Advancing Synthesis Methods with Fluoroantimonic Acid
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Synthesis Background and Objectives
Fluoroantimonic acid, a superacid with extraordinary chemical properties, has been a subject of intense research and development in the field of synthetic chemistry for several decades. The evolution of synthesis methods for this powerful compound has been driven by its potential applications in various industrial processes, particularly in the petrochemical industry.
The history of fluoroantimonic acid synthesis dates back to the mid-20th century when George A. Olah and his colleagues first reported its preparation. Initially, the synthesis involved the reaction of hydrogen fluoride with antimony pentafluoride under strictly controlled conditions. This groundbreaking discovery opened up new possibilities in organic synthesis and catalysis.
As research progressed, the focus shifted towards improving the efficiency, safety, and scalability of the synthesis process. Scientists explored alternative precursors and reaction conditions to optimize yield and purity. The development of more sophisticated handling techniques and specialized equipment also played a crucial role in advancing the synthesis methods.
In recent years, there has been a growing interest in developing greener and more sustainable approaches to fluoroantimonic acid synthesis. This trend aligns with the broader movement towards environmentally friendly chemical processes in the industry. Researchers are investigating novel catalysts and reaction pathways that could potentially reduce the environmental impact of production while maintaining or enhancing the acid's potency.
The primary objectives of advancing synthesis methods for fluoroantimonic acid are multifaceted. Firstly, there is a continuous effort to increase the yield and purity of the final product. This involves fine-tuning reaction parameters and exploring new synthetic routes that minimize side reactions and impurities.
Secondly, researchers aim to enhance the safety aspects of the synthesis process. Given the highly corrosive and reactive nature of fluoroantimonic acid, developing safer handling and storage protocols is crucial for its widespread adoption in industrial settings. This includes the design of specialized containment systems and the implementation of advanced safety measures throughout the production chain.
Another key objective is to scale up the synthesis for industrial production. While laboratory-scale synthesis has been well-established, translating these methods to large-scale manufacturing presents unique challenges. Engineers and chemists are working collaboratively to design and optimize industrial-scale reactors and processes that can maintain the acid's quality while meeting commercial demand.
Lastly, there is a growing emphasis on exploring new applications for fluoroantimonic acid beyond its traditional use in petrochemistry. This includes investigating its potential in advanced materials synthesis, pharmaceutical manufacturing, and other emerging fields. As such, the development of tailored synthesis methods that cater to specific application requirements is becoming increasingly important.
The history of fluoroantimonic acid synthesis dates back to the mid-20th century when George A. Olah and his colleagues first reported its preparation. Initially, the synthesis involved the reaction of hydrogen fluoride with antimony pentafluoride under strictly controlled conditions. This groundbreaking discovery opened up new possibilities in organic synthesis and catalysis.
As research progressed, the focus shifted towards improving the efficiency, safety, and scalability of the synthesis process. Scientists explored alternative precursors and reaction conditions to optimize yield and purity. The development of more sophisticated handling techniques and specialized equipment also played a crucial role in advancing the synthesis methods.
In recent years, there has been a growing interest in developing greener and more sustainable approaches to fluoroantimonic acid synthesis. This trend aligns with the broader movement towards environmentally friendly chemical processes in the industry. Researchers are investigating novel catalysts and reaction pathways that could potentially reduce the environmental impact of production while maintaining or enhancing the acid's potency.
The primary objectives of advancing synthesis methods for fluoroantimonic acid are multifaceted. Firstly, there is a continuous effort to increase the yield and purity of the final product. This involves fine-tuning reaction parameters and exploring new synthetic routes that minimize side reactions and impurities.
Secondly, researchers aim to enhance the safety aspects of the synthesis process. Given the highly corrosive and reactive nature of fluoroantimonic acid, developing safer handling and storage protocols is crucial for its widespread adoption in industrial settings. This includes the design of specialized containment systems and the implementation of advanced safety measures throughout the production chain.
