How to Implement Cutting‑Edge Reactions with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Research Objectives
Fluoroantimonic acid, a superacid formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful tool in cutting-edge chemical reactions. This compound's exceptional acidity, surpassing that of 100% sulfuric acid by over a trillion times, has revolutionized our understanding of superacidity and opened new avenues for chemical synthesis and catalysis.
The development of fluoroantimonic acid can be traced back to the early 20th century, with significant advancements made in the 1960s and 1970s. Its discovery and subsequent refinement have been pivotal in expanding the boundaries of acid-catalyzed reactions and enabling previously impossible transformations in organic synthesis.
The primary objective of research into fluoroantimonic acid implementation is to harness its unprecedented acidity for novel chemical reactions and industrial applications. Scientists aim to exploit its unique properties to catalyze challenging transformations, such as the activation of typically unreactive C-H bonds and the isomerization of hydrocarbons.
One key area of focus is the development of safer handling and containment methods for this highly corrosive substance. Researchers are exploring innovative reactor designs and materials that can withstand the extreme acidity while maintaining reaction efficiency. This includes investigating specialized fluoropolymer coatings and advanced alloys resistant to superacid corrosion.
Another critical research objective is to expand the scope of fluoroantimonic acid-mediated reactions in organic synthesis. This involves identifying new substrate classes amenable to superacid catalysis and developing selective transformation protocols. Researchers are particularly interested in applying fluoroantimonic acid to the synthesis of complex pharmaceutical intermediates and advanced materials.
The environmental impact of fluoroantimonic acid usage is also a significant concern. Current research efforts are directed towards developing more sustainable processes, including the recycling and regeneration of the superacid catalyst. Scientists are exploring methods to minimize waste generation and reduce the overall environmental footprint of reactions involving this powerful reagent.
Furthermore, researchers are investigating the fundamental chemistry of fluoroantimonic acid to gain deeper insights into its behavior at the molecular level. This includes studying its solvation properties, proton transfer mechanisms, and interactions with various organic and inorganic substrates. Such knowledge is crucial for optimizing reaction conditions and predicting new applications.
As the field progresses, there is a growing interest in combining fluoroantimonic acid with other cutting-edge technologies, such as flow chemistry and microreactor systems. These synergistic approaches aim to enhance reaction control, improve safety, and increase the scalability of superacid-mediated processes for industrial applications.
The development of fluoroantimonic acid can be traced back to the early 20th century, with significant advancements made in the 1960s and 1970s. Its discovery and subsequent refinement have been pivotal in expanding the boundaries of acid-catalyzed reactions and enabling previously impossible transformations in organic synthesis.
The primary objective of research into fluoroantimonic acid implementation is to harness its unprecedented acidity for novel chemical reactions and industrial applications. Scientists aim to exploit its unique properties to catalyze challenging transformations, such as the activation of typically unreactive C-H bonds and the isomerization of hydrocarbons.
One key area of focus is the development of safer handling and containment methods for this highly corrosive substance. Researchers are exploring innovative reactor designs and materials that can withstand the extreme acidity while maintaining reaction efficiency. This includes investigating specialized fluoropolymer coatings and advanced alloys resistant to superacid corrosion.
Another critical research objective is to expand the scope of fluoroantimonic acid-mediated reactions in organic synthesis. This involves identifying new substrate classes amenable to superacid catalysis and developing selective transformation protocols. Researchers are particularly interested in applying fluoroantimonic acid to the synthesis of complex pharmaceutical intermediates and advanced materials.
The environmental impact of fluoroantimonic acid usage is also a significant concern. Current research efforts are directed towards developing more sustainable processes, including the recycling and regeneration of the superacid catalyst. Scientists are exploring methods to minimize waste generation and reduce the overall environmental footprint of reactions involving this powerful reagent.
Furthermore, researchers are investigating the fundamental chemistry of fluoroantimonic acid to gain deeper insights into its behavior at the molecular level. This includes studying its solvation properties, proton transfer mechanisms, and interactions with various organic and inorganic substrates. Such knowledge is crucial for optimizing reaction conditions and predicting new applications.
As the field progresses, there is a growing interest in combining fluoroantimonic acid with other cutting-edge technologies, such as flow chemistry and microreactor systems. These synergistic approaches aim to enhance reaction control, improve safety, and increase the scalability of superacid-mediated processes for industrial applications.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has been experiencing significant growth in recent years. This growth is primarily driven by the increasing demand for advanced materials and chemicals in various industries, including pharmaceuticals, petrochemicals, and electronics.
