How to Break New Ground in Chemistry with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, a superacid with extraordinary chemical properties, has been a subject of fascination and intensive research in the field of chemistry for decades. This compound, formed by mixing hydrogen fluoride (HF) and antimony pentafluoride (SbF5), is recognized as one of the strongest acids known to science, with a Hammett acidity function estimated to be as low as -28.
The development of fluoroantimonic acid can be traced back to the early 20th century when the concept of superacids was first introduced. However, it wasn't until the 1960s that significant progress was made in understanding and synthesizing this powerful compound. The groundbreaking work of George A. Olah in the field of superacids, which eventually led to his Nobel Prize in Chemistry in 1994, played a crucial role in advancing our knowledge of fluoroantimonic acid and its potential applications.
Over the years, the study of fluoroantimonic acid has evolved from purely theoretical investigations to practical applications in various fields of chemistry. Its extreme acidity and unique chemical properties have opened up new possibilities in organic synthesis, catalysis, and materials science. The ability of fluoroantimonic acid to protonate even very weak bases and generate highly reactive carbocations has made it an invaluable tool in the hands of chemists seeking to push the boundaries of molecular transformations.
The primary objective in exploring new frontiers with fluoroantimonic acid is to harness its exceptional properties for innovative chemical processes and reactions. Researchers aim to develop novel synthetic methodologies that can enable the creation of complex molecules, particularly those that are challenging or impossible to synthesize using conventional acids. Additionally, there is a growing interest in utilizing fluoroantimonic acid as a catalyst for industrial processes, potentially leading to more efficient and environmentally friendly chemical production methods.
Another key goal is to expand our fundamental understanding of superacidity and its effects on molecular structures and reactivity. By studying the behavior of fluoroantimonic acid at the molecular level, scientists hope to gain deeper insights into extreme acid-base chemistry and potentially discover new phenomena in chemical bonding and reactivity.
Furthermore, the research community is actively exploring ways to enhance the stability and handling of fluoroantimonic acid, as its extreme corrosiveness and reactivity pose significant challenges in practical applications. Developing safer and more manageable forms of this superacid, without compromising its unique properties, is a critical objective that could greatly expand its utility in both research and industrial settings.
The development of fluoroantimonic acid can be traced back to the early 20th century when the concept of superacids was first introduced. However, it wasn't until the 1960s that significant progress was made in understanding and synthesizing this powerful compound. The groundbreaking work of George A. Olah in the field of superacids, which eventually led to his Nobel Prize in Chemistry in 1994, played a crucial role in advancing our knowledge of fluoroantimonic acid and its potential applications.
Over the years, the study of fluoroantimonic acid has evolved from purely theoretical investigations to practical applications in various fields of chemistry. Its extreme acidity and unique chemical properties have opened up new possibilities in organic synthesis, catalysis, and materials science. The ability of fluoroantimonic acid to protonate even very weak bases and generate highly reactive carbocations has made it an invaluable tool in the hands of chemists seeking to push the boundaries of molecular transformations.
The primary objective in exploring new frontiers with fluoroantimonic acid is to harness its exceptional properties for innovative chemical processes and reactions. Researchers aim to develop novel synthetic methodologies that can enable the creation of complex molecules, particularly those that are challenging or impossible to synthesize using conventional acids. Additionally, there is a growing interest in utilizing fluoroantimonic acid as a catalyst for industrial processes, potentially leading to more efficient and environmentally friendly chemical production methods.
Another key goal is to expand our fundamental understanding of superacidity and its effects on molecular structures and reactivity. By studying the behavior of fluoroantimonic acid at the molecular level, scientists hope to gain deeper insights into extreme acid-base chemistry and potentially discover new phenomena in chemical bonding and reactivity.
Furthermore, the research community is actively exploring ways to enhance the stability and handling of fluoroantimonic acid, as its extreme corrosiveness and reactivity pose significant challenges in practical applications. Developing safer and more manageable forms of this superacid, without compromising its unique properties, is a critical objective that could greatly expand its utility in both research and industrial settings.
Market Demand Analysis for Superacid Applications
The market demand for superacid applications, particularly those involving fluoroantimonic acid, has been steadily growing across various industries. This powerful superacid, known for its extreme acidity and unique chemical properties, has found increasing relevance in sectors such as petrochemicals, pharmaceuticals, and materials science.
