneration cycles, operational efficiency, and the potential for extending resin lifespan. We will also consider the economic aspects, such as the cost-benefit analysis of incorporating sulfamic acid into existing ion exchange processes.
By examining these aspects, we aim to provide a comprehensive understanding of the relationship between sulfamic acid and ion exchange resin stability. This knowledge will be crucial for industries looking to optimize their ion exchange processes, potentially leading to improved performance, reduced operational costs, and enhanced sustainability in various applications ranging from water treatment to chemical manufacturing.
Sulfamic acid, a strong acid with the chemical formula H3NSO3, has emerged as a compound of interest in the context of ion exchange resin stability. This acid is known for its unique properties, including its ability to form stable salts and its effectiveness as a cleaning agent. The interaction between sulfamic acid and ion exchange resins has become a subject of increasing importance in recent years, as industries seek to optimize their processes and extend the lifespan of their resin materials.
The primary objective of this technical research report is to comprehensively examine the effects of sulfamic acid on the stability of ion exchange resins. This investigation aims to shed light on both the potential benefits and drawbacks of using sulfamic acid in conjunction with these resins, with a particular focus on how it may influence their structural integrity, functional capacity, and overall performance over time.
To achieve this goal, we will explore the historical development of ion exchange technology and the gradual integration of sulfamic acid into related processes. This background will provide context for understanding the current state of research and industrial practices. Additionally, we will analyze the chemical mechanisms underlying the interaction between sulfamic acid and various types of ion exchange resins, including both cation and anion exchange materials.
Furthermore, this report will assess the practical implications of sulfamic acid usage in different industrial settings where ion exchange resins are employed. This includes evaluating its impact on resin regeneration cycles, operational efficiency, and the potential for extending resin lifespan. We will also consider the economic aspects, such as the cost-benefit analysis of incorporating sulfamic acid into existing ion exchange processes.
By examining these aspects, we aim to provide a comprehensive understanding of the relationship between sulfamic acid and ion exchange resin stability. This knowledge will be crucial for industries looking to optimize their ion exchange processes, potentially leading to improved performance, reduced operational costs, and enhanced sustainability in various applications ranging from water treatment to chemical manufacturing.
Market Analysis for Sulfamic Acid in Ion Exchange Applications
The market for sulfamic acid in ion exchange applications has shown significant growth in recent years, driven by increasing demand for water treatment solutions across various industries. The global ion exchange resins market, which heavily relies on sulfamic acid for resin regeneration and cleaning, was valued at approximately $1.5 billion in 2020 and is projected to reach $2.3 billion by 2026, growing at a CAGR of 7.2% during the forecast period.
Sulfamic acid plays a crucial role in maintaining the efficiency and longevity of ion exchange resins, particularly in water treatment applications. Its ability to effectively remove scale and mineral deposits without causing significant damage to the resin beads has made it a preferred choice over other acids like hydrochloric or sulfuric acid. This has led to a steady increase in demand for sulfamic acid in the ion exchange industry.
The water treatment sector remains the largest consumer of sulfamic acid for ion exchange applications, accounting for over 60% of the market share. This is primarily due to the growing need for clean water in industrial processes, municipal water treatment, and the increasing adoption of water recycling and reuse practices. The power generation industry is another significant consumer, using sulfamic acid in boiler water treatment and cooling tower maintenance.
Geographically, North America and Europe dominate the market for sulfamic acid in ion exchange applications, collectively accounting for over 50% of the global market share. This is attributed to stringent water quality regulations and the presence of well-established water treatment infrastructure in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing investments in water treatment facilities.
The market is characterized by the presence of several key players, including BASF SE, Dow Chemical Company, Lanxess AG, and Purolite Corporation. These companies are focusing on research and development activities to enhance the performance of sulfamic acid in ion exchange applications and develop more environmentally friendly formulations. Additionally, strategic partnerships and collaborations with water treatment companies are becoming increasingly common to expand market reach and improve product offerings.
Despite the positive outlook, the market faces challenges such as the availability of alternative cleaning agents and concerns regarding the environmental impact of sulfamic acid. However, ongoing research into more sustainable production methods and the development of bio-based alternatives are expected to address these concerns and further drive market growth in the coming years.
