Riding the Crest of Catalysis Innovations with Fluoroantimonic Acid
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Background and Objectives
Fluoroantimonic acid, a superacid formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful catalyst in various chemical reactions. Its exceptional acidity, surpassing that of conventional acids, has sparked significant interest in the scientific community for its potential to revolutionize catalytic processes.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for their unique properties. The field gained momentum in the 1960s and 1970s with the work of George A. Olah, who extensively studied superacid chemistry and its applications in hydrocarbon transformations.
Over the years, fluoroantimonic acid has demonstrated remarkable catalytic activity in numerous reactions, including isomerization, alkylation, and cracking of hydrocarbons. Its ability to protonate even weak bases and generate highly reactive carbocations has opened up new possibilities in organic synthesis and petrochemical processes.
The primary objective of research in fluoroantimonic acid catalysis is to harness its exceptional acidity for more efficient and selective chemical transformations. Scientists aim to develop novel catalytic systems that can operate under milder conditions, reduce energy consumption, and minimize waste generation in industrial processes.
Current research efforts focus on several key areas. First, there is a push to better understand the fundamental mechanisms of fluoroantimonic acid catalysis at the molecular level. This knowledge is crucial for optimizing reaction conditions and designing more effective catalytic systems.
Secondly, researchers are exploring ways to stabilize and immobilize fluoroantimonic acid on various support materials. This approach aims to enhance the acid's recyclability and facilitate its use in continuous flow processes, which are increasingly important in industrial applications.
Another significant objective is to expand the scope of fluoroantimonic acid catalysis beyond traditional hydrocarbon chemistry. Scientists are investigating its potential in the synthesis of fine chemicals, pharmaceuticals, and advanced materials, where highly selective and efficient catalysts are in high demand.
Environmental considerations also play a crucial role in shaping the research landscape. As sustainability becomes increasingly important, there is a growing emphasis on developing greener alternatives to fluoroantimonic acid catalysis or finding ways to mitigate its environmental impact while maintaining its catalytic efficiency.
In conclusion, the field of fluoroantimonic acid catalysis continues to evolve, driven by the need for more efficient and sustainable chemical processes. As researchers delve deeper into its mechanisms and applications, this superacid catalyst holds promise for transforming various sectors of the chemical industry and contributing to the development of next-generation catalytic technologies.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for their unique properties. The field gained momentum in the 1960s and 1970s with the work of George A. Olah, who extensively studied superacid chemistry and its applications in hydrocarbon transformations.
Over the years, fluoroantimonic acid has demonstrated remarkable catalytic activity in numerous reactions, including isomerization, alkylation, and cracking of hydrocarbons. Its ability to protonate even weak bases and generate highly reactive carbocations has opened up new possibilities in organic synthesis and petrochemical processes.
The primary objective of research in fluoroantimonic acid catalysis is to harness its exceptional acidity for more efficient and selective chemical transformations. Scientists aim to develop novel catalytic systems that can operate under milder conditions, reduce energy consumption, and minimize waste generation in industrial processes.
Current research efforts focus on several key areas. First, there is a push to better understand the fundamental mechanisms of fluoroantimonic acid catalysis at the molecular level. This knowledge is crucial for optimizing reaction conditions and designing more effective catalytic systems.
Secondly, researchers are exploring ways to stabilize and immobilize fluoroantimonic acid on various support materials. This approach aims to enhance the acid's recyclability and facilitate its use in continuous flow processes, which are increasingly important in industrial applications.
Another significant objective is to expand the scope of fluoroantimonic acid catalysis beyond traditional hydrocarbon chemistry. Scientists are investigating its potential in the synthesis of fine chemicals, pharmaceuticals, and advanced materials, where highly selective and efficient catalysts are in high demand.
Environmental considerations also play a crucial role in shaping the research landscape. As sustainability becomes increasingly important, there is a growing emphasis on developing greener alternatives to fluoroantimonic acid catalysis or finding ways to mitigate its environmental impact while maintaining its catalytic efficiency.
