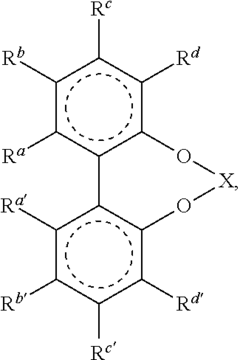
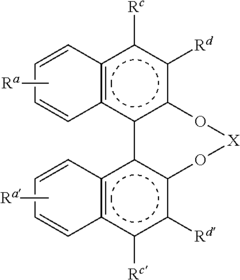
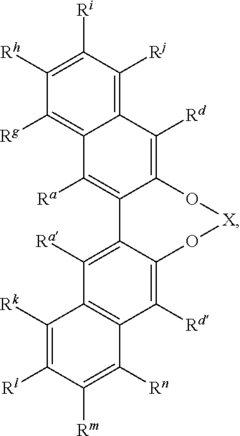
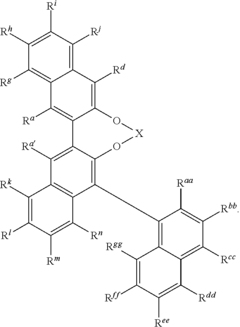
Catalysis Frontiers with Fluoroantimonic Acid
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Background and Objectives
Fluoroantimonic acid, a superacid formed by combining hydrogen fluoride and antimony pentafluoride, has emerged as a powerful catalyst in various chemical reactions. Its exceptional acidity, surpassing that of conventional acids, has revolutionized the field of catalysis and opened new frontiers in chemical synthesis and industrial processes.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for their unique properties. The breakthrough came in the 1960s when George A. Olah discovered the potential of fluoroantimonic acid as a catalyst, leading to his Nobel Prize in Chemistry in 1994 for his work on carbocations.
Over the past decades, fluoroantimonic acid catalysis has evolved significantly, finding applications in diverse areas such as petrochemical processing, pharmaceutical synthesis, and materials science. Its ability to catalyze reactions under mild conditions and with high selectivity has made it an attractive option for industrial-scale processes.
The primary objective of research in fluoroantimonic acid catalysis is to harness its exceptional acidity for more efficient and environmentally friendly chemical transformations. Scientists aim to develop novel catalytic systems that can activate inert molecules, facilitate challenging bond formations, and enable previously impossible reactions.
Current research trends focus on understanding the fundamental mechanisms of fluoroantimonic acid catalysis at the molecular level. This includes investigating the formation of reactive intermediates, studying the role of counterions, and exploring the influence of reaction conditions on catalytic performance.
Another key objective is to address the challenges associated with handling and using fluoroantimonic acid, given its highly corrosive nature and sensitivity to moisture. Researchers are working on developing safer and more stable forms of the catalyst, such as supported or encapsulated versions, to enhance its practicality in industrial settings.
The future of fluoroantimonic acid catalysis lies in expanding its application scope and improving its sustainability. This includes exploring its potential in green chemistry, developing recyclable catalyst systems, and integrating it with other emerging technologies such as flow chemistry and artificial intelligence for reaction optimization.
As we delve deeper into the frontiers of fluoroantimonic acid catalysis, the field promises to unlock new possibilities in chemical synthesis, potentially revolutionizing industries ranging from energy to healthcare. The ongoing research and development in this area are expected to yield innovative solutions to some of the most pressing challenges in modern chemistry and chemical engineering.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for their unique properties. The breakthrough came in the 1960s when George A. Olah discovered the potential of fluoroantimonic acid as a catalyst, leading to his Nobel Prize in Chemistry in 1994 for his work on carbocations.
Over the past decades, fluoroantimonic acid catalysis has evolved significantly, finding applications in diverse areas such as petrochemical processing, pharmaceutical synthesis, and materials science. Its ability to catalyze reactions under mild conditions and with high selectivity has made it an attractive option for industrial-scale processes.
The primary objective of research in fluoroantimonic acid catalysis is to harness its exceptional acidity for more efficient and environmentally friendly chemical transformations. Scientists aim to develop novel catalytic systems that can activate inert molecules, facilitate challenging bond formations, and enable previously impossible reactions.
Current research trends focus on understanding the fundamental mechanisms of fluoroantimonic acid catalysis at the molecular level. This includes investigating the formation of reactive intermediates, studying the role of counterions, and exploring the influence of reaction conditions on catalytic performance.
