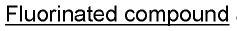Pioneering New Synthesis Pathways with Fluoroantimonic Acid
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Synthesis Background
Fluoroantimonic acid, a superacid with extraordinary chemical properties, has been a subject of intense research and development in the field of synthetic chemistry for several decades. Its synthesis background can be traced back to the early 20th century when the concept of superacids was first introduced by James Bryant Conant in 1927. However, it wasn't until the 1960s that George A. Olah and his team made significant breakthroughs in the synthesis and characterization of fluoroantimonic acid.
The development of fluoroantimonic acid synthesis techniques has been driven by the need for powerful catalysts in various industrial processes, particularly in the petrochemical industry. The unique ability of this superacid to protonate even weak bases and its extreme acidity (estimated to be more than a quadrillion times stronger than 100% sulfuric acid) have made it an invaluable tool in organic synthesis and hydrocarbon chemistry.
Traditional synthesis methods for fluoroantimonic acid involve the combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5) in specific ratios. This process requires extreme caution due to the highly corrosive and reactive nature of the components. Over the years, researchers have focused on optimizing the synthesis conditions, including temperature control, pressure regulation, and the use of specialized containment materials to handle the aggressive nature of the acid.
The evolution of fluoroantimonic acid synthesis has been closely tied to advancements in materials science and chemical engineering. The development of fluoropolymers and other resistant materials has been crucial in enabling safer and more efficient production methods. Additionally, the introduction of advanced analytical techniques, such as NMR spectroscopy and computational chemistry, has allowed for a deeper understanding of the acid's structure and behavior, further refining synthesis protocols.
Recent years have seen a growing interest in exploring alternative synthesis pathways for fluoroantimonic acid. This interest is driven by several factors, including the need for more environmentally friendly production methods, improved safety protocols, and the desire to enhance the acid's properties for specific applications. Researchers are investigating novel precursors, catalytic systems, and reaction conditions that could potentially lead to more efficient and sustainable synthesis routes.
The ongoing research in fluoroantimonic acid synthesis is not only focused on improving the production process but also on expanding its applications. As new fields such as nanotechnology and advanced materials science emerge, the demand for superacids with tailored properties continues to grow. This has led to exploration of modified synthesis pathways that could yield fluoroantimonic acid derivatives with enhanced stability, selectivity, or reactivity for specific chemical transformations.
The development of fluoroantimonic acid synthesis techniques has been driven by the need for powerful catalysts in various industrial processes, particularly in the petrochemical industry. The unique ability of this superacid to protonate even weak bases and its extreme acidity (estimated to be more than a quadrillion times stronger than 100% sulfuric acid) have made it an invaluable tool in organic synthesis and hydrocarbon chemistry.
Traditional synthesis methods for fluoroantimonic acid involve the combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5) in specific ratios. This process requires extreme caution due to the highly corrosive and reactive nature of the components. Over the years, researchers have focused on optimizing the synthesis conditions, including temperature control, pressure regulation, and the use of specialized containment materials to handle the aggressive nature of the acid.
The evolution of fluoroantimonic acid synthesis has been closely tied to advancements in materials science and chemical engineering. The development of fluoropolymers and other resistant materials has been crucial in enabling safer and more efficient production methods. Additionally, the introduction of advanced analytical techniques, such as NMR spectroscopy and computational chemistry, has allowed for a deeper understanding of the acid's structure and behavior, further refining synthesis protocols.
Recent years have seen a growing interest in exploring alternative synthesis pathways for fluoroantimonic acid. This interest is driven by several factors, including the need for more environmentally friendly production methods, improved safety protocols, and the desire to enhance the acid's properties for specific applications. Researchers are investigating novel precursors, catalytic systems, and reaction conditions that could potentially lead to more efficient and sustainable synthesis routes.
The ongoing research in fluoroantimonic acid synthesis is not only focused on improving the production process but also on expanding its applications. As new fields such as nanotechnology and advanced materials science emerge, the demand for superacids with tailored properties continues to grow. This has led to exploration of modified synthesis pathways that could yield fluoroantimonic acid derivatives with enhanced stability, selectivity, or reactivity for specific chemical transformations.