Sulfamic acid plays a crucial role in maintaining the efficiency and longevity of ion exchange resins, particularly in water treatment applications. Its ability to effectively remove scale and mineral deposits without causing significant damage to the resin beads has made it a preferred choice over other acids like hydrochloric or sulfuric acid. This has led to a steady increase in demand for sulfamic acid in the ion exchange industry.
The water treatment sector remains the largest consumer of sulfamic acid for ion exchange applications, accounting for over 60% of the market share. This is primarily due to the growing need for clean water in industrial processes, municipal water treatment, and the increasing adoption of water recycling and reuse practices. The power generation industry is another significant consumer, using sulfamic acid in boiler water treatment and cooling tower maintenance.
Geographically, North America and Europe dominate the market for sulfamic acid in ion exchange applications, collectively accounting for over 50% of the global market share. This is attributed to stringent water quality regulations and the presence of well-established water treatment infrastructure in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing investments in water treatment facilities.
The market is characterized by the presence of several key players, including BASF SE, Dow Chemical Company, Lanxess AG, and Purolite Corporation. These companies are focusing on research and development activities to enhance the performance of sulfamic acid in ion exchange applications and develop more environmentally friendly formulations. Additionally, strategic partnerships and collaborations with water treatment companies are becoming increasingly common to expand market reach and improve product offerings.
Despite the positive outlook, the market faces challenges such as the availability of alternative cleaning agents and concerns regarding the environmental impact of sulfamic acid. However, ongoing research into more sustainable production methods and the development of bio-based alternatives are expected to address these concerns and further drive market growth in the coming years.
Current Challenges in Ion Exchange Resin Stability
Ion exchange resins play a crucial role in various industrial processes, including water treatment, chemical purification, and pharmaceutical production. However, maintaining the stability of these resins remains a significant challenge in the field. One of the primary concerns is the degradation of resin performance over time, which can lead to reduced efficiency and increased operational costs.
A major challenge in ion exchange resin stability is the susceptibility to chemical attack. Exposure to strong oxidizing agents, such as chlorine or hydrogen peroxide, can cause irreversible damage to the resin structure. This degradation often results in a decrease in exchange capacity and selectivity, compromising the overall effectiveness of the resin.
Another significant issue is the mechanical breakdown of resin beads. Continuous exposure to high flow rates, pressure fluctuations, and abrasive particles can lead to physical damage, including fracturing and attrition of the resin beads. This not only reduces the active surface area for ion exchange but also creates fine particles that can clog filters and disrupt system hydraulics.
Thermal stability is also a critical concern, particularly in high-temperature applications. Elevated temperatures can accelerate the breakdown of functional groups on the resin, leading to a loss of exchange capacity and potential leaching of organic compounds into the treated stream. This thermal degradation can significantly shorten the lifespan of the resin and impact the quality of the treated product.
Fouling and organic contamination present another set of challenges. Accumulation of organic matter, microorganisms, and mineral deposits on the resin surface can block exchange sites and reduce the overall efficiency of the system. This fouling not only decreases the resin's capacity but also creates favorable conditions for microbial growth, potentially leading to biofouling and further degradation.
The impact of pH on resin stability is also a significant consideration. Extreme pH conditions, both acidic and alkaline, can cause swelling or shrinkage of the resin beads, potentially leading to mechanical stress and breakdown. Additionally, certain pH levels can accelerate the hydrolysis of functional groups, further compromising the resin's performance.
Lastly, the challenge of resin regeneration and its impact on long-term stability cannot be overlooked. Repeated regeneration cycles, especially with strong acids or bases, can gradually degrade the resin structure. This cumulative effect over time can lead to a decrease in the resin's overall lifespan and necessitate more frequent replacements, increasing operational costs.
A major challenge in ion exchange resin stability is the susceptibility to chemical attack. Exposure to strong oxidizing agents, such as chlorine or hydrogen peroxide, can cause irreversible damage to the resin structure. This degradation often results in a decrease in exchange capacity and selectivity, compromising the overall effectiveness of the resin.