In the pharmaceutical sector, superacids like fluoroantimonic acid play a crucial role in the synthesis of complex drug molecules. The ability of these acids to catalyze reactions that are otherwise difficult or impossible has led to their increased adoption in drug discovery and development processes. This trend is expected to continue as pharmaceutical companies seek more efficient and cost-effective ways to produce novel therapeutic compounds.
The petrochemical industry represents another major market for superacid applications. Fluoroantimonic acid and other superacids are used in various refining processes, including isomerization and alkylation. These processes are essential for producing high-octane gasoline and other valuable petroleum products. As global energy demand continues to rise, the market for superacid applications in this sector is projected to expand further.
In the electronics industry, superacids find applications in the production of advanced materials for semiconductors and display technologies. The extreme acidity of fluoroantimonic acid makes it useful for etching and cleaning processes in the manufacture of microchips and other electronic components. With the ongoing miniaturization of electronic devices and the development of new technologies like 5G, the demand for superacids in this sector is expected to grow substantially.
The market for superacid applications also extends to other industries, such as polymer production, where fluoroantimonic acid is used as a catalyst in the synthesis of high-performance plastics. Additionally, the aerospace and automotive industries utilize superacids in the development of advanced materials with improved strength-to-weight ratios and corrosion resistance.
Despite the growing market opportunities, there are challenges that need to be addressed. The extreme reactivity and corrosiveness of fluoroantimonic acid pose significant safety and handling concerns. This has led to increased focus on developing safer handling protocols and specialized equipment for working with superacids. Additionally, environmental regulations regarding the use and disposal of such powerful acids are becoming more stringent, driving research into more environmentally friendly alternatives or improved containment methods.
Overall, the market for superacid applications, particularly those involving fluoroantimonic acid, is poised for continued growth across multiple industries. The unique properties of these acids make them indispensable in various cutting-edge applications, driving innovation and technological advancements in diverse fields.
In the pharmaceutical sector, superacids like fluoroantimonic acid play a crucial role in the synthesis of complex drug molecules. The ability of these acids to catalyze reactions that are otherwise difficult or impossible has led to their increased adoption in drug discovery and development processes. This trend is expected to continue as pharmaceutical companies seek more efficient and cost-effective ways to produce novel therapeutic compounds.
The petrochemical industry represents another major market for superacid applications. Fluoroantimonic acid and other superacids are used in various refining processes, including isomerization and alkylation. These processes are essential for producing high-octane gasoline and other valuable petroleum products. As global energy demand continues to rise, the market for superacid applications in this sector is projected to expand further.
In the electronics industry, superacids find applications in the production of advanced materials for semiconductors and display technologies. The extreme acidity of fluoroantimonic acid makes it useful for etching and cleaning processes in the manufacture of microchips and other electronic components. With the ongoing miniaturization of electronic devices and the development of new technologies like 5G, the demand for superacids in this sector is expected to grow substantially.
The market for superacid applications also extends to other industries, such as polymer production, where fluoroantimonic acid is used as a catalyst in the synthesis of high-performance plastics. Additionally, the aerospace and automotive industries utilize superacids in the development of advanced materials with improved strength-to-weight ratios and corrosion resistance.
Despite the growing market opportunities, there are challenges that need to be addressed. The extreme reactivity and corrosiveness of fluoroantimonic acid pose significant safety and handling concerns. This has led to increased focus on developing safer handling protocols and specialized equipment for working with superacids. Additionally, environmental regulations regarding the use and disposal of such powerful acids are becoming more stringent, driving research into more environmentally friendly alternatives or improved containment methods.
Overall, the market for superacid applications, particularly those involving fluoroantimonic acid, is poised for continued growth across multiple industries. The unique properties of these acids make them indispensable in various cutting-edge applications, driving innovation and technological advancements in diverse fields.
Current Challenges in Fluoroantimonic Acid Reactions
Despite the remarkable potential of fluoroantimonic acid in cutting-edge reactions, several significant challenges hinder its widespread implementation in industrial and research settings. One of the primary obstacles is the extreme corrosiveness of fluoroantimonic acid, which necessitates specialized handling and containment measures. This corrosiveness limits the choice of reaction vessels and equipment, often requiring the use of expensive fluoropolymer-based materials or specially treated glassware.