Another key objective is to address the challenges associated with handling and using fluoroantimonic acid, given its highly corrosive nature and sensitivity to moisture. Researchers are working on developing safer and more stable forms of the catalyst, such as supported or encapsulated versions, to enhance its practicality in industrial settings.
The future of fluoroantimonic acid catalysis lies in expanding its application scope and improving its sustainability. This includes exploring its potential in green chemistry, developing recyclable catalyst systems, and integrating it with other emerging technologies such as flow chemistry and artificial intelligence for reaction optimization.
As we delve deeper into the frontiers of fluoroantimonic acid catalysis, the field promises to unlock new possibilities in chemical synthesis, potentially revolutionizing industries ranging from energy to healthcare. The ongoing research and development in this area are expected to yield innovative solutions to some of the most pressing challenges in modern chemistry and chemical engineering.
Industrial Applications and Market Demand
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in the field of catalysis due to its exceptional proton-donating ability. The industrial applications of this powerful catalyst span across various sectors, driving a growing market demand for its utilization in chemical processes.
In the petrochemical industry, fluoroantimonic acid has found extensive use in alkylation reactions, particularly in the production of high-octane gasoline components. Its ability to catalyze the reaction between isobutane and light olefins has led to improved efficiency and product quality in fuel production. This application alone has created a substantial market for fluoroantimonic acid, with major oil companies investing in its implementation.
The polymer industry has also embraced fluoroantimonic acid as a catalyst for polymerization reactions. Its strong acidic properties enable the production of high-performance polymers with unique characteristics, such as enhanced thermal stability and chemical resistance. This has opened up new possibilities in the development of advanced materials for aerospace, automotive, and electronics applications.
In the pharmaceutical sector, fluoroantimonic acid has shown promise in the synthesis of complex organic compounds. Its ability to catalyze challenging reactions that were previously difficult or impossible to achieve has attracted the attention of drug manufacturers. This has led to increased research and development efforts to explore its potential in creating novel pharmaceutical intermediates and active ingredients.
The fine chemicals industry has also recognized the value of fluoroantimonic acid in producing specialty chemicals and intermediates. Its catalytic properties have enabled more efficient and selective synthesis routes, reducing production costs and improving product purity. This has resulted in a growing demand for fluoroantimonic acid in the manufacture of fragrances, flavors, and other high-value chemical products.
As environmental regulations become more stringent, there is an increasing focus on developing cleaner and more sustainable chemical processes. Fluoroantimonic acid has shown potential in this area, particularly in the field of biomass conversion. Its ability to catalyze the breakdown of cellulose and lignin has opened up new possibilities for the production of biofuels and bio-based chemicals, aligning with the global push towards renewable resources.
The market demand for fluoroantimonic acid is driven by its versatility and effectiveness as a catalyst. However, its highly corrosive nature and sensitivity to moisture present challenges in handling and storage, which has led to the development of specialized equipment and safety protocols. Despite these challenges, the unique catalytic properties of fluoroantimonic acid continue to attract interest from various industries, fueling ongoing research and development efforts to expand its applications and overcome its limitations.
In the petrochemical industry, fluoroantimonic acid has found extensive use in alkylation reactions, particularly in the production of high-octane gasoline components. Its ability to catalyze the reaction between isobutane and light olefins has led to improved efficiency and product quality in fuel production. This application alone has created a substantial market for fluoroantimonic acid, with major oil companies investing in its implementation.
The polymer industry has also embraced fluoroantimonic acid as a catalyst for polymerization reactions. Its strong acidic properties enable the production of high-performance polymers with unique characteristics, such as enhanced thermal stability and chemical resistance. This has opened up new possibilities in the development of advanced materials for aerospace, automotive, and electronics applications.
In the pharmaceutical sector, fluoroantimonic acid has shown promise in the synthesis of complex organic compounds. Its ability to catalyze challenging reactions that were previously difficult or impossible to achieve has attracted the attention of drug manufacturers. This has led to increased research and development efforts to explore its potential in creating novel pharmaceutical intermediates and active ingredients.
The fine chemicals industry has also recognized the value of fluoroantimonic acid in producing specialty chemicals and intermediates. Its catalytic properties have enabled more efficient and selective synthesis routes, reducing production costs and improving product purity. This has resulted in a growing demand for fluoroantimonic acid in the manufacture of fragrances, flavors, and other high-value chemical products.