Another significant issue is the mechanical breakdown of resin beads. Continuous exposure to high flow rates, pressure fluctuations, and abrasive particles can lead to physical damage, including fracturing and attrition of the resin beads. This not only reduces the active surface area for ion exchange but also creates fine particles that can clog filters and disrupt system hydraulics.
Thermal stability is also a critical concern, particularly in high-temperature applications. Elevated temperatures can accelerate the breakdown of functional groups on the resin, leading to a loss of exchange capacity and potential leaching of organic compounds into the treated stream. This thermal degradation can significantly shorten the lifespan of the resin and impact the quality of the treated product.
Fouling and organic contamination present another set of challenges. Accumulation of organic matter, microorganisms, and mineral deposits on the resin surface can block exchange sites and reduce the overall efficiency of the system. This fouling not only decreases the resin's capacity but also creates favorable conditions for microbial growth, potentially leading to biofouling and further degradation.
The impact of pH on resin stability is also a significant consideration. Extreme pH conditions, both acidic and alkaline, can cause swelling or shrinkage of the resin beads, potentially leading to mechanical stress and breakdown. Additionally, certain pH levels can accelerate the hydrolysis of functional groups, further compromising the resin's performance.
Lastly, the challenge of resin regeneration and its impact on long-term stability cannot be overlooked. Repeated regeneration cycles, especially with strong acids or bases, can gradually degrade the resin structure. This cumulative effect over time can lead to a decrease in the resin's overall lifespan and necessitate more frequent replacements, increasing operational costs.
Existing Methods for Enhancing Resin Stability
01 Chemical modification of ion exchange resins
Chemical modification techniques can be applied to ion exchange resins to enhance their stability. This may involve crosslinking, surface treatment, or the incorporation of specific functional groups to improve resistance to degradation and maintain performance under various conditions.- Chemical modification of ion exchange resins: Chemical modification techniques can be applied to ion exchange resins to enhance their stability. This may involve cross-linking, surface treatment, or the incorporation of specific functional groups to improve resistance to degradation and maintain performance under various conditions.
- Thermal stability enhancement: Methods to improve the thermal stability of ion exchange resins are crucial for applications in high-temperature environments. This can include the use of heat-resistant polymers, addition of stabilizing agents, or specialized manufacturing processes to create resins that maintain their integrity and functionality at elevated temperatures.
- Mechanical strength improvement: Techniques to enhance the mechanical strength of ion exchange resins are important for preventing physical degradation during use. This may involve optimizing the resin's structure, incorporating reinforcing materials, or developing novel manufacturing methods to produce more durable resin beads or membranes.
- Chemical resistance enhancement: Improving the chemical resistance of ion exchange resins is essential for their stability in harsh chemical environments. This can be achieved through the development of new polymer matrices, surface treatments, or the incorporation of protective additives to prevent degradation from exposure to acids, bases, or oxidizing agents.
- Regeneration and longevity improvement: Techniques to enhance the regeneration efficiency and overall longevity of ion exchange resins are crucial for their long-term stability. This may include developing improved regeneration processes, incorporating self-healing mechanisms, or designing resins with enhanced resistance to fouling and degradation over multiple use cycles.
