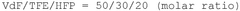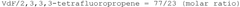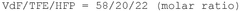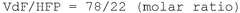How to Enhance Catalytic Scalability with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful catalyst in various chemical processes. Its exceptional acidity, surpassing that of 100% sulfuric acid by over a trillion times, has garnered significant attention in the field of catalysis. The evolution of fluoroantimonic acid catalysis can be traced back to the mid-20th century, with pioneering work by George A. Olah and his colleagues.
The primary objective in enhancing catalytic scalability with fluoroantimonic acid is to harness its unique properties for large-scale industrial applications while addressing the challenges associated with its corrosive nature and handling difficulties. Researchers aim to develop innovative methods to stabilize and control the acid's reactivity, enabling its use in continuous flow processes and heterogeneous catalysis systems.
One of the key technological trends in this field is the development of supported fluoroantimonic acid catalysts. These systems aim to immobilize the superacid on solid supports, such as silica or polymeric materials, to improve handling and recyclability while maintaining catalytic activity. Another emerging trend is the exploration of ionic liquid-based systems incorporating fluoroantimonic acid, which could potentially offer a more stable and easily manageable form of the superacid for catalytic applications.
The scalability of fluoroantimonic acid catalysis is closely tied to advancements in materials science and process engineering. Researchers are investigating novel reactor designs and materials that can withstand the extreme acidity while allowing for efficient heat and mass transfer. Additionally, the development of in-situ monitoring techniques for reaction progress and catalyst activity is crucial for optimizing large-scale processes.
Environmental considerations and safety concerns are driving efforts to develop greener alternatives or modified versions of fluoroantimonic acid catalysts. This includes research into encapsulation technologies and the design of less corrosive superacid systems that retain high catalytic activity. The ultimate goal is to bridge the gap between the remarkable catalytic potential of fluoroantimonic acid and its practical implementation in industrial-scale processes.
As the field progresses, interdisciplinary collaboration between chemists, materials scientists, and chemical engineers will be essential to overcome the technical challenges and realize the full potential of fluoroantimonic acid catalysis in large-scale applications. The successful enhancement of catalytic scalability with this superacid could revolutionize various industries, from petrochemicals to fine chemical synthesis, by enabling more efficient and selective transformations.
The primary objective in enhancing catalytic scalability with fluoroantimonic acid is to harness its unique properties for large-scale industrial applications while addressing the challenges associated with its corrosive nature and handling difficulties. Researchers aim to develop innovative methods to stabilize and control the acid's reactivity, enabling its use in continuous flow processes and heterogeneous catalysis systems.
One of the key technological trends in this field is the development of supported fluoroantimonic acid catalysts. These systems aim to immobilize the superacid on solid supports, such as silica or polymeric materials, to improve handling and recyclability while maintaining catalytic activity. Another emerging trend is the exploration of ionic liquid-based systems incorporating fluoroantimonic acid, which could potentially offer a more stable and easily manageable form of the superacid for catalytic applications.
The scalability of fluoroantimonic acid catalysis is closely tied to advancements in materials science and process engineering. Researchers are investigating novel reactor designs and materials that can withstand the extreme acidity while allowing for efficient heat and mass transfer. Additionally, the development of in-situ monitoring techniques for reaction progress and catalyst activity is crucial for optimizing large-scale processes.
Environmental considerations and safety concerns are driving efforts to develop greener alternatives or modified versions of fluoroantimonic acid catalysts. This includes research into encapsulation technologies and the design of less corrosive superacid systems that retain high catalytic activity. The ultimate goal is to bridge the gap between the remarkable catalytic potential of fluoroantimonic acid and its practical implementation in industrial-scale processes.
As the field progresses, interdisciplinary collaboration between chemists, materials scientists, and chemical engineers will be essential to overcome the technical challenges and realize the full potential of fluoroantimonic acid catalysis in large-scale applications. The successful enhancement of catalytic scalability with this superacid could revolutionize various industries, from petrochemicals to fine chemical synthesis, by enabling more efficient and selective transformations.
Industrial Demand for Enhanced Catalytic Processes
The industrial demand for enhanced catalytic processes has been steadily growing across various sectors, driven by the need for more efficient, cost-effective, and environmentally friendly production methods. Fluoroantimonic acid, known as one of the strongest superacids, has garnered significant attention for its potential to revolutionize catalytic processes in industrial applications.