As environmental regulations become more stringent, there is an increasing focus on developing cleaner and more sustainable chemical processes. Fluoroantimonic acid has shown potential in this area, particularly in the field of biomass conversion. Its ability to catalyze the breakdown of cellulose and lignin has opened up new possibilities for the production of biofuels and bio-based chemicals, aligning with the global push towards renewable resources.
The market demand for fluoroantimonic acid is driven by its versatility and effectiveness as a catalyst. However, its highly corrosive nature and sensitivity to moisture present challenges in handling and storage, which has led to the development of specialized equipment and safety protocols. Despite these challenges, the unique catalytic properties of fluoroantimonic acid continue to attract interest from various industries, fueling ongoing research and development efforts to expand its applications and overcome its limitations.
Current State and Challenges in Superacid Catalysis
Superacid catalysis, particularly with fluoroantimonic acid, represents a cutting-edge frontier in the field of catalytic chemistry. The current state of this technology is characterized by significant advancements in reaction efficiency and selectivity, yet it faces substantial challenges that hinder widespread industrial adoption.
Fluoroantimonic acid, recognized as the strongest known superacid, has demonstrated remarkable catalytic properties in various organic transformations. Its extreme acidity enables the activation of typically unreactive substrates, opening new pathways for chemical synthesis. Recent research has shown its efficacy in alkane isomerization, aromatic alkylation, and the cracking of heavy hydrocarbons, processes crucial for the petrochemical industry.
Despite these promising applications, the corrosive nature of fluoroantimonic acid poses a major challenge. Its extreme reactivity necessitates specialized handling and containment systems, significantly increasing operational costs and safety concerns. This has limited its use primarily to laboratory-scale experiments and specialized industrial processes.
Another significant challenge lies in the control and modulation of the acid's catalytic activity. The extreme acidity often leads to over-reaction and unwanted side products, necessitating the development of sophisticated reaction control mechanisms. Researchers are actively exploring methods to temper the acid's reactivity while maintaining its catalytic efficiency.
Environmental concerns also present a substantial hurdle. The production and use of fluoroantimonic acid involve highly toxic and environmentally hazardous compounds, raising questions about its long-term sustainability. This has spurred research into more environmentally benign superacid catalysts, though none have yet matched the catalytic power of fluoroantimonic acid.
The scalability of superacid catalysis processes remains a critical challenge. While effective on a small scale, many reactions catalyzed by fluoroantimonic acid face difficulties in scaling up to industrial levels. Issues such as heat management, product separation, and catalyst recovery become increasingly complex at larger scales.
Recent advancements in materials science have opened new avenues for addressing these challenges. The development of novel reactor materials resistant to superacid corrosion and innovative catalyst support systems show promise in enhancing the practicality of fluoroantimonic acid catalysis. Additionally, research into heterogeneous superacid catalysts aims to combine the power of fluoroantimonic acid with the ease of handling associated with solid catalysts.
In conclusion, while superacid catalysis with fluoroantimonic acid offers unprecedented catalytic potential, its current state is marked by a delicate balance between remarkable reactivity and significant practical challenges. Overcoming these hurdles through innovative engineering and chemical approaches will be crucial for realizing the full potential of this powerful catalytic system in industrial applications.
Fluoroantimonic acid, recognized as the strongest known superacid, has demonstrated remarkable catalytic properties in various organic transformations. Its extreme acidity enables the activation of typically unreactive substrates, opening new pathways for chemical synthesis. Recent research has shown its efficacy in alkane isomerization, aromatic alkylation, and the cracking of heavy hydrocarbons, processes crucial for the petrochemical industry.
Despite these promising applications, the corrosive nature of fluoroantimonic acid poses a major challenge. Its extreme reactivity necessitates specialized handling and containment systems, significantly increasing operational costs and safety concerns. This has limited its use primarily to laboratory-scale experiments and specialized industrial processes.
Another significant challenge lies in the control and modulation of the acid's catalytic activity. The extreme acidity often leads to over-reaction and unwanted side products, necessitating the development of sophisticated reaction control mechanisms. Researchers are actively exploring methods to temper the acid's reactivity while maintaining its catalytic efficiency.
Environmental concerns also present a substantial hurdle. The production and use of fluoroantimonic acid involve highly toxic and environmentally hazardous compounds, raising questions about its long-term sustainability. This has spurred research into more environmentally benign superacid catalysts, though none have yet matched the catalytic power of fluoroantimonic acid.