In conclusion, the field of fluoroantimonic acid catalysis continues to evolve, driven by the need for more efficient and sustainable chemical processes. As researchers delve deeper into its mechanisms and applications, this superacid catalyst holds promise for transforming various sectors of the chemical industry and contributing to the development of next-generation catalytic technologies.
Market Analysis for Superacid Catalysts
The market for superacid catalysts, particularly those utilizing fluoroantimonic acid, has shown significant growth potential in recent years. This growth is primarily driven by the increasing demand for efficient catalytic processes in various industries, including petrochemicals, pharmaceuticals, and fine chemicals. Fluoroantimonic acid, being one of the strongest known superacids, offers unique catalytic properties that make it attractive for specialized applications.
In the petrochemical industry, superacid catalysts are crucial for processes such as alkylation, isomerization, and cracking. The global petrochemical market is projected to expand steadily, with a compound annual growth rate (CAGR) of around 5% over the next five years. This growth directly translates to increased demand for advanced catalysts, including those based on fluoroantimonic acid.
The pharmaceutical sector represents another significant market for superacid catalysts. As the industry continues to seek more efficient synthesis routes for complex molecules, the use of superacids in organic transformations is gaining traction. The global pharmaceutical market is expected to grow at a CAGR of approximately 6% through 2025, indicating a parallel increase in demand for specialized catalysts.
Fine chemical manufacturing is also a key area where superacid catalysts find application. The market for fine chemicals is highly diverse and includes segments such as flavors and fragrances, specialty polymers, and electronic chemicals. This sector is anticipated to grow at a CAGR of about 5.5% in the coming years, further driving the demand for advanced catalytic solutions.
However, it's important to note that the market for fluoroantimonic acid-based catalysts faces certain challenges. The extreme corrosiveness and reactivity of fluoroantimonic acid necessitate specialized handling and equipment, which can increase operational costs. Additionally, environmental and safety concerns associated with superacids may limit their adoption in some applications.
Despite these challenges, ongoing research and development efforts are focused on harnessing the unique properties of fluoroantimonic acid while mitigating its drawbacks. Innovations in catalyst design, such as supported catalysts or ionic liquid systems incorporating fluoroantimonic acid, are opening up new possibilities for safer and more efficient applications.
The geographical distribution of the superacid catalyst market shows a concentration in regions with strong chemical and petrochemical industries. North America and Europe currently lead in terms of research and development, while Asia-Pacific is emerging as a significant market due to rapid industrialization and increasing investments in chemical manufacturing.
In conclusion, the market for superacid catalysts, particularly those involving fluoroantimonic acid, presents a complex landscape of opportunities and challenges. While the demand is driven by the need for more efficient and selective chemical processes, successful market penetration will depend on addressing safety concerns and developing innovative formulations that maximize the catalytic potential of these powerful acids.
In the petrochemical industry, superacid catalysts are crucial for processes such as alkylation, isomerization, and cracking. The global petrochemical market is projected to expand steadily, with a compound annual growth rate (CAGR) of around 5% over the next five years. This growth directly translates to increased demand for advanced catalysts, including those based on fluoroantimonic acid.
The pharmaceutical sector represents another significant market for superacid catalysts. As the industry continues to seek more efficient synthesis routes for complex molecules, the use of superacids in organic transformations is gaining traction. The global pharmaceutical market is expected to grow at a CAGR of approximately 6% through 2025, indicating a parallel increase in demand for specialized catalysts.
Fine chemical manufacturing is also a key area where superacid catalysts find application. The market for fine chemicals is highly diverse and includes segments such as flavors and fragrances, specialty polymers, and electronic chemicals. This sector is anticipated to grow at a CAGR of about 5.5% in the coming years, further driving the demand for advanced catalytic solutions.
However, it's important to note that the market for fluoroantimonic acid-based catalysts faces certain challenges. The extreme corrosiveness and reactivity of fluoroantimonic acid necessitate specialized handling and equipment, which can increase operational costs. Additionally, environmental and safety concerns associated with superacids may limit their adoption in some applications.