In the petrochemical industry, there is a pressing need for improved catalysts to enhance the efficiency of hydrocarbon cracking and isomerization processes. Fluoroantimonic acid's exceptional acidity offers promising opportunities to increase reaction rates and selectivity, potentially leading to higher yields and reduced energy consumption in refinery operations.
The pharmaceutical sector has also expressed keen interest in advanced catalytic processes to streamline the synthesis of complex drug molecules. The ability of fluoroantimonic acid to catalyze challenging reactions at milder conditions could significantly reduce production costs and time-to-market for new medications.
In the polymer industry, there is a growing demand for catalysts that can facilitate the production of high-performance materials with specific properties. Fluoroantimonic acid's unique catalytic properties may enable the synthesis of novel polymers with enhanced thermal stability, mechanical strength, or chemical resistance.
The fine chemicals industry is constantly seeking ways to improve the synthesis of specialty chemicals and intermediates. The use of fluoroantimonic acid as a catalyst could potentially unlock new reaction pathways, allowing for the production of previously challenging or impossible-to-synthesize compounds.
Environmental concerns have also fueled the demand for more efficient catalytic processes in waste treatment and pollution control. Fluoroantimonic acid's strong oxidizing properties could be harnessed to develop advanced catalytic systems for the breakdown of persistent organic pollutants or the treatment of industrial effluents.
The electronics industry has shown interest in exploring fluoroantimonic acid's potential in semiconductor manufacturing processes, particularly in etching and surface modification applications. Its ability to generate highly reactive species could lead to more precise and efficient microfabrication techniques.
As industries strive to meet sustainability goals and reduce their carbon footprint, there is a growing emphasis on developing catalytic processes that operate at lower temperatures and pressures. Fluoroantimonic acid's exceptional catalytic activity could contribute to the development of such energy-efficient processes across multiple sectors.
In the petrochemical industry, there is a pressing need for improved catalysts to enhance the efficiency of hydrocarbon cracking and isomerization processes. Fluoroantimonic acid's exceptional acidity offers promising opportunities to increase reaction rates and selectivity, potentially leading to higher yields and reduced energy consumption in refinery operations.
The pharmaceutical sector has also expressed keen interest in advanced catalytic processes to streamline the synthesis of complex drug molecules. The ability of fluoroantimonic acid to catalyze challenging reactions at milder conditions could significantly reduce production costs and time-to-market for new medications.
In the polymer industry, there is a growing demand for catalysts that can facilitate the production of high-performance materials with specific properties. Fluoroantimonic acid's unique catalytic properties may enable the synthesis of novel polymers with enhanced thermal stability, mechanical strength, or chemical resistance.
The fine chemicals industry is constantly seeking ways to improve the synthesis of specialty chemicals and intermediates. The use of fluoroantimonic acid as a catalyst could potentially unlock new reaction pathways, allowing for the production of previously challenging or impossible-to-synthesize compounds.
Environmental concerns have also fueled the demand for more efficient catalytic processes in waste treatment and pollution control. Fluoroantimonic acid's strong oxidizing properties could be harnessed to develop advanced catalytic systems for the breakdown of persistent organic pollutants or the treatment of industrial effluents.
The electronics industry has shown interest in exploring fluoroantimonic acid's potential in semiconductor manufacturing processes, particularly in etching and surface modification applications. Its ability to generate highly reactive species could lead to more precise and efficient microfabrication techniques.
As industries strive to meet sustainability goals and reduce their carbon footprint, there is a growing emphasis on developing catalytic processes that operate at lower temperatures and pressures. Fluoroantimonic acid's exceptional catalytic activity could contribute to the development of such energy-efficient processes across multiple sectors.
Current Challenges in Fluoroantimonic Acid Catalysis
Fluoroantimonic acid (HSbF6) is renowned as one of the strongest superacids, with exceptional catalytic properties. However, its widespread industrial application faces several significant challenges that hinder its scalability and practical use.
One of the primary obstacles is the extreme reactivity and corrosiveness of fluoroantimonic acid. This superacid is capable of protonating even weak bases, making it highly aggressive towards most materials. Consequently, finding suitable containment materials that can withstand its corrosive nature without degradation or contamination is a major challenge. Traditional materials like glass, most metals, and many polymers are rapidly attacked by this superacid, limiting options for reactor design and storage.
The sensitivity of fluoroantimonic acid to moisture presents another significant hurdle. Even trace amounts of water can lead to rapid decomposition, releasing toxic hydrogen fluoride gas. This necessitates stringent handling protocols and sophisticated moisture exclusion systems, which are difficult to maintain at industrial scales. The requirement for anhydrous conditions also complicates the integration of fluoroantimonic acid catalysis into existing industrial processes that may involve water-containing feedstocks or solvents.