Another key objective is to scale up the synthesis for industrial production. While laboratory-scale synthesis has been well-established, translating these methods to large-scale manufacturing presents unique challenges. Engineers and chemists are working collaboratively to design and optimize industrial-scale reactors and processes that can maintain the acid's quality while meeting commercial demand.
Lastly, there is a growing emphasis on exploring new applications for fluoroantimonic acid beyond its traditional use in petrochemistry. This includes investigating its potential in advanced materials synthesis, pharmaceutical manufacturing, and other emerging fields. As such, the development of tailored synthesis methods that cater to specific application requirements is becoming increasingly important.
Market Analysis for Fluoroantimonic Acid Applications
The market for fluoroantimonic acid applications is experiencing significant growth, driven by its unique properties as one of the strongest known superacids. This compound finds extensive use in various industrial processes, particularly in the petrochemical and pharmaceutical sectors. In the petrochemical industry, fluoroantimonic acid plays a crucial role in catalyzing alkylation reactions, isomerization processes, and the production of high-octane gasoline components.
The pharmaceutical sector represents another major market for fluoroantimonic acid, where it is utilized in the synthesis of complex organic compounds and active pharmaceutical ingredients (APIs). Its ability to catalyze reactions under mild conditions and promote selective transformations makes it invaluable in drug discovery and development processes.
Emerging applications in materials science and nanotechnology are also contributing to market expansion. Fluoroantimonic acid is being explored for its potential in surface modification of materials, etching of semiconductors, and the synthesis of advanced polymers and composites.
The global market for fluoroantimonic acid is projected to grow steadily over the next five years. This growth is attributed to increasing demand from end-use industries, particularly in developing economies. Asia-Pacific region, led by China and India, is expected to be the fastest-growing market due to rapid industrialization and expanding chemical manufacturing sectors.
However, the market faces challenges related to the handling and transportation of fluoroantimonic acid due to its highly corrosive nature. Stringent regulations regarding its use and disposal also impact market dynamics. These factors have led to increased focus on developing safer handling protocols and exploring alternative synthesis methods that minimize environmental risks.
The competitive landscape of the fluoroantimonic acid market is characterized by a few key players dominating global production. These companies are investing in research and development to improve production efficiency and explore new applications. Strategic partnerships between manufacturers and end-users are becoming more common to ensure stable supply chains and drive innovation in application development.
As environmental concerns gain prominence, there is a growing trend towards developing greener alternatives or modified versions of fluoroantimonic acid with reduced environmental impact. This shift is likely to shape the future market landscape, potentially leading to the emergence of new market segments and opportunities for sustainable chemistry solutions.
The pharmaceutical sector represents another major market for fluoroantimonic acid, where it is utilized in the synthesis of complex organic compounds and active pharmaceutical ingredients (APIs). Its ability to catalyze reactions under mild conditions and promote selective transformations makes it invaluable in drug discovery and development processes.
Emerging applications in materials science and nanotechnology are also contributing to market expansion. Fluoroantimonic acid is being explored for its potential in surface modification of materials, etching of semiconductors, and the synthesis of advanced polymers and composites.
The global market for fluoroantimonic acid is projected to grow steadily over the next five years. This growth is attributed to increasing demand from end-use industries, particularly in developing economies. Asia-Pacific region, led by China and India, is expected to be the fastest-growing market due to rapid industrialization and expanding chemical manufacturing sectors.
However, the market faces challenges related to the handling and transportation of fluoroantimonic acid due to its highly corrosive nature. Stringent regulations regarding its use and disposal also impact market dynamics. These factors have led to increased focus on developing safer handling protocols and exploring alternative synthesis methods that minimize environmental risks.
The competitive landscape of the fluoroantimonic acid market is characterized by a few key players dominating global production. These companies are investing in research and development to improve production efficiency and explore new applications. Strategic partnerships between manufacturers and end-users are becoming more common to ensure stable supply chains and drive innovation in application development.