Despite these challenges, ongoing research and development efforts are focused on harnessing the unique properties of fluoroantimonic acid while mitigating its drawbacks. Innovations in catalyst design, such as supported catalysts or ionic liquid systems incorporating fluoroantimonic acid, are opening up new possibilities for safer and more efficient applications.
The geographical distribution of the superacid catalyst market shows a concentration in regions with strong chemical and petrochemical industries. North America and Europe currently lead in terms of research and development, while Asia-Pacific is emerging as a significant market due to rapid industrialization and increasing investments in chemical manufacturing.
In conclusion, the market for superacid catalysts, particularly those involving fluoroantimonic acid, presents a complex landscape of opportunities and challenges. While the demand is driven by the need for more efficient and selective chemical processes, successful market penetration will depend on addressing safety concerns and developing innovative formulations that maximize the catalytic potential of these powerful acids.
Current State and Challenges in Fluoroantimonic Acid Catalysis
Fluoroantimonic acid (H2FSbF6) stands as the strongest known superacid, with a Hammett acidity function estimated at -28. Its exceptional acidity has positioned it as a powerful catalyst in various chemical processes, particularly in hydrocarbon chemistry. The current state of fluoroantimonic acid catalysis is characterized by significant advancements in petrochemical industries, organic synthesis, and materials science.
In the petrochemical sector, fluoroantimonic acid catalysis has revolutionized the cracking and isomerization of hydrocarbons, enabling more efficient production of high-octane fuels and valuable chemical intermediates. Recent innovations have focused on optimizing reaction conditions to enhance selectivity and reduce side product formation, thereby improving overall process efficiency.
Organic synthesis has benefited greatly from fluoroantimonic acid catalysis, particularly in carbon-carbon bond formation reactions. Researchers have successfully employed this superacid in Friedel-Crafts alkylations and acylations, achieving higher yields and shorter reaction times compared to traditional Lewis acid catalysts. Moreover, its application in the synthesis of complex pharmaceutical intermediates has opened new pathways for drug discovery and development.
Despite these advancements, fluoroantimonic acid catalysis faces several significant challenges. The extreme corrosiveness of the acid necessitates specialized handling and reactor materials, limiting its widespread industrial adoption. Current research efforts are focused on developing more stable and easily manageable forms of the catalyst, such as supported or encapsulated versions, to mitigate these issues.
Another major challenge lies in controlling the reactivity of fluoroantimonic acid. Its exceptional strength can lead to over-activation of substrates, resulting in unwanted side reactions and product degradation. Scientists are exploring the use of co-catalysts and reaction modifiers to fine-tune the acid's activity and improve reaction selectivity.
Environmental concerns pose a significant hurdle for the broader application of fluoroantimonic acid catalysis. The production and use of this superacid involve highly toxic and environmentally hazardous compounds, including hydrogen fluoride and antimony pentafluoride. Developing greener alternatives or closed-loop systems for catalyst recovery and reuse is a priority for sustainable implementation.
The scalability of fluoroantimonic acid-catalyzed processes remains a challenge, particularly for fine chemical and pharmaceutical applications. Researchers are investigating continuous flow reactors and microreactor technologies to address this issue, aiming to enhance process control and safety while facilitating scale-up.
In the petrochemical sector, fluoroantimonic acid catalysis has revolutionized the cracking and isomerization of hydrocarbons, enabling more efficient production of high-octane fuels and valuable chemical intermediates. Recent innovations have focused on optimizing reaction conditions to enhance selectivity and reduce side product formation, thereby improving overall process efficiency.
Organic synthesis has benefited greatly from fluoroantimonic acid catalysis, particularly in carbon-carbon bond formation reactions. Researchers have successfully employed this superacid in Friedel-Crafts alkylations and acylations, achieving higher yields and shorter reaction times compared to traditional Lewis acid catalysts. Moreover, its application in the synthesis of complex pharmaceutical intermediates has opened new pathways for drug discovery and development.
Despite these advancements, fluoroantimonic acid catalysis faces several significant challenges. The extreme corrosiveness of the acid necessitates specialized handling and reactor materials, limiting its widespread industrial adoption. Current research efforts are focused on developing more stable and easily manageable forms of the catalyst, such as supported or encapsulated versions, to mitigate these issues.