Safety concerns pose a substantial challenge to scaling up fluoroantimonic acid catalysis. The extreme acidity and potential for generating hazardous byproducts demand rigorous safety measures, specialized equipment, and extensive personnel training. These requirements significantly increase operational costs and complexity, making it less attractive for large-scale industrial adoption.
The high cost of production and purification of fluoroantimonic acid is another limiting factor. The synthesis process involves expensive precursors and requires specialized equipment, contributing to elevated production costs. This economic barrier makes it challenging to justify its use in many industrial applications, especially when competing with more established and cost-effective catalytic systems.
Furthermore, the environmental impact of fluoroantimonic acid production and use is a growing concern. The potential release of fluorine-containing compounds and antimony species raises environmental and health issues, necessitating complex waste treatment and disposal procedures. This aspect becomes increasingly problematic as regulations on chemical processes become more stringent.
Lastly, the limited understanding of the precise reaction mechanisms in fluoroantimonic acid catalysis hinders optimization efforts. The extreme acidity regime creates unique reaction environments that are not fully characterized, making it challenging to predict and control reaction outcomes at larger scales. This knowledge gap impedes the development of more efficient and selective catalytic processes using fluoroantimonic acid.
One of the primary obstacles is the extreme reactivity and corrosiveness of fluoroantimonic acid. This superacid is capable of protonating even weak bases, making it highly aggressive towards most materials. Consequently, finding suitable containment materials that can withstand its corrosive nature without degradation or contamination is a major challenge. Traditional materials like glass, most metals, and many polymers are rapidly attacked by this superacid, limiting options for reactor design and storage.
The sensitivity of fluoroantimonic acid to moisture presents another significant hurdle. Even trace amounts of water can lead to rapid decomposition, releasing toxic hydrogen fluoride gas. This necessitates stringent handling protocols and sophisticated moisture exclusion systems, which are difficult to maintain at industrial scales. The requirement for anhydrous conditions also complicates the integration of fluoroantimonic acid catalysis into existing industrial processes that may involve water-containing feedstocks or solvents.
Safety concerns pose a substantial challenge to scaling up fluoroantimonic acid catalysis. The extreme acidity and potential for generating hazardous byproducts demand rigorous safety measures, specialized equipment, and extensive personnel training. These requirements significantly increase operational costs and complexity, making it less attractive for large-scale industrial adoption.
The high cost of production and purification of fluoroantimonic acid is another limiting factor. The synthesis process involves expensive precursors and requires specialized equipment, contributing to elevated production costs. This economic barrier makes it challenging to justify its use in many industrial applications, especially when competing with more established and cost-effective catalytic systems.
Furthermore, the environmental impact of fluoroantimonic acid production and use is a growing concern. The potential release of fluorine-containing compounds and antimony species raises environmental and health issues, necessitating complex waste treatment and disposal procedures. This aspect becomes increasingly problematic as regulations on chemical processes become more stringent.
Lastly, the limited understanding of the precise reaction mechanisms in fluoroantimonic acid catalysis hinders optimization efforts. The extreme acidity regime creates unique reaction environments that are not fully characterized, making it challenging to predict and control reaction outcomes at larger scales. This knowledge gap impedes the development of more efficient and selective catalytic processes using fluoroantimonic acid.
Existing Scalability Solutions for Fluoroantimonic Acid
01 Catalytic process optimization
Optimization of catalytic processes involving fluoroantimonic acid for improved scalability. This includes adjusting reaction parameters, developing more efficient reactor designs, and implementing advanced control systems to enhance the overall catalytic performance and yield at larger scales.- Catalytic process optimization: Optimization of catalytic processes involving fluoroantimonic acid for improved scalability. This includes adjusting reaction parameters, developing more efficient reactor designs, and implementing advanced control systems to enhance the overall catalytic performance and yield at larger scales.
- Handling and safety considerations: Development of specialized handling and safety protocols for working with fluoroantimonic acid at industrial scales. This involves designing corrosion-resistant equipment, implementing robust containment systems, and establishing comprehensive safety procedures to mitigate risks associated with the highly reactive nature of the acid.
- Purification and recycling techniques: Implementation of advanced purification and recycling methods for fluoroantimonic acid to improve its reusability in large-scale catalytic processes. This includes developing efficient separation techniques, minimizing waste generation, and optimizing the recovery of the acid to enhance overall process economics and sustainability.