The high reactivity of fluoroantimonic acid also poses challenges in terms of selectivity and control over reaction outcomes. While its superacidity can catalyze a wide range of transformations, it may lead to undesired side reactions or over-functionalization of substrates. This lack of selectivity can result in complex product mixtures, making purification and isolation of target compounds difficult and reducing overall reaction efficiency.
Another significant challenge is the moisture sensitivity of fluoroantimonic acid. Exposure to even trace amounts of water can lead to rapid decomposition and loss of activity. This necessitates stringent anhydrous conditions and specialized techniques for reagent preparation and reaction setup, which can be technically demanding and resource-intensive.
The environmental and safety concerns associated with fluoroantimonic acid present additional hurdles. Its extreme acidity and potential to generate toxic hydrogen fluoride gas require robust safety protocols and specialized waste disposal procedures. These factors contribute to increased operational costs and regulatory compliance challenges, particularly in industrial settings.
Scale-up of reactions involving fluoroantimonic acid from laboratory to industrial scale remains a significant challenge. The heat generation during large-scale reactions and the need for efficient mixing while maintaining containment integrity pose engineering challenges. Additionally, the cost of fluoroantimonic acid and its precursors can be prohibitive for large-scale applications, limiting its use to high-value, small-volume products.
The limited compatibility of fluoroantimonic acid with many common organic solvents and reagents further restricts its applicability. This incompatibility narrows the range of possible reaction conditions and substrates, potentially limiting the scope of accessible transformations. Researchers must often develop novel reaction protocols or find alternative reagent systems to overcome these limitations.
Lastly, the characterization of reaction intermediates and mechanistic studies in fluoroantimonic acid-mediated reactions present significant analytical challenges. The extreme acidity and reactivity of the medium complicate the use of many standard spectroscopic and analytical techniques, making it difficult to elucidate reaction pathways and optimize processes based on mechanistic understanding.
The high reactivity of fluoroantimonic acid also poses challenges in terms of selectivity and control over reaction outcomes. While its superacidity can catalyze a wide range of transformations, it may lead to undesired side reactions or over-functionalization of substrates. This lack of selectivity can result in complex product mixtures, making purification and isolation of target compounds difficult and reducing overall reaction efficiency.
Another significant challenge is the moisture sensitivity of fluoroantimonic acid. Exposure to even trace amounts of water can lead to rapid decomposition and loss of activity. This necessitates stringent anhydrous conditions and specialized techniques for reagent preparation and reaction setup, which can be technically demanding and resource-intensive.
The environmental and safety concerns associated with fluoroantimonic acid present additional hurdles. Its extreme acidity and potential to generate toxic hydrogen fluoride gas require robust safety protocols and specialized waste disposal procedures. These factors contribute to increased operational costs and regulatory compliance challenges, particularly in industrial settings.
Scale-up of reactions involving fluoroantimonic acid from laboratory to industrial scale remains a significant challenge. The heat generation during large-scale reactions and the need for efficient mixing while maintaining containment integrity pose engineering challenges. Additionally, the cost of fluoroantimonic acid and its precursors can be prohibitive for large-scale applications, limiting its use to high-value, small-volume products.
The limited compatibility of fluoroantimonic acid with many common organic solvents and reagents further restricts its applicability. This incompatibility narrows the range of possible reaction conditions and substrates, potentially limiting the scope of accessible transformations. Researchers must often develop novel reaction protocols or find alternative reagent systems to overcome these limitations.
Lastly, the characterization of reaction intermediates and mechanistic studies in fluoroantimonic acid-mediated reactions present significant analytical challenges. The extreme acidity and reactivity of the medium complicate the use of many standard spectroscopic and analytical techniques, making it difficult to elucidate reaction pathways and optimize processes based on mechanistic understanding.
Existing Methodologies for Fluoroantimonic Acid Reactions
01 Synthesis and properties of fluoroantimonic acid
Fluoroantimonic acid is a superacid composed of hydrogen fluoride (HF) and antimony pentafluoride (SbF5). It is known for its extremely high acidity and is often used as a powerful catalyst in various chemical reactions. The synthesis and characterization of fluoroantimonic acid involve careful handling due to its corrosive nature and reactivity.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in chemical reactions and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. Its extreme acidity allows for the activation of otherwise unreactive compounds, making it valuable in organic synthesis and petrochemical industries.
- Material compatibility and storage: Due to its highly corrosive nature, fluoroantimonic acid requires specialized materials for handling and storage. Research has been conducted on developing resistant materials, such as certain fluoropolymers and specially treated metals, that can withstand its corrosive effects. Proper containment and storage methods are crucial for safety and maintaining the acid's effectiveness.