The scalability of superacid catalysis processes remains a critical challenge. While effective on a small scale, many reactions catalyzed by fluoroantimonic acid face difficulties in scaling up to industrial levels. Issues such as heat management, product separation, and catalyst recovery become increasingly complex at larger scales.
Recent advancements in materials science have opened new avenues for addressing these challenges. The development of novel reactor materials resistant to superacid corrosion and innovative catalyst support systems show promise in enhancing the practicality of fluoroantimonic acid catalysis. Additionally, research into heterogeneous superacid catalysts aims to combine the power of fluoroantimonic acid with the ease of handling associated with solid catalysts.
In conclusion, while superacid catalysis with fluoroantimonic acid offers unprecedented catalytic potential, its current state is marked by a delicate balance between remarkable reactivity and significant practical challenges. Overcoming these hurdles through innovative engineering and chemical approaches will be crucial for realizing the full potential of this powerful catalytic system in industrial applications.
Existing Fluoroantimonic Acid Catalytic Methodologies
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in catalysis and chemical reactions: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. Its extreme acidity allows for the activation of otherwise unreactive compounds, enabling unique transformations in organic synthesis and petrochemical industries.
- Use in materials science and surface treatments: Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. This superacid's unique characteristics make it valuable in developing advanced materials with specific surface properties.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and controlled environments. Proper neutralization and disposal methods are crucial to prevent environmental contamination and ensure worker safety.
- Analytical and research applications: Fluoroantimonic acid is used in various analytical and research applications. Its superacidic properties make it valuable in studying reaction mechanisms, probing molecular structures, and developing new analytical techniques. It can be employed in spectroscopic studies, materials characterization, and as a reference compound for acidity measurements.
02 Applications in catalysis and chemical reactions
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts.Expand Specific Solutions03 Use in materials science and surface treatments
Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It can be used to etch or modify surfaces of various materials, including metals and semiconductors. The acid's unique properties allow for precise control of surface characteristics in advanced materials and electronic components.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme reactivity and corrosiveness, fluoroantimonic acid requires specialized safety measures and handling procedures. This includes the use of specific containment materials, personal protective equipment, and controlled environments. Proper storage, transportation, and disposal methods are crucial to prevent accidents and environmental contamination.Expand Specific Solutions05 Analytical and research applications
Fluoroantimonic acid is employed in various analytical and research applications. Its unique properties make it valuable in spectroscopic studies, chemical analysis, and as a reference material for superacidity. The acid is also used in fundamental research to explore the limits of acidity and develop new chemical reactions and materials.Expand Specific Solutions
Key Players in Superacid Catalysis Research and Industry
The field of catalysis using fluoroantimonic acid is in a nascent stage of development, with significant potential for growth. The market size is relatively small but expanding rapidly as researchers explore its applications in various industries. Technologically, it's still in the early phases of maturity, with ongoing research to fully understand and harness its catalytic properties. Key players like BASF Corp., DuPont de Nemours, Inc., and Johnson Matthey Plc are at the forefront, investing in R&D to advance this technology. Academic institutions such as the California Institute of Technology and Massachusetts Institute of Technology are also contributing significantly to the fundamental research. The competitive landscape is characterized by a mix of established chemical companies and innovative research institutions, all vying to unlock the full potential of fluoroantimonic acid catalysis.
BASF Corp.
Technical Solution: BASF has developed a novel catalytic system using fluoroantimonic acid as a superacid catalyst for various organic transformations. Their approach involves stabilizing the highly reactive fluoroantimonic acid in a specialized fluoropolymer matrix, allowing for controlled release and enhanced catalytic activity. This system enables efficient alkylation, isomerization, and cracking reactions under milder conditions compared to traditional acid catalysts. BASF has also implemented advanced safety protocols and containment strategies to handle the corrosive nature of fluoroantimonic acid.
Strengths: Highly efficient catalysis, milder reaction conditions, versatile application in petrochemical industry. Weaknesses: Safety concerns due to extreme acidity, potential environmental risks, high cost of specialized handling equipment.
Novartis AG
Technical Solution: Novartis has pioneered the use of fluoroantimonic acid in pharmaceutical synthesis, particularly for the activation of challenging substrates in drug discovery. Their approach involves using microfluidic reactors to precisely control the exposure of reactants to fluoroantimonic acid, minimizing side reactions and improving yield. This technology has been crucial in synthesizing complex heterocyclic compounds that are potential drug candidates. Novartis has also developed a novel neutralization process to safely quench the acid after reactions, addressing environmental concerns.