In the petrochemical industry, fluoroantimonic acid has become a crucial catalyst for various processes, including isomerization and alkylation reactions. The demand for more efficient and environmentally friendly catalysts has driven research into superacid applications, with fluoroantimonic acid emerging as a promising candidate for improving process efficiency and reducing energy consumption.
The pharmaceutical sector has also shown significant interest in superacid applications. Fluoroantimonic acid's ability to facilitate complex organic syntheses has made it valuable in drug discovery and development processes. As the industry continues to seek novel compounds and more efficient synthesis routes, the demand for superacid-based methodologies is expected to rise.
Materials science represents another key area of growth for superacid applications. Researchers are exploring the use of fluoroantimonic acid in the development of advanced materials, such as high-performance polymers and nanostructured materials. The unique properties of this superacid enable the creation of materials with enhanced characteristics, driving demand in industries ranging from aerospace to electronics.
The global push for sustainability and green chemistry has paradoxically increased interest in superacid applications. While fluoroantimonic acid itself is highly corrosive and challenging to handle, its potential to enable more efficient chemical processes could lead to reduced waste and energy consumption in various industrial applications. This has sparked research into developing safer handling methods and exploring the acid's role in green chemistry initiatives.
Market analysts project a compound annual growth rate (CAGR) for superacid applications in the mid-single digits over the next five years. This growth is attributed to increasing research and development activities, expanding industrial applications, and the continuous quest for process improvements across multiple sectors.
However, the market for superacid applications faces challenges, including safety concerns, regulatory hurdles, and the need for specialized equipment and handling procedures. These factors may limit widespread adoption in some industries but also present opportunities for innovation in safety technologies and application methodologies.
As industries continue to seek breakthrough solutions in chemistry, the demand for fluoroantimonic acid and other superacid applications is expected to evolve. The market's growth will likely be driven by advancements in handling technologies, discovery of novel applications, and the ongoing need for more efficient and sustainable chemical processes across various sectors.
In the petrochemical industry, fluoroantimonic acid has become a crucial catalyst for various processes, including isomerization and alkylation reactions. The demand for more efficient and environmentally friendly catalysts has driven research into superacid applications, with fluoroantimonic acid emerging as a promising candidate for improving process efficiency and reducing energy consumption.
The pharmaceutical sector has also shown significant interest in superacid applications. Fluoroantimonic acid's ability to facilitate complex organic syntheses has made it valuable in drug discovery and development processes. As the industry continues to seek novel compounds and more efficient synthesis routes, the demand for superacid-based methodologies is expected to rise.
Materials science represents another key area of growth for superacid applications. Researchers are exploring the use of fluoroantimonic acid in the development of advanced materials, such as high-performance polymers and nanostructured materials. The unique properties of this superacid enable the creation of materials with enhanced characteristics, driving demand in industries ranging from aerospace to electronics.
The global push for sustainability and green chemistry has paradoxically increased interest in superacid applications. While fluoroantimonic acid itself is highly corrosive and challenging to handle, its potential to enable more efficient chemical processes could lead to reduced waste and energy consumption in various industrial applications. This has sparked research into developing safer handling methods and exploring the acid's role in green chemistry initiatives.
Market analysts project a compound annual growth rate (CAGR) for superacid applications in the mid-single digits over the next five years. This growth is attributed to increasing research and development activities, expanding industrial applications, and the continuous quest for process improvements across multiple sectors.
However, the market for superacid applications faces challenges, including safety concerns, regulatory hurdles, and the need for specialized equipment and handling procedures. These factors may limit widespread adoption in some industries but also present opportunities for innovation in safety technologies and application methodologies.
As industries continue to seek breakthrough solutions in chemistry, the demand for fluoroantimonic acid and other superacid applications is expected to evolve. The market's growth will likely be driven by advancements in handling technologies, discovery of novel applications, and the ongoing need for more efficient and sustainable chemical processes across various sectors.
Current State and Challenges in Superacid Chemistry
Superacid chemistry has made significant strides in recent years, with fluoroantimonic acid (HSbF6) at the forefront of research and applications. This powerful superacid, with a Hammett acidity function (H0) of -31.3, far surpasses the acidity of pure sulfuric acid and has opened new avenues in chemical synthesis and catalysis.