- Safety measures and environmental considerations: Handling fluoroantimonic acid requires strict safety protocols due to its extreme reactivity and corrosiveness. Research has focused on developing improved safety measures, including specialized personal protective equipment, containment systems, and neutralization methods. Environmental impact assessments and waste management strategies have also been studied to minimize potential hazards.
- Analytical applications and detection methods: Fluoroantimonic acid has found use in analytical chemistry for its unique properties. Research has been conducted on developing methods for its detection and quantification in various matrices. Additionally, it has been explored as a reagent for analyzing complex organic compounds and in spectroscopic studies due to its ability to generate highly reactive species.
02 Applications in organic synthesis
Fluoroantimonic acid finds extensive use in organic synthesis as a catalyst for various reactions, including alkylation, acylation, and isomerization of hydrocarbons. Its strong acidity enables it to catalyze reactions that are difficult to achieve with conventional acids, making it valuable in the production of specialty chemicals and pharmaceuticals.Expand Specific Solutions03 Use in materials science and surface treatment
In materials science, fluoroantimonic acid is employed for surface treatment of various materials, including metals and polymers. It can be used to etch surfaces, modify surface properties, or create specific surface structures. This application is particularly relevant in the semiconductor industry and in the development of advanced materials with unique surface characteristics.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme acidity and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are necessary when working with this superacid. Proper disposal and neutralization procedures must be followed to prevent environmental contamination and ensure worker safety.Expand Specific Solutions05 Analytical applications and instrumentation
Fluoroantimonic acid is used in certain analytical applications and specialized instrumentation. Its unique properties make it suitable for specific analytical techniques, particularly in the field of mass spectrometry and in the analysis of complex organic compounds. It can also be used in the development of novel analytical methods for challenging chemical systems.Expand Specific Solutions
Key Players in Superacid Research and Industry
The implementation of cutting-edge reactions with fluoroantimonic acid is in a nascent stage of development, characterized by a highly specialized and limited market. The global market size for this technology is relatively small, primarily driven by research institutions and advanced chemical industries. The technical maturity is still evolving, with companies like DAIKIN INDUSTRIES Ltd., Sinochem Lantian Co., Ltd., and LANXESS Deutschland GmbH leading the way in research and development. Universities such as the University of Kansas and Central South University are also contributing significantly to advancing the field. The competitive landscape is currently focused on overcoming challenges related to handling and application of this super-acid, with potential for breakthrough innovations in catalysis and materials processing.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN INDUSTRIES Ltd. has pioneered the use of fluoroantimonic acid in the production of advanced fluorochemicals. Their proprietary process utilizes the superacid as a catalyst for the synthesis of complex fluorinated compounds, including specialty lubricants and heat transfer fluids. DAIKIN has developed a unique reactor design that allows for precise control of reaction conditions, ensuring optimal product quality and yield. The company has also implemented a comprehensive safety management system, including remote handling techniques and sophisticated containment measures, to mitigate the risks associated with fluoroantimonic acid use.
Strengths: Expertise in fluorochemicals, advanced reactor technology, comprehensive safety measures. Weaknesses: High production costs, limited scalability, potential environmental impact.
LANXESS Deutschland GmbH
Technical Solution: LANXESS Deutschland GmbH has developed an innovative application of fluoroantimonic acid in the production of high-performance rubber compounds. Their process uses the superacid as a catalyst to create unique cross-linking structures in specialty elastomers, resulting in materials with exceptional chemical resistance and mechanical properties. LANXESS has invested in state-of-the-art reaction vessels with specialized linings to withstand the corrosive nature of fluoroantimonic acid. The company has also implemented advanced process control systems to ensure precise dosing and reaction conditions, maximizing product quality while minimizing safety risks.
Strengths: Novel application in rubber technology, advanced process control, unique product properties. Weaknesses: High material costs, specialized equipment requirements, limited market demand.