Market Demand Analysis
The market demand for fluoroantimonic acid and its potential applications in pioneering new synthesis pathways is experiencing significant growth across various industries. This superacid, known for its extreme acidity and unique chemical properties, is finding increasing relevance in sectors such as petrochemicals, pharmaceuticals, and advanced materials manufacturing.
In the petrochemical industry, fluoroantimonic acid is gaining traction as a catalyst for isomerization and alkylation processes. Its ability to facilitate these reactions under milder conditions than traditional catalysts is driving interest from major oil and gas companies seeking to optimize their refining processes. The global petrochemical market, valued at over $450 billion, is expected to grow at a CAGR of 5% through 2025, indicating a substantial potential market for fluoroantimonic acid applications.
The pharmaceutical sector represents another key area of demand growth. As drug discovery becomes increasingly complex, researchers are exploring novel synthesis pathways to create new molecular entities. Fluoroantimonic acid's unique properties make it a valuable tool in developing these pathways, particularly for the synthesis of complex organic compounds. With the global pharmaceutical market projected to reach $1.5 trillion by 2023, the demand for innovative synthesis methods is likely to surge.
In advanced materials manufacturing, fluoroantimonic acid is finding applications in the production of high-performance polymers and specialty chemicals. Its ability to catalyze reactions that are difficult or impossible with conventional acids is opening new avenues for material scientists. The global specialty chemicals market, valued at $630 billion in 2019, is expected to grow at a CAGR of 4% through 2024, providing a substantial market opportunity for fluoroantimonic acid-based processes.
The electronics industry is also showing interest in fluoroantimonic acid for potential applications in semiconductor etching and surface modification. As the demand for smaller, more powerful electronic devices continues to grow, the need for advanced manufacturing processes increases. The global semiconductor market, projected to reach $726 billion by 2027, represents a significant potential market for fluoroantimonic acid applications.
Despite the growing demand, challenges remain in the widespread adoption of fluoroantimonic acid. Safety concerns and handling difficulties due to its extreme reactivity are primary obstacles. However, ongoing research into safer handling methods and containment systems is expected to address these issues, potentially leading to broader market acceptance and application.
In the petrochemical industry, fluoroantimonic acid is gaining traction as a catalyst for isomerization and alkylation processes. Its ability to facilitate these reactions under milder conditions than traditional catalysts is driving interest from major oil and gas companies seeking to optimize their refining processes. The global petrochemical market, valued at over $450 billion, is expected to grow at a CAGR of 5% through 2025, indicating a substantial potential market for fluoroantimonic acid applications.
The pharmaceutical sector represents another key area of demand growth. As drug discovery becomes increasingly complex, researchers are exploring novel synthesis pathways to create new molecular entities. Fluoroantimonic acid's unique properties make it a valuable tool in developing these pathways, particularly for the synthesis of complex organic compounds. With the global pharmaceutical market projected to reach $1.5 trillion by 2023, the demand for innovative synthesis methods is likely to surge.
In advanced materials manufacturing, fluoroantimonic acid is finding applications in the production of high-performance polymers and specialty chemicals. Its ability to catalyze reactions that are difficult or impossible with conventional acids is opening new avenues for material scientists. The global specialty chemicals market, valued at $630 billion in 2019, is expected to grow at a CAGR of 4% through 2024, providing a substantial market opportunity for fluoroantimonic acid-based processes.
The electronics industry is also showing interest in fluoroantimonic acid for potential applications in semiconductor etching and surface modification. As the demand for smaller, more powerful electronic devices continues to grow, the need for advanced manufacturing processes increases. The global semiconductor market, projected to reach $726 billion by 2027, represents a significant potential market for fluoroantimonic acid applications.