As environmental concerns gain prominence, there is a growing trend towards developing greener alternatives or modified versions of fluoroantimonic acid with reduced environmental impact. This shift is likely to shape the future market landscape, potentially leading to the emergence of new market segments and opportunities for sustainable chemistry solutions.
Current Challenges in Fluoroantimonic Acid Production
The production of fluoroantimonic acid, one of the strongest known superacids, faces several significant challenges that hinder its widespread application and large-scale synthesis. These challenges stem from the inherent properties of the acid and the complexities involved in its production process.
One of the primary obstacles is the extreme reactivity of fluoroantimonic acid. Its corrosive nature makes it exceptionally difficult to handle and store safely. Traditional containment materials, including most metals and glass, are rapidly degraded by the acid, necessitating the use of specialized materials such as Teflon or fluorinated polymers. This requirement significantly increases production costs and limits the scalability of manufacturing processes.
The synthesis of fluoroantimonic acid also presents considerable safety concerns. The reaction between hydrogen fluoride and antimony pentafluoride, the two precursors used in its production, is highly exothermic and potentially explosive. This necessitates stringent safety protocols and specialized equipment, further complicating the production process and increasing associated costs.
Environmental and health risks pose another significant challenge. The acid's precursors, particularly hydrogen fluoride, are highly toxic and environmentally hazardous. Ensuring proper containment and disposal of these materials is crucial but adds layers of complexity to the production process. Additionally, the potential for accidental release during production or transportation raises serious environmental and public health concerns.
The purity of the final product is another critical issue in fluoroantimonic acid production. Even trace amounts of impurities can significantly affect the acid's properties and performance in various applications. Achieving and maintaining high purity levels requires sophisticated purification techniques and quality control measures, which are both technically challenging and cost-intensive.
Scale-up of production processes presents its own set of challenges. The highly exothermic nature of the synthesis reaction makes heat management a critical factor in larger-scale production. Ensuring uniform mixing and temperature control becomes increasingly difficult as batch sizes increase, potentially leading to quality inconsistencies or safety hazards.
Lastly, the limited commercial demand for fluoroantimonic acid poses economic challenges to its production. The specialized nature of its applications means that demand is relatively low compared to other industrial chemicals. This limited market size makes it difficult to justify large investments in production facilities and research, potentially slowing advancements in synthesis methods and applications.
Addressing these challenges requires a multifaceted approach, combining innovations in materials science, process engineering, and safety protocols. Developing more efficient and safer synthesis methods, improving containment and handling technologies, and exploring new applications to expand the market are key areas of focus for advancing fluoroantimonic acid production.
One of the primary obstacles is the extreme reactivity of fluoroantimonic acid. Its corrosive nature makes it exceptionally difficult to handle and store safely. Traditional containment materials, including most metals and glass, are rapidly degraded by the acid, necessitating the use of specialized materials such as Teflon or fluorinated polymers. This requirement significantly increases production costs and limits the scalability of manufacturing processes.
The synthesis of fluoroantimonic acid also presents considerable safety concerns. The reaction between hydrogen fluoride and antimony pentafluoride, the two precursors used in its production, is highly exothermic and potentially explosive. This necessitates stringent safety protocols and specialized equipment, further complicating the production process and increasing associated costs.
Environmental and health risks pose another significant challenge. The acid's precursors, particularly hydrogen fluoride, are highly toxic and environmentally hazardous. Ensuring proper containment and disposal of these materials is crucial but adds layers of complexity to the production process. Additionally, the potential for accidental release during production or transportation raises serious environmental and public health concerns.
The purity of the final product is another critical issue in fluoroantimonic acid production. Even trace amounts of impurities can significantly affect the acid's properties and performance in various applications. Achieving and maintaining high purity levels requires sophisticated purification techniques and quality control measures, which are both technically challenging and cost-intensive.