Innovative Approaches in Fluoroantimonic Acid Chemistry
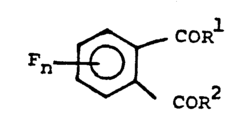
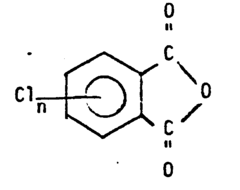
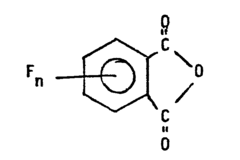
4,5-difluorophthaloyl fluoride and its preparation
PatentInactiveEP0148366A2
Innovation
- A process involving the reaction of chlorophthalic anhydrides with potassium fluoride or cesium fluoride, using polyether catalysts at specific temperature ranges and conditions, to produce fluorophthalic anhydrides, followed by conversion to fluorophthalamic acid, imide, and finally alkali metal fluorophthalimide, effectively generating 4,5-difluorophthaloyl fluoride as a versatile intermediate.
Treatment of waste water containing fluorinated acids or the salts thereof
PatentWO2011101342A1
Innovation
- The process involves using anion exchangers that are at least partially in the fluoride form, which are contacted with dilute aqueous solutions of fluorinated acids or their salts, allowing for effective binding and subsequent regeneration, with the anion exchanger being conditioned with hydrogen fluoride or fluoride anions to enhance selectivity and efficiency.
Safety Protocols for Handling Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated at -21.6. Its extreme corrosiveness and reactivity necessitate stringent safety protocols for handling and use. Proper personal protective equipment (PPE) is paramount when working with this substance. A fully encapsulating chemical suit with a self-contained breathing apparatus is essential. The suit material must be resistant to both hydrofluoric acid and antimony pentafluoride, the components of fluoroantimonic acid.
Laboratory infrastructure must be specially designed for superacid handling. All work should be conducted in a fume hood with a face velocity of at least 100 feet per minute. The hood should be constructed of fluoropolymer-lined materials resistant to both HF and SbF5. Glassware is unsuitable; instead, PTFE or PFA containers and transfer lines should be used exclusively. All equipment must be thoroughly dried, as fluoroantimonic acid reacts violently with water.
Strict containment and spill response procedures are critical. Work areas should be equipped with spill containment berms and neutralizing agents such as calcium carbonate or sodium bicarbonate. A safety shower and eyewash station must be immediately accessible. Detailed emergency response plans should be in place, including evacuation procedures and decontamination protocols.
Storage and transport of fluoroantimonic acid require specialized containers made of fluoropolymer materials. These must be kept in a cool, dry area away from incompatible substances, particularly water and bases. Double containment is recommended to prevent accidental release. Inventory control and restricted access to storage areas are essential to minimize risk.
Training is a crucial aspect of safety protocols. All personnel working with or near fluoroantimonic acid must undergo comprehensive safety training, including hands-on practice with PPE and emergency procedures. Regular refresher courses and safety drills should be conducted to maintain preparedness.
Waste disposal presents unique challenges. Neutralization with large quantities of ice-cold sodium or calcium carbonate solution is typically required before disposal. This process must be performed with extreme caution due to the highly exothermic nature of the reaction. Specialized waste management services may be necessary for larger quantities.
Implementing these safety protocols is essential for conducting cutting-edge reactions with fluoroantimonic acid. The extreme hazards associated with this superacid demand unwavering adherence to safety measures, constant vigilance, and a culture of safety prioritization in the laboratory environment.
Laboratory infrastructure must be specially designed for superacid handling. All work should be conducted in a fume hood with a face velocity of at least 100 feet per minute. The hood should be constructed of fluoropolymer-lined materials resistant to both HF and SbF5. Glassware is unsuitable; instead, PTFE or PFA containers and transfer lines should be used exclusively. All equipment must be thoroughly dried, as fluoroantimonic acid reacts violently with water.
Strict containment and spill response procedures are critical. Work areas should be equipped with spill containment berms and neutralizing agents such as calcium carbonate or sodium bicarbonate. A safety shower and eyewash station must be immediately accessible. Detailed emergency response plans should be in place, including evacuation procedures and decontamination protocols.
Storage and transport of fluoroantimonic acid require specialized containers made of fluoropolymer materials. These must be kept in a cool, dry area away from incompatible substances, particularly water and bases. Double containment is recommended to prevent accidental release. Inventory control and restricted access to storage areas are essential to minimize risk.
Training is a crucial aspect of safety protocols. All personnel working with or near fluoroantimonic acid must undergo comprehensive safety training, including hands-on practice with PPE and emergency procedures. Regular refresher courses and safety drills should be conducted to maintain preparedness.
Waste disposal presents unique challenges. Neutralization with large quantities of ice-cold sodium or calcium carbonate solution is typically required before disposal. This process must be performed with extreme caution due to the highly exothermic nature of the reaction. Specialized waste management services may be necessary for larger quantities.