Despite the growing demand, challenges remain in the widespread adoption of fluoroantimonic acid. Safety concerns and handling difficulties due to its extreme reactivity are primary obstacles. However, ongoing research into safer handling methods and containment systems is expected to address these issues, potentially leading to broader market acceptance and application.
Current Challenges
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its application for pioneering new synthesis pathways. The primary obstacle lies in its extreme reactivity and corrosiveness, which severely limits the range of materials that can safely contain and handle it. Traditional laboratory glassware and most metals are rapidly degraded upon contact, necessitating specialized equipment made from highly resistant materials such as Teflon or certain fluoropolymers.
The high reactivity of fluoroantimonic acid also poses substantial safety risks to researchers and laboratory personnel. Stringent safety protocols and advanced protective equipment are essential, significantly increasing the complexity and cost of experiments. Moreover, the acid's extreme moisture sensitivity complicates its storage and handling, requiring rigorously anhydrous conditions that are challenging to maintain consistently in practical laboratory settings.
Another major challenge is the difficulty in controlling and fine-tuning reactions involving fluoroantimonic acid. Its exceptional strength often leads to over-fluorination or unwanted side reactions, making it challenging to achieve selective transformations. This lack of selectivity limits its applicability in complex organic synthesis where precise control over reaction outcomes is crucial.
The environmental impact of fluoroantimonic acid is also a significant concern. Its production, use, and disposal require extensive safety measures to prevent environmental contamination. The acid's potential for causing severe ecological damage if accidentally released has led to strict regulations governing its use, further complicating research efforts.
From an analytical perspective, characterizing reaction intermediates and products formed in fluoroantimonic acid media presents unique challenges. Many standard analytical techniques are incompatible with such an aggressive medium, necessitating the development of specialized analytical methods and equipment.
The scalability of processes involving fluoroantimonic acid is another critical issue. While small-scale laboratory experiments may be feasible, scaling up to industrial production levels introduces significant engineering and safety challenges that have yet to be fully addressed. This limitation hinders the practical application of potentially valuable synthetic pathways discovered using this superacid.
Lastly, the high cost and limited availability of fluoroantimonic acid restrict its widespread use in research and development. This economic barrier, combined with the technical challenges, has led many researchers to seek alternative approaches or milder reaction conditions, potentially overlooking valuable synthetic opportunities that this unique reagent might offer.
The high reactivity of fluoroantimonic acid also poses substantial safety risks to researchers and laboratory personnel. Stringent safety protocols and advanced protective equipment are essential, significantly increasing the complexity and cost of experiments. Moreover, the acid's extreme moisture sensitivity complicates its storage and handling, requiring rigorously anhydrous conditions that are challenging to maintain consistently in practical laboratory settings.
Another major challenge is the difficulty in controlling and fine-tuning reactions involving fluoroantimonic acid. Its exceptional strength often leads to over-fluorination or unwanted side reactions, making it challenging to achieve selective transformations. This lack of selectivity limits its applicability in complex organic synthesis where precise control over reaction outcomes is crucial.
The environmental impact of fluoroantimonic acid is also a significant concern. Its production, use, and disposal require extensive safety measures to prevent environmental contamination. The acid's potential for causing severe ecological damage if accidentally released has led to strict regulations governing its use, further complicating research efforts.
From an analytical perspective, characterizing reaction intermediates and products formed in fluoroantimonic acid media presents unique challenges. Many standard analytical techniques are incompatible with such an aggressive medium, necessitating the development of specialized analytical methods and equipment.
The scalability of processes involving fluoroantimonic acid is another critical issue. While small-scale laboratory experiments may be feasible, scaling up to industrial production levels introduces significant engineering and safety challenges that have yet to be fully addressed. This limitation hinders the practical application of potentially valuable synthetic pathways discovered using this superacid.
Lastly, the high cost and limited availability of fluoroantimonic acid restrict its widespread use in research and development. This economic barrier, combined with the technical challenges, has led many researchers to seek alternative approaches or milder reaction conditions, potentially overlooking valuable synthetic opportunities that this unique reagent might offer.