The current state of superacid chemistry is characterized by intense research into novel applications and improved synthesis methods. Fluoroantimonic acid, being the strongest known superacid, has garnered particular attention due to its exceptional protonating ability and potential for activating inert compounds. Its use in organic synthesis has led to breakthroughs in the formation of carbocations and the activation of C-H bonds, enabling reactions that were previously unfeasible.
However, the field faces several significant challenges. The extreme reactivity of fluoroantimonic acid poses substantial handling and storage difficulties. Its corrosive nature necessitates specialized equipment and safety protocols, limiting widespread adoption in industrial settings. Moreover, the environmental impact of superacids remains a concern, with ongoing efforts to develop more sustainable and eco-friendly alternatives.
Another major challenge lies in the scalability of superacid-based processes. While laboratory-scale experiments have shown promising results, translating these findings to industrial-scale applications presents numerous engineering and safety hurdles. Researchers are actively working on developing reactor designs and process controls that can safely harness the power of superacids at larger scales.
The synthesis and purification of fluoroantimonic acid itself remain complex processes, requiring careful control of reaction conditions and sophisticated purification techniques. Improving the efficiency and yield of these processes is crucial for expanding the accessibility and applicability of this superacid in various chemical industries.
Furthermore, the theoretical understanding of superacid behavior in extreme acidity regimes is still evolving. Computational studies and advanced spectroscopic techniques are being employed to elucidate the molecular-level interactions and reaction mechanisms in superacidic media. This fundamental research is essential for predicting and optimizing superacid-catalyzed reactions.
In the realm of materials science, superacids like fluoroantimonic acid are being explored for their potential in developing novel materials with unique properties. From super-strong polymers to advanced catalysts, the ability of superacids to facilitate unconventional chemical transformations is opening new frontiers in materials engineering.
The current state of superacid chemistry is characterized by intense research into novel applications and improved synthesis methods. Fluoroantimonic acid, being the strongest known superacid, has garnered particular attention due to its exceptional protonating ability and potential for activating inert compounds. Its use in organic synthesis has led to breakthroughs in the formation of carbocations and the activation of C-H bonds, enabling reactions that were previously unfeasible.
However, the field faces several significant challenges. The extreme reactivity of fluoroantimonic acid poses substantial handling and storage difficulties. Its corrosive nature necessitates specialized equipment and safety protocols, limiting widespread adoption in industrial settings. Moreover, the environmental impact of superacids remains a concern, with ongoing efforts to develop more sustainable and eco-friendly alternatives.
Another major challenge lies in the scalability of superacid-based processes. While laboratory-scale experiments have shown promising results, translating these findings to industrial-scale applications presents numerous engineering and safety hurdles. Researchers are actively working on developing reactor designs and process controls that can safely harness the power of superacids at larger scales.
The synthesis and purification of fluoroantimonic acid itself remain complex processes, requiring careful control of reaction conditions and sophisticated purification techniques. Improving the efficiency and yield of these processes is crucial for expanding the accessibility and applicability of this superacid in various chemical industries.
Furthermore, the theoretical understanding of superacid behavior in extreme acidity regimes is still evolving. Computational studies and advanced spectroscopic techniques are being employed to elucidate the molecular-level interactions and reaction mechanisms in superacidic media. This fundamental research is essential for predicting and optimizing superacid-catalyzed reactions.
In the realm of materials science, superacids like fluoroantimonic acid are being explored for their potential in developing novel materials with unique properties. From super-strong polymers to advanced catalysts, the ability of superacids to facilitate unconventional chemical transformations is opening new frontiers in materials engineering.
Existing Methodologies for Fluoroantimonic Acid Synthesis
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in chemical reactions and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in organic synthesis and petrochemical industries.
- Use in materials science and surface treatments: Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It can be used to etch or modify surfaces of various materials, including metals and semiconductors. The acid's unique properties allow for precise control of surface characteristics, which is crucial in the development of advanced materials and electronic components.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are essential when working with this superacid. Proper storage, transportation, and disposal protocols must be followed to prevent accidents and environmental contamination.