Another major challenge lies in controlling the reactivity of fluoroantimonic acid. Its exceptional strength can lead to over-activation of substrates, resulting in unwanted side reactions and product degradation. Scientists are exploring the use of co-catalysts and reaction modifiers to fine-tune the acid's activity and improve reaction selectivity.
Environmental concerns pose a significant hurdle for the broader application of fluoroantimonic acid catalysis. The production and use of this superacid involve highly toxic and environmentally hazardous compounds, including hydrogen fluoride and antimony pentafluoride. Developing greener alternatives or closed-loop systems for catalyst recovery and reuse is a priority for sustainable implementation.
The scalability of fluoroantimonic acid-catalyzed processes remains a challenge, particularly for fine chemical and pharmaceutical applications. Researchers are investigating continuous flow reactors and microreactor technologies to address this issue, aiming to enhance process control and safety while facilitating scale-up.
Existing Fluoroantimonic Acid Catalytic Solutions
01 Fluoroantimonic acid as a catalyst in hydrocarbon processing
Fluoroantimonic acid is utilized as a powerful catalyst in various hydrocarbon processing applications, including isomerization, alkylation, and cracking reactions. Its super-acidic properties enable efficient conversion of hydrocarbons under milder conditions compared to traditional catalysts.- Fluoroantimonic acid as a catalyst in hydrocarbon processing: Fluoroantimonic acid is utilized as a powerful catalyst in various hydrocarbon processing applications, including isomerization, alkylation, and cracking reactions. Its super-acidic properties enable efficient conversion of hydrocarbons under milder conditions compared to traditional catalysts.
- Fluoroantimonic acid in polymer synthesis and modification: The catalytic activity of fluoroantimonic acid is exploited in polymer synthesis and modification processes. It can initiate polymerization reactions, facilitate polymer cross-linking, and enable the production of specialized polymers with unique properties.
- Application of fluoroantimonic acid in organic synthesis: Fluoroantimonic acid serves as a potent catalyst in various organic synthesis reactions, including Friedel-Crafts reactions, rearrangements, and carbon-carbon bond formations. Its strong acidity enables challenging transformations under mild conditions.
- Fluoroantimonic acid in materials science and nanotechnology: The unique properties of fluoroantimonic acid are utilized in materials science and nanotechnology applications. It can be used for surface modification of materials, etching processes, and the synthesis of nanostructures with specific properties.
- Safety and handling considerations for fluoroantimonic acid catalysis: Due to its extreme acidity and reactivity, special safety measures and handling protocols are necessary when working with fluoroantimonic acid as a catalyst. This includes the use of specialized equipment, containment systems, and proper neutralization procedures.
02 Fluoroantimonic acid in polymer synthesis and modification
The catalytic activity of fluoroantimonic acid is exploited in polymer chemistry for initiating polymerization reactions and modifying existing polymers. It can facilitate the synthesis of high-performance polymers and enable the functionalization of polymer chains.Expand Specific Solutions03 Application in organic synthesis and fine chemical production
Fluoroantimonic acid catalysis is employed in the synthesis of complex organic molecules and fine chemicals. Its strong acidity promotes various organic transformations, including rearrangements, additions, and eliminations, leading to the formation of valuable chemical intermediates and products.Expand Specific Solutions04 Use in electrochemical applications and energy storage
Fluoroantimonic acid finds applications in electrochemical systems and energy storage devices. It can be used as an electrolyte component or catalyst in fuel cells, batteries, and other electrochemical technologies to enhance performance and efficiency.Expand Specific Solutions05 Safety and handling considerations in fluoroantimonic acid catalysis
Due to its extreme acidity and reactivity, special safety measures and handling protocols are necessary when working with fluoroantimonic acid as a catalyst. This includes the development of specialized equipment, containment systems, and neutralization procedures to ensure safe and controlled use in industrial and laboratory settings.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research and Industry