Existing Synthesis Methods
01 Direct synthesis from antimony pentafluoride and hydrogen fluoride
The most common method for synthesizing fluoroantimonic acid involves the direct reaction of antimony pentafluoride (SbF5) with anhydrous hydrogen fluoride (HF). This reaction is typically carried out under controlled conditions, often at low temperatures, to produce the highly reactive and corrosive fluoroantimonic acid.- Direct synthesis from antimony pentafluoride and hydrogen fluoride: The most common method for synthesizing fluoroantimonic acid involves the direct reaction of antimony pentafluoride (SbF5) with anhydrous hydrogen fluoride (HF). This reaction is typically carried out under controlled conditions, often at low temperatures, to produce the highly reactive and corrosive fluoroantimonic acid.
- Electrochemical synthesis methods: Electrochemical techniques can be employed to synthesize fluoroantimonic acid. These methods often involve the electrolysis of antimony-containing compounds in the presence of fluoride-rich electrolytes. The electrochemical approach allows for better control over the reaction conditions and can potentially yield higher purity products.
- Synthesis using alternative fluorinating agents: Some pathways for fluoroantimonic acid synthesis utilize alternative fluorinating agents instead of hydrogen fluoride. These may include compounds such as xenon difluoride or other strong fluorinating agents. This approach can offer advantages in terms of handling and safety compared to the use of highly corrosive HF.
- Continuous flow synthesis methods: Continuous flow reactors have been developed for the synthesis of fluoroantimonic acid. These systems allow for better control of reaction parameters, improved safety, and potentially higher yields. The continuous nature of the process can also facilitate easier scale-up for industrial production.
- Purification and stabilization techniques: Various methods have been developed for the purification and stabilization of fluoroantimonic acid after synthesis. These techniques aim to remove impurities and increase the shelf life of the highly reactive compound. Approaches may include distillation, recrystallization, or the use of stabilizing additives.
02 Electrochemical synthesis methods
Electrochemical techniques can be employed to synthesize fluoroantimonic acid. These methods often involve the electrolysis of antimony-containing compounds in the presence of fluoride-rich electrolytes. The electrochemical approach allows for better control over the reaction conditions and can potentially yield higher purity products.Expand Specific Solutions03 Use of alternative fluorinating agents
Some synthesis pathways for fluoroantimonic acid explore the use of alternative fluorinating agents instead of hydrogen fluoride. These may include other fluorine-containing compounds or complexes that can react with antimony compounds to produce the desired acid. This approach aims to improve safety and handling during the synthesis process.Expand Specific Solutions04 Continuous flow synthesis techniques
Continuous flow reactors and microfluidic systems have been developed for the synthesis of fluoroantimonic acid. These techniques allow for better control of reaction parameters, improved heat management, and potentially safer handling of the highly reactive intermediates and products involved in the synthesis.Expand Specific Solutions05 Purification and stabilization methods
Various methods have been developed for the purification and stabilization of fluoroantimonic acid after synthesis. These may include distillation techniques, use of stabilizing additives, or specialized storage and handling procedures to maintain the purity and reactivity of the acid for extended periods.Expand Specific Solutions
Key Industry Players
The development of new synthesis pathways using fluoroantimonic acid is in its early stages, with significant potential for growth. The market size is relatively small but expanding as researchers explore applications in organic synthesis and catalysis. Technological maturity varies among key players, with companies like Merck Sharp & Dohme Corp., BASF Corp., and 3M Innovative Properties Co. leading in industrial applications. Academic institutions such as Hunan University and the University of Kansas are advancing fundamental research. Collaborations between industry and academia, exemplified by partnerships involving Forschungszentrum Jülich GmbH and Westfälische Wilhelms-Universität Münster, are driving innovation in this challenging field. The competitive landscape is characterized by a mix of established chemical companies and emerging research-focused entities, all vying to unlock the full potential of fluoroantimonic acid in synthetic chemistry.
Merck Sharp & Dohme Corp.