Scale-up of production processes presents its own set of challenges. The highly exothermic nature of the synthesis reaction makes heat management a critical factor in larger-scale production. Ensuring uniform mixing and temperature control becomes increasingly difficult as batch sizes increase, potentially leading to quality inconsistencies or safety hazards.
Lastly, the limited commercial demand for fluoroantimonic acid poses economic challenges to its production. The specialized nature of its applications means that demand is relatively low compared to other industrial chemicals. This limited market size makes it difficult to justify large investments in production facilities and research, potentially slowing advancements in synthesis methods and applications.
Addressing these challenges requires a multifaceted approach, combining innovations in materials science, process engineering, and safety protocols. Developing more efficient and safer synthesis methods, improving containment and handling technologies, and exploring new applications to expand the market are key areas of focus for advancing fluoroantimonic acid production.
Existing Fluoroantimonic Acid Synthesis Techniques
01 Direct synthesis from antimony pentafluoride and hydrogen fluoride
Fluoroantimonic acid can be synthesized by directly combining antimony pentafluoride with anhydrous hydrogen fluoride. This method typically involves careful mixing of the two components under controlled conditions, often at low temperatures, to produce the highly reactive superacid.- Direct synthesis from antimony pentafluoride and hydrogen fluoride: Fluoroantimonic acid can be synthesized by directly combining antimony pentafluoride with anhydrous hydrogen fluoride. This method typically involves careful mixing of the two components under controlled conditions, often at low temperatures, to produce the highly reactive superacid.
- Electrochemical synthesis methods: Electrochemical techniques can be employed to synthesize fluoroantimonic acid. This approach may involve the electrolysis of antimony-containing compounds in a fluoride-rich environment, potentially offering more controlled and efficient production of the superacid.
- Use of stabilizing agents in synthesis: Incorporating stabilizing agents during the synthesis of fluoroantimonic acid can enhance its stability and handling properties. These agents may include certain organic compounds or inert materials that help to moderate the extreme reactivity of the acid without compromising its superacidic properties.
- Low-temperature synthesis techniques: Employing low-temperature conditions during the synthesis of fluoroantimonic acid can improve yield and purity. This method often involves specialized equipment for maintaining cryogenic temperatures and may utilize precursor compounds that react more controllably at reduced temperatures.
- Purification and handling methods post-synthesis: After synthesis, fluoroantimonic acid requires specialized purification and handling techniques due to its extreme reactivity. This may involve distillation under inert atmospheres, use of fluoropolymer containers, and rigorous exclusion of moisture to maintain the acid's potency and prevent degradation.
02 Electrochemical synthesis methods
Electrochemical techniques can be employed to synthesize fluoroantimonic acid. This approach may involve the electrolysis of antimony-containing compounds in a fluoride-rich environment, potentially offering more controlled production of the superacid.Expand Specific Solutions03 Use of catalysts in fluoroantimonic acid synthesis
Catalysts can be utilized to enhance the synthesis of fluoroantimonic acid. These catalysts may facilitate the reaction between antimony compounds and fluorine-containing substances, potentially improving yield and reaction efficiency.Expand Specific Solutions04 Continuous flow synthesis methods
Continuous flow reactors can be employed for the synthesis of fluoroantimonic acid. This approach allows for better control of reaction parameters and can potentially improve safety in handling the highly reactive components involved in the synthesis.Expand Specific Solutions05 Purification and stabilization techniques
Various methods can be used to purify and stabilize fluoroantimonic acid after synthesis. These techniques may include distillation, crystallization, or the use of stabilizing additives to maintain the acid's potency and prevent degradation during storage or use.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research and Production
The advancement of synthesis methods using fluoroantimonic acid is in a nascent stage, with significant potential for growth. The market size is relatively small but expanding as researchers explore its applications in various industries. The technology's maturity is still developing, with key players like DuPont de Nemours, Inc., Evonik Operations GmbH, and Clariant Finance (BVI) Ltd. leading research efforts. Academic institutions such as Central South University and Zhejiang University are also contributing to the field's progress. The competitive landscape is characterized by a mix of established chemical companies and emerging research institutions, each striving to unlock the full potential of fluoroantimonic acid in synthesis applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a novel synthesis method using fluoroantimonic acid as a super-acid catalyst. This approach involves a controlled reaction environment where fluoroantimonic acid facilitates the formation of carbocations, enabling rapid and selective carbon-carbon bond formation. The process is carried out in specially designed corrosion-resistant reactors with precise temperature and pressure control. DuPont's method also incorporates a proprietary quenching system to neutralize the acid and isolate the desired products efficiently.