The research on catalysis innovations using fluoroantimonic acid is in a nascent stage, with significant potential for growth. The market size is relatively small but expanding as industries recognize the acid's superacidity properties. Technologically, it's still developing, with varying levels of maturity across companies. Leaders like 3M Innovative Properties Co., DuPont de Nemours, Inc., and BASF Corp. are at the forefront, leveraging their extensive R&D capabilities. Academic institutions such as The Regents of the University of California and Osaka University are contributing fundamental research. Emerging players like Zhejiang Shangyu Lixing Chemical Co., Ltd and Jiangsu Hengli Chemical Fiber Co., Ltd are also making strides, particularly in application-specific developments. The competitive landscape is diverse, with both established chemical giants and specialized firms vying for breakthroughs in this challenging yet promising field.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a novel catalytic system using fluoroantimonic acid for the production of high-performance fluoropolymers. Their approach involves a controlled reaction environment where fluoroantimonic acid acts as a super-acid catalyst, enabling the polymerization of fluorinated monomers under milder conditions. This innovative process allows for better control over molecular weight distribution and reduces the formation of unwanted by-products, resulting in fluoropolymers with enhanced thermal stability and chemical resistance. DuPont's research also focuses on optimizing the recovery and recycling of the fluoroantimonic acid catalyst, addressing environmental concerns associated with its use.
Strengths: Improved product quality, reduced energy consumption, and potential for catalyst recycling. Weaknesses: High cost of fluoroantimonic acid and potential safety concerns due to its corrosive nature.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has pioneered a fluoroantimonic acid-based catalytic system for the synthesis of specialty chemicals and advanced materials. Their approach utilizes a proprietary stabilization technique that allows for the controlled use of fluoroantimonic acid in organic transformations. This innovation enables the company to perform challenging carbon-carbon bond formations and isomerizations under relatively mild conditions. Eastman's research has also led to the development of novel reactor designs that can withstand the highly corrosive nature of fluoroantimonic acid, allowing for scaled-up production of high-value chemicals. Additionally, they have made significant progress in the recovery and purification of the acid catalyst, improving the overall efficiency and sustainability of their processes.
Strengths: Enables production of unique specialty chemicals, improved reaction selectivity, and potential for process intensification. Weaknesses: High equipment costs due to corrosion-resistant materials and potential environmental concerns.
Core Innovations in Fluoroantimonic Acid Catalysis
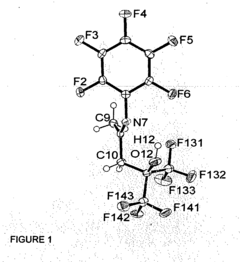
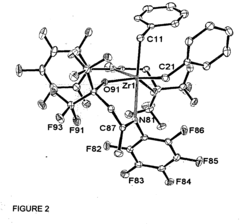
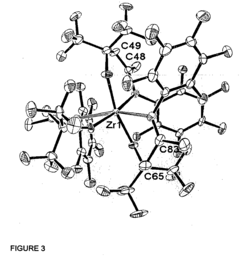
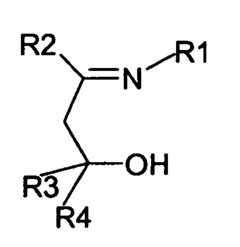
Fluorinated alkoxy-imino catalyst components
PatentInactiveEP2064218B1
Innovation
- The development of fluorinated alcohol-imine pro-ligands with perfluorinated alkyl groups, which act as strongly electron-withdrawing groups to inhibit aggregation and increase metal electrophilicity, leading to the formation of active catalyst components for olefin polymerization or oligomerization, including the metallation of these pro-ligands with metal salts and activation with aluminoxane or boron-containing agents.
Asymmetric electrophilic fluorination using an anionic chiral phase-transfer catalyst
PatentActiveUS20140350253A1
Innovation
- Development of chiral anion phase-transfer catalysts that facilitate electrophilic addition reactions, allowing for enantioselective fluorination using stable and inexpensive reagents, and enabling the formation of fluorinated compounds that can be further functionalized.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated at -21.6. Its extreme corrosiveness and reactivity necessitate rigorous safety protocols and specialized handling procedures. When working with this compound, researchers must prioritize personal protection equipment (PPE) including chemical-resistant suits, gloves, and full-face respirators with appropriate filters. All operations involving fluoroantimonic acid should be conducted in a properly functioning fume hood with a face velocity of at least 100 feet per minute.