Technical Solution: Merck Sharp & Dohme has pioneered a fluoroantimonic acid-mediated synthesis pathway for complex pharmaceutical intermediates. Their approach utilizes a controlled-release system for fluoroantimonic acid, allowing for precise regulation of acid strength during different stages of the reaction. This method incorporates a series of neutralization and extraction steps to isolate the desired products efficiently. The company has also developed a novel recycling process for the acid, significantly reducing waste and improving the overall sustainability of the synthesis.
Strengths: Precise control over reaction conditions, efficient product isolation, and improved sustainability. Weaknesses: Complex process control requirements, potential scalability issues.
AbbVie, Inc.
Technical Solution: AbbVie has pioneered a fluoroantimonic acid-mediated synthesis pathway for creating novel pharmaceutical scaffolds. Their approach involves a stepwise process where fluoroantimonic acid is used to activate inert functional groups, followed by a series of controlled additions and cyclizations to build complex molecular structures. The company has developed a unique microreactor system that allows for precise control over reaction parameters and minimizes exposure risks. Additionally, AbbVie has implemented an advanced purification process that effectively removes trace amounts of fluoroantimonic acid from the final products, ensuring their safety for pharmaceutical applications.
Strengths: Enables synthesis of complex pharmaceutical scaffolds, high purity of final products. Weaknesses: High development costs, potential regulatory challenges due to the use of highly reactive reagents.
Core Innovations
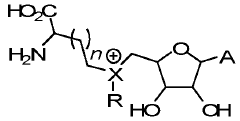
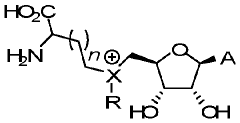
Methods and cells for the production of fluorinated compounds
PatentWO2023170058A1
Innovation
- A cell expressing specific enzymes such as isomerase, aldolase, acetylating acetaldehyde dehydrogenase, fluoroacetaldehyde dehydrogenase, and a non-conventional fluorinase from Methanosaeta sp. Ptall1.BinO55, capable of producing fluorinated compounds like F-acetaldehyde, F-ethanol, and F-acetyl-CoA from glucose and fluoride, using pathways that include phosphorylation and fluorination steps.
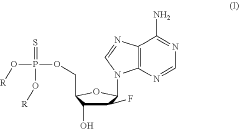
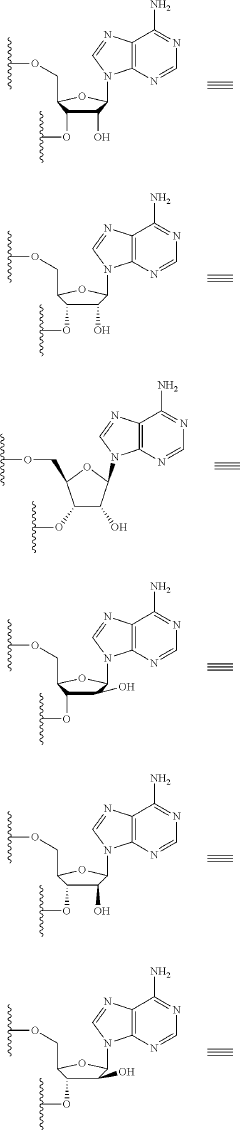
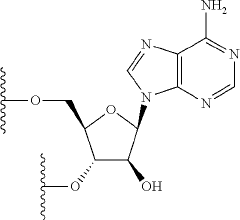
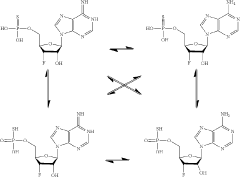
Synthesis of fluorinated nucleotides
PatentPendingUS20230287032A1
Innovation
- A more efficient synthetic route using stereo-controlled electrophilic fluorination and glycosylation, employing a new class of organocatalysts to reduce dimeric and polymeric impurities, and avoiding corrosive aminosulfurane-based fluorinating reagents, resulting in a shorter process with improved stereoselectivity and the use of stable crystalline intermediates.