Implementing these safety protocols is essential for conducting cutting-edge reactions with fluoroantimonic acid. The extreme hazards associated with this superacid demand unwavering adherence to safety measures, constant vigilance, and a culture of safety prioritization in the laboratory environment.
Environmental Impact of Superacid Chemistry
The implementation of cutting-edge reactions using fluoroantimonic acid, while offering significant potential for chemical synthesis, raises substantial environmental concerns. As a superacid, fluoroantimonic acid poses severe risks to ecosystems and human health if not properly managed. Its extreme corrosiveness and reactivity can lead to devastating effects on soil, water bodies, and air quality if released into the environment.
When used in industrial processes, the production and handling of fluoroantimonic acid require stringent safety measures to prevent accidental releases. Even small spills can cause rapid and extensive damage to surrounding ecosystems, potentially leading to long-term environmental degradation. The acid's ability to react violently with water makes it particularly hazardous to aquatic environments, where it can cause immediate and severe pH changes, destroying aquatic life and disrupting entire food chains.
The environmental impact extends beyond direct contamination. The production of fluoroantimonic acid involves the use of hydrofluoric acid and antimony pentafluoride, both of which have their own significant environmental footprints. The mining and processing of antimony, in particular, can lead to soil and water pollution, as well as contribute to greenhouse gas emissions through energy-intensive extraction processes.
Furthermore, the disposal of waste products from reactions involving fluoroantimonic acid presents additional environmental challenges. Neutralization processes must be carefully controlled to prevent the release of toxic byproducts, and the resulting waste often requires specialized treatment and disposal methods to minimize environmental harm.
The use of fluoroantimonic acid in chemical reactions also raises concerns about the potential for atmospheric pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants may be generated during reactions, necessitating advanced air filtration and scrubbing systems to prevent their release into the atmosphere.
From a sustainability perspective, the environmental costs associated with the use of fluoroantimonic acid must be carefully weighed against its benefits in chemical synthesis. Researchers and industry professionals are increasingly exploring greener alternatives and process modifications to reduce the environmental impact of superacid chemistry. This includes the development of less hazardous catalysts, the implementation of closed-loop systems to minimize waste, and the adoption of more environmentally friendly reaction media.
As regulations surrounding environmental protection become more stringent, the chemical industry faces growing pressure to address the environmental implications of superacid chemistry. This has spurred innovation in safer handling techniques, more efficient reaction processes, and improved waste management strategies. However, significant challenges remain in balancing the technological advantages of fluoroantimonic acid with the imperative of environmental stewardship.
When used in industrial processes, the production and handling of fluoroantimonic acid require stringent safety measures to prevent accidental releases. Even small spills can cause rapid and extensive damage to surrounding ecosystems, potentially leading to long-term environmental degradation. The acid's ability to react violently with water makes it particularly hazardous to aquatic environments, where it can cause immediate and severe pH changes, destroying aquatic life and disrupting entire food chains.
The environmental impact extends beyond direct contamination. The production of fluoroantimonic acid involves the use of hydrofluoric acid and antimony pentafluoride, both of which have their own significant environmental footprints. The mining and processing of antimony, in particular, can lead to soil and water pollution, as well as contribute to greenhouse gas emissions through energy-intensive extraction processes.
Furthermore, the disposal of waste products from reactions involving fluoroantimonic acid presents additional environmental challenges. Neutralization processes must be carefully controlled to prevent the release of toxic byproducts, and the resulting waste often requires specialized treatment and disposal methods to minimize environmental harm.
The use of fluoroantimonic acid in chemical reactions also raises concerns about the potential for atmospheric pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants may be generated during reactions, necessitating advanced air filtration and scrubbing systems to prevent their release into the atmosphere.
From a sustainability perspective, the environmental costs associated with the use of fluoroantimonic acid must be carefully weighed against its benefits in chemical synthesis. Researchers and industry professionals are increasingly exploring greener alternatives and process modifications to reduce the environmental impact of superacid chemistry. This includes the development of less hazardous catalysts, the implementation of closed-loop systems to minimize waste, and the adoption of more environmentally friendly reaction media.
As regulations surrounding environmental protection become more stringent, the chemical industry faces growing pressure to address the environmental implications of superacid chemistry. This has spurred innovation in safer handling techniques, more efficient reaction processes, and improved waste management strategies. However, significant challenges remain in balancing the technological advantages of fluoroantimonic acid with the imperative of environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!