- Analytical and research applications: Fluoroantimonic acid is used in various analytical and research applications. Its unique properties make it valuable in spectroscopic studies, particularly in NMR spectroscopy of highly acidic systems. The acid also serves as a reference point in acidity scales and is used in fundamental studies of superacidity and related phenomena in physical chemistry.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are challenging with conventional acid catalysts, making it valuable in the production of specialty chemicals and advanced materials.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It is used for etching, cleaning, and activating surfaces of metals, semiconductors, and other materials. The acid's strong protonating ability allows for the creation of unique surface properties and the development of advanced coatings and thin films.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires specialized equipment and strict safety protocols. Researchers have developed containment systems, protective gear, and neutralization methods to mitigate risks associated with its use. Proper storage, transportation, and disposal procedures are crucial to ensure safe handling of this powerful superacid.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analysis, and computational modeling. Researchers employ these techniques to understand the acid's structure, properties, and behavior in different chemical environments, which is crucial for optimizing its applications and developing new uses.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research and Production
The field of fluoroantimonic acid research is in its early development stage, with significant potential for groundbreaking discoveries. The market size remains relatively small but is expected to grow as applications in catalysis and materials science expand. Technologically, it is still in the experimental phase, with varying levels of maturity across different companies. 3M Innovative Properties Co. and Central Glass Co., Ltd. are leading in industrial applications, while academic institutions like Beijing Normal University and Yale University are pushing fundamental research boundaries. Companies such as Toyota Motor Corp. and DAIKIN INDUSTRIES Ltd. are exploring potential uses in advanced materials and manufacturing processes. The highly specialized nature of fluoroantimonic acid research creates a competitive yet collaborative environment, with both industry and academia contributing to its development.
Central Glass Co., Ltd.
Technical Solution: Central Glass has pioneered a method for using fluoroantimonic acid in the etching and surface modification of specialty glass products. Their technique involves carefully controlled exposure of glass surfaces to the superacid, allowing for precise etching patterns and the creation of unique surface properties. The company has developed specialized containment and application systems that enable the safe use of fluoroantimonic acid in industrial settings. Central Glass is also exploring the potential of the superacid in the synthesis of novel fluorine-containing compounds for use in the electronics and pharmaceutical industries.
Strengths: Specialized expertise in glass technology, innovative surface modification techniques, potential for new material development. Weaknesses: Highly niche application, significant safety and handling challenges, limited market for superacid-treated glass products.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has developed an innovative approach to using fluoroantimonic acid in the production of high-performance fluorinated materials. Their method involves using the superacid as a reaction medium for the synthesis of complex fluoropolymers and fluorinated organic compounds. DAIKIN's process includes a proprietary containment system that allows for the safe handling of large quantities of fluoroantimonic acid in industrial settings. The company is also exploring the use of the superacid in the development of new refrigerants and heat transfer fluids with exceptional thermal properties.
Strengths: Extensive experience with fluorine chemistry, potential for new high-performance materials, applications in HVAC industry. Weaknesses: High production costs, safety concerns in large-scale use, potential environmental impact of fluorinated products.
Breakthrough Innovations in Fluoroantimonic Acid Chemistry
A method for functionalization of an aromatic amino acid or a nucleobase
PatentActiveUS20220177514A1
Innovation
- A transition metal-free method using hypervalent iodine fluoroalkyl reagents with reductants to rapidly functionalize aromatic amino acids and nucleobases, forming fluoroalkylating radicals that selectively react with target residues, enabling fast and versatile bioconjugation.
Novel Fluorescent Amino Acid Derivative and Production Method Of The Same
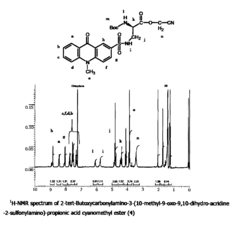
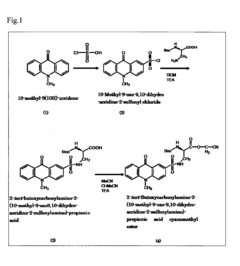
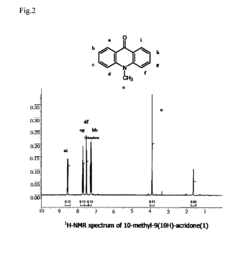
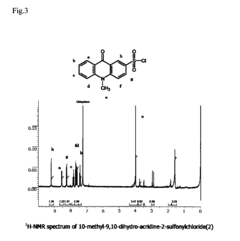
PatentInactiveUS20090240026A1
Innovation
- A novel fluorescent amino acid derivative is synthesized using a fluorescent acridone derivative with an electrophilic substitution reaction, incorporating an electron-withdrawing group such as a sulfonyl group, allowing excitation in the blue laser region with improved light stability and simplified production.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid, known as the world's strongest superacid, requires exceptionally stringent safety and handling protocols due to its extreme corrosiveness and reactivity. These protocols are crucial for protecting personnel, equipment, and the environment from potential hazards associated with its use.