Safety and Handling Protocols
Fluoroantimonic acid, recognized as one of the strongest superacids, demands rigorous safety and handling protocols due to its extreme corrosiveness and reactivity. Proper personal protective equipment (PPE) is paramount when working with this substance. Researchers must wear fully encapsulating chemical-resistant suits, including gloves, boots, and face shields. Respiratory protection with supplied air or self-contained breathing apparatus is essential to prevent inhalation of toxic fumes.
Laboratory infrastructure for handling fluoroantimonic acid requires specialized materials resistant to its corrosive nature. All containment vessels, transfer lines, and reaction chambers must be constructed from materials such as fluoropolymers (e.g., PTFE, PFA) or certain high-purity metals like tantalum or iridium. Standard glassware is unsuitable and will rapidly degrade upon contact.
Strict environmental controls are necessary to mitigate risks associated with potential releases. Work should be conducted in a dedicated fume hood with a robust ventilation system capable of handling corrosive fumes. Emergency shower and eyewash stations must be readily accessible in the immediate vicinity of the work area.
Storage protocols for fluoroantimonic acid are equally critical. The acid must be kept in tightly sealed, fluoropolymer containers within secondary containment systems. Storage areas should be cool, dry, and well-ventilated, with restricted access limited to trained personnel only. Regular inspections of storage containers for signs of degradation or leakage are essential.
Waste management and disposal procedures require careful consideration. Neutralization of fluoroantimonic acid waste should be performed with extreme caution, typically involving slow addition to large volumes of crushed ice or very cold water, followed by neutralization with bases such as sodium hydroxide or calcium hydroxide. Disposal must comply with all relevant local, state, and federal regulations for hazardous waste.
Emergency response planning is crucial when working with fluoroantimonic acid. Detailed spill response procedures must be established and regularly practiced. This includes the availability of appropriate spill control materials, such as fluorinated absorbents, and specific decontamination protocols for affected areas and personnel.
Training programs for all personnel involved in handling fluoroantimonic acid should be comprehensive and ongoing. These programs must cover not only the specific handling procedures but also the chemical properties, health hazards, and emergency response measures associated with the acid.
Laboratory infrastructure for handling fluoroantimonic acid requires specialized materials resistant to its corrosive nature. All containment vessels, transfer lines, and reaction chambers must be constructed from materials such as fluoropolymers (e.g., PTFE, PFA) or certain high-purity metals like tantalum or iridium. Standard glassware is unsuitable and will rapidly degrade upon contact.
Strict environmental controls are necessary to mitigate risks associated with potential releases. Work should be conducted in a dedicated fume hood with a robust ventilation system capable of handling corrosive fumes. Emergency shower and eyewash stations must be readily accessible in the immediate vicinity of the work area.
Storage protocols for fluoroantimonic acid are equally critical. The acid must be kept in tightly sealed, fluoropolymer containers within secondary containment systems. Storage areas should be cool, dry, and well-ventilated, with restricted access limited to trained personnel only. Regular inspections of storage containers for signs of degradation or leakage are essential.
Waste management and disposal procedures require careful consideration. Neutralization of fluoroantimonic acid waste should be performed with extreme caution, typically involving slow addition to large volumes of crushed ice or very cold water, followed by neutralization with bases such as sodium hydroxide or calcium hydroxide. Disposal must comply with all relevant local, state, and federal regulations for hazardous waste.
Emergency response planning is crucial when working with fluoroantimonic acid. Detailed spill response procedures must be established and regularly practiced. This includes the availability of appropriate spill control materials, such as fluorinated absorbents, and specific decontamination protocols for affected areas and personnel.
Training programs for all personnel involved in handling fluoroantimonic acid should be comprehensive and ongoing. These programs must cover not only the specific handling procedures but also the chemical properties, health hazards, and emergency response measures associated with the acid.
Environmental Impact Assessment
The use of fluoroantimonic acid in pioneering new synthesis pathways raises significant environmental concerns that require careful assessment. As one of the strongest known superacids, fluoroantimonic acid poses potential risks to ecosystems and human health if not properly managed. Its highly corrosive nature and ability to react violently with water necessitate stringent containment measures to prevent accidental releases.