Due to its highly hygroscopic nature, fluoroantimonic acid must be stored and handled under strictly anhydrous conditions. Exposure to moisture can lead to violent reactions and the release of toxic hydrogen fluoride gas. Therefore, storage containers must be airtight and composed of materials resistant to both hydrofluoric acid and antimony pentafluoride, such as PTFE (Teflon) or certain fluorinated polymers. Regular inspections of storage vessels and transfer equipment are crucial to prevent leaks or degradation.
When transporting fluoroantimonic acid, even within the laboratory, secondary containment is essential. Double containment systems made of compatible materials should be used, with absorbent materials placed between the primary and secondary containers to capture any potential leaks. Proper labeling and hazard communication are vital, including clear identification of the compound, associated hazards, and necessary precautions.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill control kits specifically designed for superacids. Neutralization agents such as sodium bicarbonate or calcium oxide should be available in sufficient quantities. All personnel working with or in proximity to fluoroantimonic acid must be thoroughly trained in emergency protocols, including evacuation procedures and the use of specialized first-aid techniques for acid exposures.
Waste management is another critical aspect of fluoroantimonic acid handling. Neutralization and disposal must be carried out with extreme caution, typically involving gradual dilution with cold water followed by neutralization with appropriate bases. This process should only be performed by trained professionals in designated areas with proper ventilation and containment systems. Disposal of neutralized waste must comply with local, state, and federal regulations for hazardous materials.
Given the catalytic potential of fluoroantimonic acid in various chemical processes, researchers must also consider the safety implications of reaction products and byproducts. Comprehensive risk assessments should be conducted for each experimental protocol, taking into account potential side reactions, heat generation, and the formation of volatile or toxic compounds. Scaling up reactions involving fluoroantimonic acid requires additional safety considerations and often necessitates the use of specialized reaction vessels and monitoring equipment.
Due to its highly hygroscopic nature, fluoroantimonic acid must be stored and handled under strictly anhydrous conditions. Exposure to moisture can lead to violent reactions and the release of toxic hydrogen fluoride gas. Therefore, storage containers must be airtight and composed of materials resistant to both hydrofluoric acid and antimony pentafluoride, such as PTFE (Teflon) or certain fluorinated polymers. Regular inspections of storage vessels and transfer equipment are crucial to prevent leaks or degradation.
When transporting fluoroantimonic acid, even within the laboratory, secondary containment is essential. Double containment systems made of compatible materials should be used, with absorbent materials placed between the primary and secondary containers to capture any potential leaks. Proper labeling and hazard communication are vital, including clear identification of the compound, associated hazards, and necessary precautions.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill control kits specifically designed for superacids. Neutralization agents such as sodium bicarbonate or calcium oxide should be available in sufficient quantities. All personnel working with or in proximity to fluoroantimonic acid must be thoroughly trained in emergency protocols, including evacuation procedures and the use of specialized first-aid techniques for acid exposures.
Waste management is another critical aspect of fluoroantimonic acid handling. Neutralization and disposal must be carried out with extreme caution, typically involving gradual dilution with cold water followed by neutralization with appropriate bases. This process should only be performed by trained professionals in designated areas with proper ventilation and containment systems. Disposal of neutralized waste must comply with local, state, and federal regulations for hazardous materials.
Given the catalytic potential of fluoroantimonic acid in various chemical processes, researchers must also consider the safety implications of reaction products and byproducts. Comprehensive risk assessments should be conducted for each experimental protocol, taking into account potential side reactions, heat generation, and the formation of volatile or toxic compounds. Scaling up reactions involving fluoroantimonic acid requires additional safety considerations and often necessitates the use of specialized reaction vessels and monitoring equipment.