- Modeling and simulation for scale-up: Utilization of advanced modeling and simulation techniques to predict and optimize the behavior of fluoroantimonic acid-catalyzed reactions at larger scales. This involves developing accurate computational models, conducting virtual experiments, and employing machine learning algorithms to guide the scale-up process and identify potential challenges.
- Alternative catalyst formulations: Exploration of modified or alternative catalyst formulations based on fluoroantimonic acid to address scalability challenges. This includes investigating supported catalysts, developing novel acid-base complexes, and synthesizing more stable derivatives to enhance the acid's catalytic performance and handling properties at industrial scales.
02 Handling and safety considerations
Development of specialized handling and safety protocols for working with fluoroantimonic acid at industrial scales. This involves designing corrosion-resistant equipment, implementing robust containment systems, and establishing comprehensive safety procedures to mitigate risks associated with the highly reactive nature of the acid.Expand Specific Solutions03 Purification and recycling techniques
Implementation of advanced purification and recycling methods for fluoroantimonic acid to improve its reusability in large-scale catalytic processes. This includes developing efficient separation techniques, minimizing waste generation, and optimizing the recovery of the acid to enhance overall process economics and sustainability.Expand Specific Solutions04 Modeling and simulation for scale-up
Utilization of advanced modeling and simulation techniques to predict and optimize the behavior of fluoroantimonic acid-catalyzed reactions at larger scales. This involves developing accurate computational models, conducting virtual experiments, and employing machine learning algorithms to guide the scale-up process and identify potential challenges.Expand Specific Solutions05 Alternative catalyst formulations
Exploration of modified or alternative catalyst formulations based on fluoroantimonic acid to address scalability challenges. This includes investigating supported catalysts, developing novel acid mixtures, and synthesizing more stable derivatives to overcome limitations associated with the pure acid while maintaining its catalytic activity.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Catalysis Industry
The catalytic scalability enhancement using fluoroantimonic acid is in a nascent stage of development, with significant potential for growth. The market size is expanding as industries seek more efficient catalytic processes. Technologically, it's still evolving, with varying levels of maturity among key players. Companies like BASF SE and BASF Corp. are at the forefront, leveraging their extensive chemical expertise. JGC Catalysts & Chemicals Ltd. and Kurita Water Industries Ltd. are making strides in application-specific developments. Emerging players such as Xi'an Modern Chemistry Research Institute and Sinochem Lantian Co., Ltd. are contributing to technological advancements. The competitive landscape is dynamic, with both established chemical giants and specialized firms vying for market share and technological breakthroughs.
BASF SE
Technical Solution: BASF SE has developed a novel catalytic system using fluoroantimonic acid as a super-acid catalyst to enhance the scalability of various chemical processes. Their approach involves immobilizing the fluoroantimonic acid on a porous support material, which allows for easier handling and recovery of the catalyst. This system is designed to operate under milder conditions compared to traditional catalysts, reducing energy consumption and improving overall process efficiency. BASF's technology also incorporates a unique stabilization method that prolongs the catalyst's lifespan and maintains its activity over multiple reaction cycles.
Strengths: High catalytic activity, improved process efficiency, and reduced energy consumption. Weaknesses: Potential corrosion issues and the need for specialized handling equipment due to the highly acidic nature of fluoroantimonic acid.
JGC Catalysts & Chemicals Ltd.
Technical Solution: JGC Catalysts & Chemicals Ltd. has developed a proprietary fluoroantimonic acid-based catalyst system that focuses on enhancing catalytic scalability in petrochemical processes. Their approach involves a carefully engineered support matrix that allows for the controlled release of fluoroantimonic acid during reactions. This system is designed to maximize catalyst surface area and accessibility, leading to improved reaction rates and selectivity. JGC's technology also incorporates a novel regeneration process that helps maintain catalyst activity over extended periods, making it particularly suitable for large-scale industrial applications.
Strengths: Improved catalyst longevity and suitability for large-scale operations. Weaknesses: Potential environmental concerns and the need for specialized waste treatment processes.
Core Innovations in Fluoroantimonic Acid Catalysis
Crosslinkable composition, member, and use of crosslinkable composition
PatentPendingEP4455475A1
Innovation
- A crosslinkable composition comprising a fluoroelastomer and a cross-linking agent, with hydrotalcite as an acid acceptor, or alternative acid acceptors like metal oxides, hydroxides, and silicates, is used to create a crosslinked fluoroelastomer with enhanced resistance to nitric acid, ensuring the system's components maintain shape and functionality.