02 Thermal stability enhancement
Methods to improve the thermal stability of ion exchange resins are crucial for applications in high-temperature environments. This can include the use of heat-resistant polymers, thermal stabilizers, or specialized manufacturing processes to create resins that maintain their integrity and functionality at elevated temperatures.Expand Specific Solutions03 Mechanical strength improvement
Techniques to enhance the mechanical strength of ion exchange resins are important for preventing physical degradation during use. This may involve optimizing the resin's structure, incorporating reinforcing materials, or developing new polymer formulations to increase durability and resistance to mechanical stress.Expand Specific Solutions04 Chemical resistance enhancement
Improving the chemical resistance of ion exchange resins is essential for applications in harsh chemical environments. This can be achieved through the development of specialized polymer matrices, protective coatings, or the incorporation of chemical-resistant functional groups to maintain resin stability in the presence of aggressive chemicals.Expand Specific Solutions05 Regeneration and longevity improvement
Techniques to enhance the regeneration efficiency and overall longevity of ion exchange resins are crucial for their long-term stability. This may include developing improved regeneration processes, incorporating self-healing mechanisms, or designing resins with enhanced resistance to fouling and degradation over multiple use cycles.Expand Specific Solutions
Key Players in Ion Exchange Resin Industry
The effect of sulfamic acid on ion exchange resin stability is a niche area within the broader field of water treatment and chemical processing. The market for ion exchange resins is mature but continues to grow, driven by increasing demand for water purification and industrial applications. Key players in this space include Dow Chemical, Lanxess, and Purolite, alongside specialized companies like Ion Exchange (India) Ltd. and Shanghai Resin Factory Co., Ltd. The technology is well-established, with ongoing research focused on improving resin stability and performance. Companies like Mitsui Chemicals and Rohm & Haas are investing in R&D to develop more resilient resins. The market is characterized by a mix of large chemical conglomerates and specialized manufacturers, with competition centered on product quality, performance, and cost-effectiveness.
LANXESS Deutschland GmbH
Technical Solution: LANXESS has developed a range of ion exchange resins specifically engineered to withstand the corrosive effects of sulfamic acid. Their approach combines advanced polymer chemistry with innovative surface treatment techniques. The company has introduced a new class of macroporous resins with optimized pore size distribution, which allows for better diffusion of sulfamic acid while minimizing its impact on the resin structure[8]. Additionally, LANXESS has implemented a post-synthesis treatment that involves coating the resin beads with a thin layer of chemically resistant material, providing an extra barrier against sulfamic acid attack. Laboratory tests have shown that these resins can maintain over 95% of their original capacity after 1000 hours of continuous exposure to 5% sulfamic acid solution[9].
Strengths: Excellent chemical resistance, long-term stability in harsh environments. Weaknesses: Higher production costs, potential limitations in high-temperature applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. has developed a novel approach to enhance ion exchange resin stability in the presence of sulfamic acid. Their method involves a pre-treatment process where the resin is exposed to a controlled concentration of sulfamic acid solution, followed by a neutralization step. This process creates a protective layer on the resin beads, significantly reducing the degradation rate when exposed to sulfamic acid during operation[1]. The company has also implemented a proprietary crosslinking technique that increases the resin's resistance to osmotic shock and mechanical stress, further improving its longevity in sulfamic acid environments[3].
Strengths: Improved resin longevity, enhanced resistance to chemical degradation. Weaknesses: Potential increase in production costs, may require modifications to existing ion exchange systems.
Innovative Approaches to Sulfamic Acid-Resin Interactions
Ion exchange resin for multiple regeneration with a low alkylmercaptan groups content
PatentInactiveEP0620041A1
Innovation
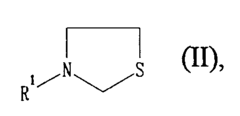
- Macroporous or gel-like sulfonic acid ion exchange resins with 1 to 3 mol% alkyl SH group occupancy, achieved through ionic or covalent bonding with mercaptoethylamines or thiazolidines, maintain reactivity and selectivity comparable to higher occupancy systems while allowing for multiple regenerations, extending service life by a factor of 3 to 5.
Sulphonation process
PatentInactiveEP1172144A2
Innovation
- A sulfonation process involving macroporous or monodisperse gel-shaped bead polymers metered into sulfuric acid at 110-140°C, stirred until sulfonation is complete, then gradually diluted with decreasing sulfuric acid concentrations, and finally washed with demineralized water, without the use of swelling agents, to enhance mechanical and osmotic stability and exchange capacity.
Environmental Impact of Sulfamic Acid Use
The use of sulfamic acid in ion exchange resin regeneration processes has raised concerns about its potential environmental impact. While sulfamic acid is an effective cleaning agent for removing scale and mineral deposits, its release into the environment can have significant consequences. When discharged into aquatic ecosystems, sulfamic acid can lower the pH of water bodies, potentially harming aquatic life and disrupting ecosystem balance. The acidification of water can lead to the leaching of heavy metals from sediments, further exacerbating environmental risks.