Personal protective equipment (PPE) is paramount when working with fluoroantimonic acid. Researchers must wear fully encapsulating chemical-resistant suits, including gloves, boots, and face shields. The suit material must be carefully selected to withstand the acid's corrosive nature, with fluoropolymer-based materials being the most suitable. Respiratory protection, such as a self-contained breathing apparatus (SCBA), is essential to prevent inhalation of toxic fumes.
Specialized containment systems are necessary for storing and handling fluoroantimonic acid. These systems should be constructed from materials that can withstand the acid's corrosive properties, such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). Double containment is often employed to provide an additional layer of protection against leaks or spills.
Proper ventilation is critical in laboratories working with fluoroantimonic acid. Fume hoods equipped with specialized scrubbers are required to capture and neutralize any vapors or fumes generated during handling or reactions. The ventilation system should be regularly inspected and maintained to ensure optimal performance.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment kits specifically designed for superacids. Personnel must be trained in the proper use of these emergency equipment and in the specific protocols for responding to fluoroantimonic acid incidents.
Waste disposal protocols for fluoroantimonic acid are equally critical. The acid must be carefully neutralized using specialized techniques before disposal. This often involves a multi-step process, including dilution with ice-cold water and gradual neutralization with bases such as sodium hydroxide or calcium hydroxide, followed by proper treatment of the resulting waste.
Regular safety audits and training sessions are essential to maintain a high level of safety awareness and compliance. These should cover the specific hazards of fluoroantimonic acid, proper handling techniques, emergency procedures, and updates on any new safety protocols or equipment.
Lastly, strict access control measures should be implemented to ensure that only trained and authorized personnel can handle or access fluoroantimonic acid. This includes secure storage areas with limited access and detailed logging of all usage and handling events.
Personal protective equipment (PPE) is paramount when working with fluoroantimonic acid. Researchers must wear fully encapsulating chemical-resistant suits, including gloves, boots, and face shields. The suit material must be carefully selected to withstand the acid's corrosive nature, with fluoropolymer-based materials being the most suitable. Respiratory protection, such as a self-contained breathing apparatus (SCBA), is essential to prevent inhalation of toxic fumes.
Specialized containment systems are necessary for storing and handling fluoroantimonic acid. These systems should be constructed from materials that can withstand the acid's corrosive properties, such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). Double containment is often employed to provide an additional layer of protection against leaks or spills.
Proper ventilation is critical in laboratories working with fluoroantimonic acid. Fume hoods equipped with specialized scrubbers are required to capture and neutralize any vapors or fumes generated during handling or reactions. The ventilation system should be regularly inspected and maintained to ensure optimal performance.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment kits specifically designed for superacids. Personnel must be trained in the proper use of these emergency equipment and in the specific protocols for responding to fluoroantimonic acid incidents.
Waste disposal protocols for fluoroantimonic acid are equally critical. The acid must be carefully neutralized using specialized techniques before disposal. This often involves a multi-step process, including dilution with ice-cold water and gradual neutralization with bases such as sodium hydroxide or calcium hydroxide, followed by proper treatment of the resulting waste.
Regular safety audits and training sessions are essential to maintain a high level of safety awareness and compliance. These should cover the specific hazards of fluoroantimonic acid, proper handling techniques, emergency procedures, and updates on any new safety protocols or equipment.
Lastly, strict access control measures should be implemented to ensure that only trained and authorized personnel can handle or access fluoroantimonic acid. This includes secure storage areas with limited access and detailed logging of all usage and handling events.