Strengths: Enables synthesis of previously inaccessible drug molecules, high precision in reaction control. Weaknesses: Limited scalability for large-scale production, high cost of specialized equipment.
Breakthrough Innovations in Fluoroantimonic Acid Catalysis
Asymmetric electrophilic fluorination using an anionic chiral phase-transfer catalyst
PatentWO2013096971A1
Innovation
- Development of chiral anionic phase-transfer catalysts that facilitate enantioselective electrophilic addition reactions by forming soluble ion pairs with insoluble cationic electrophilic reagents, enabling efficient and enantiocontrolled C-F bond formation through electrophilic addition reactions.
Asymmetric electrophilic fluorination using an anionic chiral phase-transfer catalyst
PatentActiveUS20140350253A1
Innovation
- Development of chiral anion phase-transfer catalysts that facilitate electrophilic addition reactions, allowing for enantioselective fluorination using stable and inexpensive reagents, and enabling the formation of fluorinated compounds that can be further functionalized.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that must be carefully addressed in catalysis applications. The extreme corrosiveness and reactivity of this compound necessitate stringent handling protocols and specialized containment systems to prevent accidental exposure or release.
Workers involved in processes utilizing fluoroantimonic acid require extensive training and must use appropriate personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection. Facilities must be equipped with advanced ventilation systems, acid-resistant materials, and emergency containment measures to mitigate potential spills or leaks.
The environmental impact of fluoroantimonic acid is a critical concern. Its highly reactive nature can lead to severe ecological damage if released into the environment. Proper disposal methods are essential, often involving neutralization processes and specialized waste treatment facilities. Strict regulations govern the transportation, storage, and use of this superacid to minimize environmental risks.
Monitoring systems play a crucial role in ensuring safety and environmental compliance. Continuous air quality monitoring, leak detection systems, and regular equipment inspections are necessary to prevent and quickly respond to potential hazards. Emergency response plans must be in place, with personnel trained to handle acid-related incidents effectively.
The long-term effects of fluoroantimonic acid exposure on human health and ecosystems are not fully understood, necessitating ongoing research and risk assessment. Occupational health monitoring programs for workers regularly exposed to the acid are essential to detect and prevent potential health issues.
As catalysis research with fluoroantimonic acid advances, there is a growing focus on developing safer alternatives or modified processes that reduce the risks associated with its use. This includes exploring encapsulation techniques, immobilization on solid supports, or the use of ionic liquids as safer reaction media.
Regulatory compliance is a significant aspect of working with fluoroantimonic acid. Researchers and industries must adhere to strict guidelines set by environmental protection agencies and occupational safety organizations. Regular audits and documentation of safety procedures are necessary to maintain compliance and ensure responsible use of this powerful catalyst.
Workers involved in processes utilizing fluoroantimonic acid require extensive training and must use appropriate personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection. Facilities must be equipped with advanced ventilation systems, acid-resistant materials, and emergency containment measures to mitigate potential spills or leaks.
The environmental impact of fluoroantimonic acid is a critical concern. Its highly reactive nature can lead to severe ecological damage if released into the environment. Proper disposal methods are essential, often involving neutralization processes and specialized waste treatment facilities. Strict regulations govern the transportation, storage, and use of this superacid to minimize environmental risks.
Monitoring systems play a crucial role in ensuring safety and environmental compliance. Continuous air quality monitoring, leak detection systems, and regular equipment inspections are necessary to prevent and quickly respond to potential hazards. Emergency response plans must be in place, with personnel trained to handle acid-related incidents effectively.
The long-term effects of fluoroantimonic acid exposure on human health and ecosystems are not fully understood, necessitating ongoing research and risk assessment. Occupational health monitoring programs for workers regularly exposed to the acid are essential to detect and prevent potential health issues.
As catalysis research with fluoroantimonic acid advances, there is a growing focus on developing safer alternatives or modified processes that reduce the risks associated with its use. This includes exploring encapsulation techniques, immobilization on solid supports, or the use of ionic liquids as safer reaction media.
Regulatory compliance is a significant aspect of working with fluoroantimonic acid. Researchers and industries must adhere to strict guidelines set by environmental protection agencies and occupational safety organizations. Regular audits and documentation of safety procedures are necessary to maintain compliance and ensure responsible use of this powerful catalyst.