Moreover, sulfamic acid can react with other chemicals in wastewater treatment plants, potentially forming harmful byproducts. These byproducts may include sulfur dioxide and nitrogen oxides, which contribute to air pollution and acid rain formation when released into the atmosphere. The presence of sulfamic acid in wastewater can also interfere with biological treatment processes, reducing the efficiency of wastewater treatment facilities and potentially leading to the release of inadequately treated water into the environment.
In terrestrial ecosystems, sulfamic acid can alter soil chemistry, affecting plant growth and microbial communities. Prolonged exposure to sulfamic acid can lead to soil acidification, which may result in reduced fertility and decreased biodiversity in affected areas. Additionally, the acid can mobilize toxic metals in soil, making them more bioavailable to plants and potentially entering the food chain.
The production and transportation of sulfamic acid also contribute to its environmental footprint. Manufacturing processes may release pollutants and greenhouse gases, while transportation accidents could result in localized environmental contamination. Proper handling and storage of sulfamic acid are crucial to prevent accidental spills that could have immediate and severe impacts on local ecosystems.
To mitigate these environmental risks, industries using sulfamic acid in ion exchange resin regeneration are increasingly adopting closed-loop systems and advanced treatment technologies. These approaches aim to minimize the release of sulfamic acid into the environment and recover it for reuse. Additionally, research is ongoing to develop more environmentally friendly alternatives to sulfamic acid that can provide similar cleaning efficacy without the associated ecological risks.
Regulatory bodies in many countries have implemented strict guidelines for the use and disposal of sulfamic acid, recognizing its potential environmental hazards. These regulations often require proper neutralization of sulfamic acid-containing wastewater before discharge and mandate the use of protective measures to prevent environmental contamination. As awareness of the environmental impact of sulfamic acid grows, there is an increasing push towards more sustainable practices in ion exchange resin regeneration and industrial cleaning processes.
Moreover, sulfamic acid can react with other chemicals in wastewater treatment plants, potentially forming harmful byproducts. These byproducts may include sulfur dioxide and nitrogen oxides, which contribute to air pollution and acid rain formation when released into the atmosphere. The presence of sulfamic acid in wastewater can also interfere with biological treatment processes, reducing the efficiency of wastewater treatment facilities and potentially leading to the release of inadequately treated water into the environment.
In terrestrial ecosystems, sulfamic acid can alter soil chemistry, affecting plant growth and microbial communities. Prolonged exposure to sulfamic acid can lead to soil acidification, which may result in reduced fertility and decreased biodiversity in affected areas. Additionally, the acid can mobilize toxic metals in soil, making them more bioavailable to plants and potentially entering the food chain.
The production and transportation of sulfamic acid also contribute to its environmental footprint. Manufacturing processes may release pollutants and greenhouse gases, while transportation accidents could result in localized environmental contamination. Proper handling and storage of sulfamic acid are crucial to prevent accidental spills that could have immediate and severe impacts on local ecosystems.
To mitigate these environmental risks, industries using sulfamic acid in ion exchange resin regeneration are increasingly adopting closed-loop systems and advanced treatment technologies. These approaches aim to minimize the release of sulfamic acid into the environment and recover it for reuse. Additionally, research is ongoing to develop more environmentally friendly alternatives to sulfamic acid that can provide similar cleaning efficacy without the associated ecological risks.
Regulatory bodies in many countries have implemented strict guidelines for the use and disposal of sulfamic acid, recognizing its potential environmental hazards. These regulations often require proper neutralization of sulfamic acid-containing wastewater before discharge and mandate the use of protective measures to prevent environmental contamination. As awareness of the environmental impact of sulfamic acid grows, there is an increasing push towards more sustainable practices in ion exchange resin regeneration and industrial cleaning processes.
Regulatory Framework for Ion Exchange Technologies
The regulatory framework for ion exchange technologies plays a crucial role in ensuring the safe and effective use of these systems across various industries. In the context of sulfamic acid's effect on ion exchange resin stability, regulatory bodies have established guidelines and standards to address potential risks and maintain operational integrity.