Method for producing a lithium battery material, materials and lithium battery
PatentWO2017055763A1
Innovation
- A method involving grinding fluorinated carbon nanofibers using impact friction to open up the carbon structure, reducing defects and enhancing electrochemical performance, which includes specific parameters like pressure, duration, and atmosphere control, and potentially followed by controlled fluorination to increase capacity.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated to be as low as -21.6. Due to its extreme corrosiveness and reactivity, handling this substance requires stringent safety protocols and specialized equipment. The primary concern when working with fluoroantimonic acid is its ability to react violently with water, producing toxic and corrosive fumes.
Personal protective equipment (PPE) is crucial when handling fluoroantimonic acid. This includes chemical-resistant suits, gloves, and boots made from materials such as fluorinated ethylene propylene (FEP) or polytetrafluoroethylene (PTFE). Full-face respirators with appropriate acid gas cartridges or self-contained breathing apparatus (SCBA) are essential to protect against toxic fumes.
Storage and containment of fluoroantimonic acid require specialized materials resistant to its corrosive nature. Containers should be made from fluoropolymers like PTFE or perfluoroalkoxy alkanes (PFA). Glass and metal containers are unsuitable due to the acid's ability to dissolve these materials. All storage areas must be kept dry and well-ventilated to prevent the accumulation of potentially hazardous fumes.
Handling procedures for fluoroantimonic acid should be conducted in a controlled environment, preferably within a fume hood equipped with appropriate filtration systems. Transfer of the acid should be performed using specialized pumps and tubing made from compatible materials. Any equipment that comes into contact with the acid must be thoroughly cleaned and neutralized after use.
Emergency response protocols are critical when working with fluoroantimonic acid. Spill kits containing neutralizing agents such as sodium bicarbonate or calcium carbonate should be readily available. In case of skin or eye contact, immediate flushing with copious amounts of water for at least 15 minutes is necessary, followed by urgent medical attention.
Training and education are paramount for personnel working with fluoroantimonic acid. Comprehensive safety training should cover proper handling techniques, emergency procedures, and the use of PPE. Regular safety drills and refresher courses should be conducted to ensure all staff members are prepared for potential incidents.
Waste disposal of fluoroantimonic acid and related materials requires specialized procedures. Neutralization with appropriate bases should be performed before disposal, and all waste must be handled in accordance with local, state, and federal regulations governing hazardous materials.
By implementing these rigorous safety and handling protocols, the risks associated with fluoroantimonic acid can be significantly mitigated, allowing for its safe use in catalytic processes aimed at enhancing scalability.
Personal protective equipment (PPE) is crucial when handling fluoroantimonic acid. This includes chemical-resistant suits, gloves, and boots made from materials such as fluorinated ethylene propylene (FEP) or polytetrafluoroethylene (PTFE). Full-face respirators with appropriate acid gas cartridges or self-contained breathing apparatus (SCBA) are essential to protect against toxic fumes.
Storage and containment of fluoroantimonic acid require specialized materials resistant to its corrosive nature. Containers should be made from fluoropolymers like PTFE or perfluoroalkoxy alkanes (PFA). Glass and metal containers are unsuitable due to the acid's ability to dissolve these materials. All storage areas must be kept dry and well-ventilated to prevent the accumulation of potentially hazardous fumes.
Handling procedures for fluoroantimonic acid should be conducted in a controlled environment, preferably within a fume hood equipped with appropriate filtration systems. Transfer of the acid should be performed using specialized pumps and tubing made from compatible materials. Any equipment that comes into contact with the acid must be thoroughly cleaned and neutralized after use.
Emergency response protocols are critical when working with fluoroantimonic acid. Spill kits containing neutralizing agents such as sodium bicarbonate or calcium carbonate should be readily available. In case of skin or eye contact, immediate flushing with copious amounts of water for at least 15 minutes is necessary, followed by urgent medical attention.
Training and education are paramount for personnel working with fluoroantimonic acid. Comprehensive safety training should cover proper handling techniques, emergency procedures, and the use of PPE. Regular safety drills and refresher courses should be conducted to ensure all staff members are prepared for potential incidents.
Waste disposal of fluoroantimonic acid and related materials requires specialized procedures. Neutralization with appropriate bases should be performed before disposal, and all waste must be handled in accordance with local, state, and federal regulations governing hazardous materials.