Strengths: High reaction rates, excellent selectivity, and potential for synthesizing complex molecules. Weaknesses: Requires specialized equipment due to the corrosive nature of fluoroantimonic acid and stringent safety measures.
Evonik Operations GmbH
Technical Solution: Evonik has pioneered a continuous flow synthesis technique utilizing fluoroantimonic acid. Their method employs microreactor technology, where reactants are continuously fed into a series of small channels coated with acid-resistant materials. The fluoroantimonic acid is introduced as a separate stream, allowing for precise control of reaction conditions. This continuous process enables real-time monitoring and adjustment of parameters such as residence time, temperature, and acid concentration. Evonik's approach also includes an integrated in-line purification system to remove excess acid and byproducts.
Strengths: Improved safety due to smaller reaction volumes, enhanced process control, and potential for scale-up. Weaknesses: High initial investment costs and complexity in system design and operation.
Innovative Approaches in Superacid Synthesis
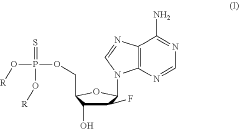
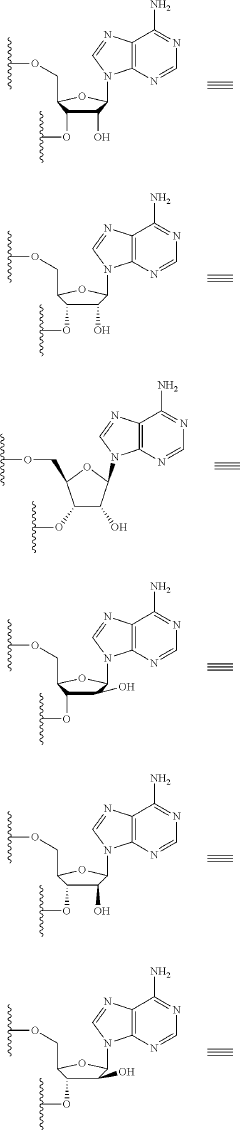
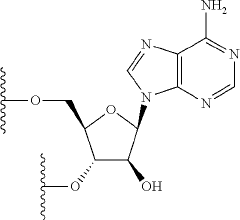
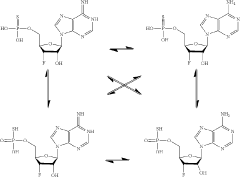
Synthesis of fluorinated nucleotides
PatentPendingUS20230287032A1
Innovation
- A more efficient synthetic route using stereo-controlled electrophilic fluorination and glycosylation, employing a new class of organocatalysts to reduce dimeric and polymeric impurities, and avoiding corrosive aminosulfurane-based fluorinating reagents, resulting in a shorter process with improved stereoselectivity and the use of stable crystalline intermediates.