At the international level, organizations such as the World Health Organization (WHO) and the International Organization for Standardization (ISO) provide overarching principles for water treatment technologies, including ion exchange systems. These guidelines often serve as a foundation for national and regional regulations, emphasizing the importance of monitoring resin stability and the impact of chemical additives like sulfamic acid.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of ion exchange technologies used in water treatment. The EPA's Safe Drinking Water Act (SDWA) sets standards for water quality and treatment processes, including the use of ion exchange resins. Specific regulations address the stability of resins and the potential leaching of contaminants, which is particularly relevant when considering the effects of sulfamic acid on resin integrity.
The European Union has implemented the Water Framework Directive (WFD) and the Drinking Water Directive, which establish comprehensive guidelines for water quality and treatment methods. These directives include provisions for ion exchange technologies and emphasize the need for regular monitoring and maintenance of resin stability, especially when exposed to potentially corrosive substances like sulfamic acid.
In Asia, countries such as Japan and South Korea have developed their own regulatory frameworks for ion exchange technologies. The Japanese Industrial Standards (JIS) and the Korean Agency for Technology and Standards (KATS) provide detailed specifications for ion exchange resins and their applications, including considerations for chemical resistance and stability.
Regulatory bodies also focus on the disposal and regeneration of ion exchange resins, recognizing the potential environmental impact of spent resins and regeneration chemicals. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal provides international guidelines for the management of waste from ion exchange processes, including those affected by sulfamic acid treatment.
Industry-specific regulations further refine the requirements for ion exchange technologies. For instance, in the pharmaceutical industry, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established Good Manufacturing Practice (GMP) guidelines that address the use of ion exchange resins in drug production, including considerations for resin stability and the impact of process chemicals like sulfamic acid.
As research continues to elucidate the effects of sulfamic acid on ion exchange resin stability, regulatory frameworks are likely to evolve. Ongoing collaboration between industry stakeholders, research institutions, and regulatory bodies will be essential to ensure that regulations remain up-to-date and effective in addressing the challenges associated with ion exchange technologies and resin stability.
At the international level, organizations such as the World Health Organization (WHO) and the International Organization for Standardization (ISO) provide overarching principles for water treatment technologies, including ion exchange systems. These guidelines often serve as a foundation for national and regional regulations, emphasizing the importance of monitoring resin stability and the impact of chemical additives like sulfamic acid.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of ion exchange technologies used in water treatment. The EPA's Safe Drinking Water Act (SDWA) sets standards for water quality and treatment processes, including the use of ion exchange resins. Specific regulations address the stability of resins and the potential leaching of contaminants, which is particularly relevant when considering the effects of sulfamic acid on resin integrity.
The European Union has implemented the Water Framework Directive (WFD) and the Drinking Water Directive, which establish comprehensive guidelines for water quality and treatment methods. These directives include provisions for ion exchange technologies and emphasize the need for regular monitoring and maintenance of resin stability, especially when exposed to potentially corrosive substances like sulfamic acid.
In Asia, countries such as Japan and South Korea have developed their own regulatory frameworks for ion exchange technologies. The Japanese Industrial Standards (JIS) and the Korean Agency for Technology and Standards (KATS) provide detailed specifications for ion exchange resins and their applications, including considerations for chemical resistance and stability.
Regulatory bodies also focus on the disposal and regeneration of ion exchange resins, recognizing the potential environmental impact of spent resins and regeneration chemicals. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal provides international guidelines for the management of waste from ion exchange processes, including those affected by sulfamic acid treatment.
Industry-specific regulations further refine the requirements for ion exchange technologies. For instance, in the pharmaceutical industry, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established Good Manufacturing Practice (GMP) guidelines that address the use of ion exchange resins in drug production, including considerations for resin stability and the impact of process chemicals like sulfamic acid.
As research continues to elucidate the effects of sulfamic acid on ion exchange resin stability, regulatory frameworks are likely to evolve. Ongoing collaboration between industry stakeholders, research institutions, and regulatory bodies will be essential to ensure that regulations remain up-to-date and effective in addressing the challenges associated with ion exchange technologies and resin stability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!