By implementing these rigorous safety and handling protocols, the risks associated with fluoroantimonic acid can be significantly mitigated, allowing for its safe use in catalytic processes aimed at enhancing scalability.
Environmental Impact of Fluoroantimonic Acid Catalysis
The environmental impact of fluoroantimonic acid catalysis is a critical consideration in the quest to enhance catalytic scalability. As one of the strongest known superacids, fluoroantimonic acid's use in catalytic processes presents both opportunities and challenges from an environmental perspective.
Fluoroantimonic acid's extreme acidity enables highly efficient catalytic reactions, potentially reducing the overall energy requirements and resource consumption in industrial processes. This efficiency can lead to decreased emissions and waste production, aligning with sustainability goals. However, the environmental benefits of improved catalytic performance must be weighed against the potential risks associated with the acid's use.
The production and handling of fluoroantimonic acid pose significant environmental concerns. The acid's components, particularly hydrogen fluoride, are highly toxic and corrosive. Accidental releases or improper disposal can have severe consequences for ecosystems and human health. Stringent safety protocols and containment measures are essential to mitigate these risks, adding complexity and cost to industrial applications.
Water contamination is a primary environmental risk associated with fluoroantimonic acid catalysis. Even small amounts of the acid can dramatically alter water pH, potentially causing widespread damage to aquatic ecosystems. The acid's high reactivity with water also complicates wastewater treatment processes, necessitating specialized handling and neutralization techniques.
Air pollution is another environmental consideration. While the acid itself has low volatility, its use in industrial processes may generate harmful gaseous byproducts, including fluorine-containing compounds. These emissions can contribute to air quality degradation and potentially impact the ozone layer, requiring robust air treatment systems in manufacturing facilities.
The long-term environmental persistence of fluoroantimonic acid and its byproducts is an area of ongoing research. The acid's extreme reactivity suggests rapid decomposition in the environment, but the fate of its constituent elements and potential secondary reaction products remains a concern. Comprehensive lifecycle assessments are crucial to fully understand the environmental footprint of fluoroantimonic acid catalysis.
Efforts to enhance the environmental sustainability of fluoroantimonic acid catalysis focus on several key areas. These include developing more efficient catalytic processes to minimize acid consumption, improving containment and handling technologies to prevent releases, and exploring recycling methods to reduce waste. Additionally, research into alternative superacid catalysts with lower environmental impact is ongoing, aiming to balance catalytic performance with ecological considerations.
Fluoroantimonic acid's extreme acidity enables highly efficient catalytic reactions, potentially reducing the overall energy requirements and resource consumption in industrial processes. This efficiency can lead to decreased emissions and waste production, aligning with sustainability goals. However, the environmental benefits of improved catalytic performance must be weighed against the potential risks associated with the acid's use.
The production and handling of fluoroantimonic acid pose significant environmental concerns. The acid's components, particularly hydrogen fluoride, are highly toxic and corrosive. Accidental releases or improper disposal can have severe consequences for ecosystems and human health. Stringent safety protocols and containment measures are essential to mitigate these risks, adding complexity and cost to industrial applications.
Water contamination is a primary environmental risk associated with fluoroantimonic acid catalysis. Even small amounts of the acid can dramatically alter water pH, potentially causing widespread damage to aquatic ecosystems. The acid's high reactivity with water also complicates wastewater treatment processes, necessitating specialized handling and neutralization techniques.
Air pollution is another environmental consideration. While the acid itself has low volatility, its use in industrial processes may generate harmful gaseous byproducts, including fluorine-containing compounds. These emissions can contribute to air quality degradation and potentially impact the ozone layer, requiring robust air treatment systems in manufacturing facilities.
The long-term environmental persistence of fluoroantimonic acid and its byproducts is an area of ongoing research. The acid's extreme reactivity suggests rapid decomposition in the environment, but the fate of its constituent elements and potential secondary reaction products remains a concern. Comprehensive lifecycle assessments are crucial to fully understand the environmental footprint of fluoroantimonic acid catalysis.
Efforts to enhance the environmental sustainability of fluoroantimonic acid catalysis focus on several key areas. These include developing more efficient catalytic processes to minimize acid consumption, improving containment and handling technologies to prevent releases, and exploring recycling methods to reduce waste. Additionally, research into alternative superacid catalysts with lower environmental impact is ongoing, aiming to balance catalytic performance with ecological considerations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!