Process For Synthesizing Fluorinated Cyclic Aliphatic Compounds
PatentActiveUS20210261478A1
Innovation
- A method involving the hydrogenation of aromatic fluorine-containing precursor substances using a catalyst with at least one rhodium atom and an N-heterocyclic carbene ligand, which prevents F—H exchange and allows for the production of fluorinated cycloaliphatic compounds with high yield and tolerance to various functional groups, particularly favoring the formation of sterically defined cis products.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated to be as low as -28. Due to its extreme corrosiveness and reactivity, handling this compound requires stringent safety protocols and specialized equipment. All operations involving fluoroantimonic acid must be conducted in a controlled environment, preferably within a fume hood equipped with acid-resistant materials.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits, gloves made of fluorinated materials such as Viton or Teflon, and a full-face respirator with appropriate acid gas cartridges. Double gloving is recommended, with frequent changes to prevent permeation. Eye protection in the form of chemical splash goggles or a face shield is mandatory.
Storage of fluoroantimonic acid requires special considerations. It must be kept in containers made of materials resistant to hydrofluoric acid, such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). Glass and metal containers are unsuitable due to the acid's corrosive nature. The storage area should be cool, dry, and well-ventilated, away from incompatible materials like water, bases, and most metals.
Handling procedures for fluoroantimonic acid involve careful transfer techniques. Use of a closed system for transfers is ideal to minimize exposure risks. When pipetting is necessary, only specialized acid-resistant pipettes should be employed. All equipment that comes into contact with the acid must be thoroughly decontaminated after use.
Emergency response protocols are essential. Spill kits specifically designed for hydrofluoric acid should be readily available. In case of skin or eye contact, immediate decontamination with copious amounts of water followed by application of calcium gluconate gel is critical. Inhalation exposure requires immediate removal to fresh air and medical attention.
Waste disposal of fluoroantimonic acid and related materials must follow strict guidelines. Neutralization should only be attempted by trained professionals using appropriate equipment. Disposal should comply with all applicable regulations and be handled by specialized chemical waste management services.
Training and education of personnel working with fluoroantimonic acid is paramount. Regular safety briefings, hands-on training sessions, and emergency drills should be conducted to ensure all staff are well-prepared for potential incidents. Documentation of all safety procedures, including Material Safety Data Sheets (MSDS), must be readily accessible in the laboratory.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits, gloves made of fluorinated materials such as Viton or Teflon, and a full-face respirator with appropriate acid gas cartridges. Double gloving is recommended, with frequent changes to prevent permeation. Eye protection in the form of chemical splash goggles or a face shield is mandatory.
Storage of fluoroantimonic acid requires special considerations. It must be kept in containers made of materials resistant to hydrofluoric acid, such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). Glass and metal containers are unsuitable due to the acid's corrosive nature. The storage area should be cool, dry, and well-ventilated, away from incompatible materials like water, bases, and most metals.
Handling procedures for fluoroantimonic acid involve careful transfer techniques. Use of a closed system for transfers is ideal to minimize exposure risks. When pipetting is necessary, only specialized acid-resistant pipettes should be employed. All equipment that comes into contact with the acid must be thoroughly decontaminated after use.
Emergency response protocols are essential. Spill kits specifically designed for hydrofluoric acid should be readily available. In case of skin or eye contact, immediate decontamination with copious amounts of water followed by application of calcium gluconate gel is critical. Inhalation exposure requires immediate removal to fresh air and medical attention.
Waste disposal of fluoroantimonic acid and related materials must follow strict guidelines. Neutralization should only be attempted by trained professionals using appropriate equipment. Disposal should comply with all applicable regulations and be handled by specialized chemical waste management services.
Training and education of personnel working with fluoroantimonic acid is paramount. Regular safety briefings, hands-on training sessions, and emergency drills should be conducted to ensure all staff are well-prepared for potential incidents. Documentation of all safety procedures, including Material Safety Data Sheets (MSDS), must be readily accessible in the laboratory.
Environmental Impact of Fluoroantimonic Acid Production
The production of fluoroantimonic acid, one of the strongest known superacids, poses significant environmental challenges that require careful consideration and management. The synthesis process involves highly reactive and corrosive materials, primarily hydrogen fluoride and antimony pentafluoride, which can have severe impacts on the environment if not properly handled and contained.