Environmental impact assessments must consider the entire lifecycle of fluoroantimonic acid use, from production to disposal. During synthesis processes, there is a risk of atmospheric emissions of hydrogen fluoride and antimony pentafluoride, both of which are toxic gases that can contribute to air pollution and acid rain formation. Proper scrubbing systems and emission controls are essential to mitigate these risks.
Water contamination is another critical concern. Even small amounts of fluoroantimonic acid can severely alter the pH of water bodies, potentially causing widespread damage to aquatic ecosystems. Groundwater contamination risks must be carefully evaluated, particularly in areas with vulnerable aquifers. Robust waste management protocols are crucial to prevent any discharge into water systems.
Soil contamination presents long-term environmental challenges. Fluoroantimonic acid can persist in soil, altering its chemical composition and potentially rendering it unsuitable for plant growth. This could have cascading effects on local flora and fauna, disrupting entire ecosystems. Remediation of contaminated soil can be extremely costly and technically challenging.
The production and use of fluoroantimonic acid also have implications for resource consumption and energy use. The synthesis process requires significant energy inputs and specialized materials, contributing to the overall environmental footprint. Life cycle assessments should quantify these impacts to inform decision-making on the sustainability of new synthesis pathways.
Biodiversity impacts must be carefully considered, especially if industrial-scale use of fluoroantimonic acid is proposed. The potential for bioaccumulation of fluorine and antimony compounds in food chains could pose risks to wildlife and ecosystem health. Long-term monitoring programs may be necessary to detect and mitigate any unforeseen ecological effects.
Given these environmental considerations, the development of new synthesis pathways using fluoroantimonic acid must be accompanied by rigorous safety protocols, containment strategies, and emergency response plans. Continuous environmental monitoring and regular impact assessments should be integral to any research or industrial application of this superacid. Additionally, exploring alternative, less environmentally hazardous synthesis methods should be a parallel priority to balance innovation with environmental stewardship.
Environmental impact assessments must consider the entire lifecycle of fluoroantimonic acid use, from production to disposal. During synthesis processes, there is a risk of atmospheric emissions of hydrogen fluoride and antimony pentafluoride, both of which are toxic gases that can contribute to air pollution and acid rain formation. Proper scrubbing systems and emission controls are essential to mitigate these risks.
Water contamination is another critical concern. Even small amounts of fluoroantimonic acid can severely alter the pH of water bodies, potentially causing widespread damage to aquatic ecosystems. Groundwater contamination risks must be carefully evaluated, particularly in areas with vulnerable aquifers. Robust waste management protocols are crucial to prevent any discharge into water systems.
Soil contamination presents long-term environmental challenges. Fluoroantimonic acid can persist in soil, altering its chemical composition and potentially rendering it unsuitable for plant growth. This could have cascading effects on local flora and fauna, disrupting entire ecosystems. Remediation of contaminated soil can be extremely costly and technically challenging.
The production and use of fluoroantimonic acid also have implications for resource consumption and energy use. The synthesis process requires significant energy inputs and specialized materials, contributing to the overall environmental footprint. Life cycle assessments should quantify these impacts to inform decision-making on the sustainability of new synthesis pathways.
Biodiversity impacts must be carefully considered, especially if industrial-scale use of fluoroantimonic acid is proposed. The potential for bioaccumulation of fluorine and antimony compounds in food chains could pose risks to wildlife and ecosystem health. Long-term monitoring programs may be necessary to detect and mitigate any unforeseen ecological effects.
Given these environmental considerations, the development of new synthesis pathways using fluoroantimonic acid must be accompanied by rigorous safety protocols, containment strategies, and emergency response plans. Continuous environmental monitoring and regular impact assessments should be integral to any research or industrial application of this superacid. Additionally, exploring alternative, less environmentally hazardous synthesis methods should be a parallel priority to balance innovation with environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!