Environmental Impact of Fluoroantimonic Acid Catalysis
Fluoroantimonic acid catalysis, while offering significant advantages in various chemical processes, poses substantial environmental concerns that require careful consideration. The use of this superacid in catalytic reactions can lead to the generation of hazardous waste streams containing highly corrosive and toxic substances. These byproducts, if not properly managed, can cause severe damage to ecosystems and pose risks to human health.
One of the primary environmental impacts of fluoroantimonic acid catalysis is the potential for soil and water contamination. The acid's extreme corrosiveness can lead to the degradation of containment materials, increasing the risk of leaks and spills. When released into the environment, fluoroantimonic acid can cause rapid acidification of soil and water bodies, disrupting pH balances and potentially harming flora and fauna. The fluoride and antimony components of the acid can also accumulate in the food chain, leading to long-term ecological consequences.
Air pollution is another significant concern associated with fluoroantimonic acid catalysis. The volatile nature of the acid and its precursors can result in the release of harmful fumes and particulates into the atmosphere. These emissions may contribute to the formation of acid rain and smog, further exacerbating environmental degradation on a broader scale. Additionally, the production and handling of fluoroantimonic acid require stringent safety measures, which often involve energy-intensive processes, indirectly contributing to greenhouse gas emissions.
The disposal of waste products from fluoroantimonic acid catalysis presents a considerable challenge. Conventional wastewater treatment methods may be inadequate for neutralizing and safely disposing of these highly acidic and toxic substances. Specialized treatment facilities and protocols are necessary to prevent environmental contamination, adding to the overall environmental footprint of processes utilizing this catalyst.
Furthermore, the production of fluoroantimonic acid itself raises environmental concerns. The synthesis of this superacid typically involves the use of hydrofluoric acid and antimony pentafluoride, both of which are associated with their own set of environmental hazards. The mining and processing of antimony, a key component of the acid, can lead to habitat destruction and the release of toxic substances into the environment.
To mitigate these environmental impacts, researchers and industry practitioners are exploring alternative catalysts and process improvements. Efforts are being made to develop greener catalytic systems that maintain high efficiency while reducing the reliance on hazardous substances. Additionally, advancements in containment technologies and waste treatment methods are crucial for minimizing the environmental risks associated with fluoroantimonic acid catalysis.
One of the primary environmental impacts of fluoroantimonic acid catalysis is the potential for soil and water contamination. The acid's extreme corrosiveness can lead to the degradation of containment materials, increasing the risk of leaks and spills. When released into the environment, fluoroantimonic acid can cause rapid acidification of soil and water bodies, disrupting pH balances and potentially harming flora and fauna. The fluoride and antimony components of the acid can also accumulate in the food chain, leading to long-term ecological consequences.
Air pollution is another significant concern associated with fluoroantimonic acid catalysis. The volatile nature of the acid and its precursors can result in the release of harmful fumes and particulates into the atmosphere. These emissions may contribute to the formation of acid rain and smog, further exacerbating environmental degradation on a broader scale. Additionally, the production and handling of fluoroantimonic acid require stringent safety measures, which often involve energy-intensive processes, indirectly contributing to greenhouse gas emissions.
The disposal of waste products from fluoroantimonic acid catalysis presents a considerable challenge. Conventional wastewater treatment methods may be inadequate for neutralizing and safely disposing of these highly acidic and toxic substances. Specialized treatment facilities and protocols are necessary to prevent environmental contamination, adding to the overall environmental footprint of processes utilizing this catalyst.
Furthermore, the production of fluoroantimonic acid itself raises environmental concerns. The synthesis of this superacid typically involves the use of hydrofluoric acid and antimony pentafluoride, both of which are associated with their own set of environmental hazards. The mining and processing of antimony, a key component of the acid, can lead to habitat destruction and the release of toxic substances into the environment.
To mitigate these environmental impacts, researchers and industry practitioners are exploring alternative catalysts and process improvements. Efforts are being made to develop greener catalytic systems that maintain high efficiency while reducing the reliance on hazardous substances. Additionally, advancements in containment technologies and waste treatment methods are crucial for minimizing the environmental risks associated with fluoroantimonic acid catalysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!