Air pollution is a primary concern in the production of fluoroantimonic acid. The volatile nature of the precursor chemicals and the acid itself can lead to the release of fluorine-containing compounds and antimony-based particulates into the atmosphere. These emissions can contribute to the formation of acid rain, ozone depletion, and local air quality degradation. Stringent air filtration and scrubbing systems are essential to mitigate these risks and comply with environmental regulations.
Water contamination is another critical environmental issue associated with fluoroantimonic acid production. The extreme corrosivity of the acid means that any accidental release or improper disposal can lead to severe contamination of water bodies. This can result in the acidification of aquatic ecosystems, causing harm to flora and fauna, and potentially impacting drinking water sources. Robust containment systems, wastewater treatment facilities, and emergency response protocols are necessary to prevent and manage potential spills or leaks.
Soil contamination is also a significant concern, particularly in areas surrounding production facilities. The acid and its precursors can leach into the soil, altering its pH and chemical composition. This can lead to long-term impacts on soil fertility, microbial communities, and plant life. Proper storage, handling, and disposal practices are crucial to minimize the risk of soil contamination and ensure the long-term environmental sustainability of production sites.
The production process also raises concerns about resource depletion and energy consumption. The synthesis of fluoroantimonic acid requires significant amounts of fluorine and antimony, both of which are finite resources. Additionally, the energy-intensive nature of the production process contributes to greenhouse gas emissions and climate change concerns. Efforts to improve process efficiency, explore alternative synthesis routes, and implement renewable energy sources in production facilities are essential for reducing the overall environmental footprint.
Waste management presents another environmental challenge in fluoroantimonic acid production. The process generates hazardous waste streams that require specialized treatment and disposal methods. Improper handling of these wastes can lead to environmental contamination and pose risks to human health. Developing and implementing comprehensive waste management strategies, including recycling and neutralization techniques, is crucial for minimizing environmental impacts and ensuring regulatory compliance.
Air pollution is a primary concern in the production of fluoroantimonic acid. The volatile nature of the precursor chemicals and the acid itself can lead to the release of fluorine-containing compounds and antimony-based particulates into the atmosphere. These emissions can contribute to the formation of acid rain, ozone depletion, and local air quality degradation. Stringent air filtration and scrubbing systems are essential to mitigate these risks and comply with environmental regulations.
Water contamination is another critical environmental issue associated with fluoroantimonic acid production. The extreme corrosivity of the acid means that any accidental release or improper disposal can lead to severe contamination of water bodies. This can result in the acidification of aquatic ecosystems, causing harm to flora and fauna, and potentially impacting drinking water sources. Robust containment systems, wastewater treatment facilities, and emergency response protocols are necessary to prevent and manage potential spills or leaks.
Soil contamination is also a significant concern, particularly in areas surrounding production facilities. The acid and its precursors can leach into the soil, altering its pH and chemical composition. This can lead to long-term impacts on soil fertility, microbial communities, and plant life. Proper storage, handling, and disposal practices are crucial to minimize the risk of soil contamination and ensure the long-term environmental sustainability of production sites.
The production process also raises concerns about resource depletion and energy consumption. The synthesis of fluoroantimonic acid requires significant amounts of fluorine and antimony, both of which are finite resources. Additionally, the energy-intensive nature of the production process contributes to greenhouse gas emissions and climate change concerns. Efforts to improve process efficiency, explore alternative synthesis routes, and implement renewable energy sources in production facilities are essential for reducing the overall environmental footprint.
Waste management presents another environmental challenge in fluoroantimonic acid production. The process generates hazardous waste streams that require specialized treatment and disposal methods. Improper handling of these wastes can lead to environmental contamination and pose risks to human health. Developing and implementing comprehensive waste management strategies, including recycling and neutralization techniques, is crucial for minimizing environmental impacts and ensuring regulatory compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!