Environmental Impact and Sustainability Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant environmental and sustainability challenges that must be carefully considered in its production, use, and disposal. The extreme corrosiveness and reactivity of this compound pose severe risks to ecosystems and human health if not properly managed.
The production of fluoroantimonic acid involves highly toxic and environmentally hazardous materials, including hydrogen fluoride and antimony pentafluoride. These precursors require stringent safety measures and containment protocols to prevent accidental releases. Any leaks or spills could result in devastating environmental consequences, including soil and water contamination, as well as potential harm to flora and fauna in the affected areas.
The use of fluoroantimonic acid in industrial processes also raises sustainability concerns. Its extreme acidity and reactivity mean that specialized equipment and materials are necessary for its handling and storage. This requirement often leads to increased resource consumption and energy expenditure, potentially offsetting any efficiency gains achieved through its use in chemical reactions.
Waste management and disposal of fluoroantimonic acid and its byproducts present another significant challenge. Traditional neutralization methods may be insufficient due to the compound's extreme acidity, necessitating specialized treatment processes. These processes must be carefully designed to ensure complete neutralization and prevent any residual environmental impact.
The long-term effects of fluoroantimonic acid on the environment are not fully understood, given its relatively limited use outside of specialized laboratory settings. However, its potential to persist in the environment and bioaccumulate in living organisms raises concerns about its long-term ecological impact. This uncertainty underscores the need for comprehensive environmental risk assessments and ongoing monitoring of areas where the acid is produced or used.
From a sustainability perspective, the use of fluoroantimonic acid in chemistry research and industrial applications must be balanced against its environmental risks. Researchers and industries must explore alternative compounds or processes that can achieve similar results with less environmental impact. This may involve developing new catalysts, reaction pathways, or entirely novel approaches to chemical synthesis that do not rely on such extreme reagents.
Efforts to improve the sustainability of fluoroantimonic acid use should focus on several key areas. These include enhancing containment and safety measures, developing more efficient and less resource-intensive production methods, improving waste treatment and neutralization techniques, and investigating potential recycling or reuse strategies for the acid and its precursors. Additionally, ongoing research into the long-term environmental fate and effects of fluoroantimonic acid is crucial for informed decision-making regarding its use and regulation.
The production of fluoroantimonic acid involves highly toxic and environmentally hazardous materials, including hydrogen fluoride and antimony pentafluoride. These precursors require stringent safety measures and containment protocols to prevent accidental releases. Any leaks or spills could result in devastating environmental consequences, including soil and water contamination, as well as potential harm to flora and fauna in the affected areas.
The use of fluoroantimonic acid in industrial processes also raises sustainability concerns. Its extreme acidity and reactivity mean that specialized equipment and materials are necessary for its handling and storage. This requirement often leads to increased resource consumption and energy expenditure, potentially offsetting any efficiency gains achieved through its use in chemical reactions.
Waste management and disposal of fluoroantimonic acid and its byproducts present another significant challenge. Traditional neutralization methods may be insufficient due to the compound's extreme acidity, necessitating specialized treatment processes. These processes must be carefully designed to ensure complete neutralization and prevent any residual environmental impact.
The long-term effects of fluoroantimonic acid on the environment are not fully understood, given its relatively limited use outside of specialized laboratory settings. However, its potential to persist in the environment and bioaccumulate in living organisms raises concerns about its long-term ecological impact. This uncertainty underscores the need for comprehensive environmental risk assessments and ongoing monitoring of areas where the acid is produced or used.
From a sustainability perspective, the use of fluoroantimonic acid in chemistry research and industrial applications must be balanced against its environmental risks. Researchers and industries must explore alternative compounds or processes that can achieve similar results with less environmental impact. This may involve developing new catalysts, reaction pathways, or entirely novel approaches to chemical synthesis that do not rely on such extreme reagents.
Efforts to improve the sustainability of fluoroantimonic acid use should focus on several key areas. These include enhancing containment and safety measures, developing more efficient and less resource-intensive production methods, improving waste treatment and neutralization techniques, and investigating potential recycling or reuse strategies for the acid and its precursors. Additionally, ongoing research into the long-term environmental fate and effects of fluoroantimonic acid is crucial for informed decision-making regarding its use and regulation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!