Economic Viability and Scalability Analysis
The economic viability and scalability of catalysis using fluoroantimonic acid present both significant opportunities and challenges. As the strongest known superacid, fluoroantimonic acid offers unparalleled catalytic potential, particularly in hydrocarbon processing and petrochemical industries. Its ability to protonate even weak bases and catalyze reactions at extremely low temperatures could lead to substantial energy savings and process efficiencies.
However, the corrosive nature of fluoroantimonic acid necessitates specialized handling and containment systems, significantly increasing initial capital investments. Materials such as PTFE (Teflon) or high-purity alloys are required for equipment, driving up costs. Additionally, the acid's extreme reactivity poses safety concerns, requiring stringent safety protocols and specialized training for personnel, further adding to operational expenses.
Scalability is a critical factor in determining the economic feasibility of fluoroantimonic acid catalysis. While laboratory-scale applications have demonstrated remarkable efficiency, translating these results to industrial-scale processes presents considerable engineering challenges. The need for precise control over reaction conditions and the difficulty in handling large quantities of the superacid may limit the size of reactors and overall production capacity.
Despite these challenges, the potential for process intensification and yield improvements in certain high-value chemical syntheses could offset the increased costs associated with fluoroantimonic acid catalysis. Industries producing specialty chemicals or pharmaceuticals, where product value is high and volumes are relatively low, may find the economic trade-offs more favorable.
The supply chain for fluoroantimonic acid components, particularly hydrogen fluoride and antimony pentafluoride, also impacts economic viability. The limited availability and high cost of these precursors could create bottlenecks in large-scale applications. Developing more efficient synthesis methods for fluoroantimonic acid or identifying alternative superacid catalysts with similar reactivity but improved handling characteristics could enhance economic viability.
Environmental considerations play a crucial role in assessing the long-term scalability of fluoroantimonic acid catalysis. Stringent regulations surrounding the use and disposal of highly corrosive substances may impose additional costs and operational constraints. Developing closed-loop systems for acid recovery and recycling could mitigate environmental impacts and improve economic feasibility, but would require significant research and development investment.
In conclusion, while fluoroantimonic acid catalysis offers transformative potential in certain chemical processes, its economic viability and scalability are contingent on overcoming substantial technical, safety, and environmental challenges. Targeted applications in high-value, low-volume sectors may prove economically viable in the near term, while broader industrial adoption will likely require further technological advancements and innovative process designs to balance costs with performance benefits.
However, the corrosive nature of fluoroantimonic acid necessitates specialized handling and containment systems, significantly increasing initial capital investments. Materials such as PTFE (Teflon) or high-purity alloys are required for equipment, driving up costs. Additionally, the acid's extreme reactivity poses safety concerns, requiring stringent safety protocols and specialized training for personnel, further adding to operational expenses.
Scalability is a critical factor in determining the economic feasibility of fluoroantimonic acid catalysis. While laboratory-scale applications have demonstrated remarkable efficiency, translating these results to industrial-scale processes presents considerable engineering challenges. The need for precise control over reaction conditions and the difficulty in handling large quantities of the superacid may limit the size of reactors and overall production capacity.
Despite these challenges, the potential for process intensification and yield improvements in certain high-value chemical syntheses could offset the increased costs associated with fluoroantimonic acid catalysis. Industries producing specialty chemicals or pharmaceuticals, where product value is high and volumes are relatively low, may find the economic trade-offs more favorable.
The supply chain for fluoroantimonic acid components, particularly hydrogen fluoride and antimony pentafluoride, also impacts economic viability. The limited availability and high cost of these precursors could create bottlenecks in large-scale applications. Developing more efficient synthesis methods for fluoroantimonic acid or identifying alternative superacid catalysts with similar reactivity but improved handling characteristics could enhance economic viability.
Environmental considerations play a crucial role in assessing the long-term scalability of fluoroantimonic acid catalysis. Stringent regulations surrounding the use and disposal of highly corrosive substances may impose additional costs and operational constraints. Developing closed-loop systems for acid recovery and recycling could mitigate environmental impacts and improve economic feasibility, but would require significant research and development investment.
In conclusion, while fluoroantimonic acid catalysis offers transformative potential in certain chemical processes, its economic viability and scalability are contingent on overcoming substantial technical, safety, and environmental challenges. Targeted applications in high-value, low-volume sectors may prove economically viable in the near term, while broader industrial adoption will likely require further technological advancements and innovative process designs to balance costs with performance benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!