How to Influence Chemical Innovation Using Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful tool in chemical innovation. This compound, first synthesized in the 1960s, has gained significant attention due to its extraordinary acidity, surpassing even the strongest mineral acids known to science.
The development of fluoroantimonic acid marks a crucial milestone in the field of superacids, pushing the boundaries of what was previously thought possible in terms of acidity. Its Hammett acidity function (H0) is estimated to be as low as -31.3, making it billions of times stronger than 100% sulfuric acid. This extreme acidity opens up new possibilities for chemical reactions and transformations that were once considered unfeasible.
The primary objective of exploring fluoroantimonic acid in chemical innovation is to harness its unique properties for advancing various areas of chemistry and materials science. Its ability to protonate even extremely weak bases and catalyze challenging reactions has the potential to revolutionize synthetic methodologies, particularly in the production of novel compounds and materials.
One of the key goals in utilizing fluoroantimonic acid is to develop more efficient and selective catalytic processes. Its exceptional proton-donating capacity can activate substrates that are typically unreactive under conventional acidic conditions, potentially leading to new reaction pathways and improved yields in organic synthesis.
Furthermore, researchers aim to exploit the superacidic properties of fluoroantimonic acid to create novel materials with enhanced properties. This includes the development of super-strong polymers, advanced ceramics, and innovative surface treatments that could find applications in industries ranging from aerospace to electronics.
Another important objective is to understand and control the fundamental chemistry of fluoroantimonic acid at a molecular level. This knowledge is crucial for predicting and manipulating its behavior in various chemical environments, ultimately leading to safer handling procedures and more precise applications in both research and industrial settings.
As the field of superacid chemistry continues to evolve, the exploration of fluoroantimonic acid also aims to push the boundaries of our understanding of acidity itself. This includes investigating the limits of the acidity scale and potentially redefining our concepts of acid-base interactions in extreme chemical environments.
The development of fluoroantimonic acid marks a crucial milestone in the field of superacids, pushing the boundaries of what was previously thought possible in terms of acidity. Its Hammett acidity function (H0) is estimated to be as low as -31.3, making it billions of times stronger than 100% sulfuric acid. This extreme acidity opens up new possibilities for chemical reactions and transformations that were once considered unfeasible.
The primary objective of exploring fluoroantimonic acid in chemical innovation is to harness its unique properties for advancing various areas of chemistry and materials science. Its ability to protonate even extremely weak bases and catalyze challenging reactions has the potential to revolutionize synthetic methodologies, particularly in the production of novel compounds and materials.
One of the key goals in utilizing fluoroantimonic acid is to develop more efficient and selective catalytic processes. Its exceptional proton-donating capacity can activate substrates that are typically unreactive under conventional acidic conditions, potentially leading to new reaction pathways and improved yields in organic synthesis.
Furthermore, researchers aim to exploit the superacidic properties of fluoroantimonic acid to create novel materials with enhanced properties. This includes the development of super-strong polymers, advanced ceramics, and innovative surface treatments that could find applications in industries ranging from aerospace to electronics.
Another important objective is to understand and control the fundamental chemistry of fluoroantimonic acid at a molecular level. This knowledge is crucial for predicting and manipulating its behavior in various chemical environments, ultimately leading to safer handling procedures and more precise applications in both research and industrial settings.
As the field of superacid chemistry continues to evolve, the exploration of fluoroantimonic acid also aims to push the boundaries of our understanding of acidity itself. This includes investigating the limits of the acidity scale and potentially redefining our concepts of acid-base interactions in extreme chemical environments.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has shown significant growth potential in recent years. This powerful superacid, known for its extreme acidity and unique chemical properties, has found increasing use across various industrial sectors. The global superacid market, which includes fluoroantimonic acid, is projected to expand at a steady rate due to rising demand in petrochemical, pharmaceutical, and materials science industries.
In the petrochemical sector, fluoroantimonic acid plays a crucial role in catalyzing reactions for the production of high-octane gasoline and other fuel additives. This application has seen consistent growth, driven by the automotive industry's need for more efficient and cleaner-burning fuels. The pharmaceutical industry has also embraced fluoroantimonic acid for its ability to facilitate complex organic syntheses, particularly in the development of novel drug compounds. This has led to increased adoption in research laboratories and pharmaceutical manufacturing facilities.
The materials science field represents another significant market for fluoroantimonic acid applications. Its use in the production of advanced polymers and specialty chemicals has grown, particularly in the development of high-performance materials for aerospace and electronics industries. The acid's unique properties allow for the creation of materials with enhanced durability, heat resistance, and electrical properties.
Despite its growth potential, the market for fluoroantimonic acid faces challenges related to handling and safety concerns. The extreme corrosiveness of the acid necessitates specialized equipment and stringent safety protocols, which can increase operational costs for end-users. This has led to ongoing research into safer handling methods and alternative superacid formulations that maintain effectiveness while reducing risks.
Geographically, North America and Europe currently dominate the superacid market, with established research institutions and chemical industries driving demand. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by expanding industrial bases in countries like China and India. This regional shift is expected to reshape the global market landscape for superacid applications in the coming years.
The market is characterized by a relatively small number of specialized producers, with competition focused on product purity, technical support, and safety features. As research into new applications continues, the market is likely to see the entry of new players and the development of innovative superacid formulations tailored to specific industrial needs.
In the petrochemical sector, fluoroantimonic acid plays a crucial role in catalyzing reactions for the production of high-octane gasoline and other fuel additives. This application has seen consistent growth, driven by the automotive industry's need for more efficient and cleaner-burning fuels. The pharmaceutical industry has also embraced fluoroantimonic acid for its ability to facilitate complex organic syntheses, particularly in the development of novel drug compounds. This has led to increased adoption in research laboratories and pharmaceutical manufacturing facilities.
The materials science field represents another significant market for fluoroantimonic acid applications. Its use in the production of advanced polymers and specialty chemicals has grown, particularly in the development of high-performance materials for aerospace and electronics industries. The acid's unique properties allow for the creation of materials with enhanced durability, heat resistance, and electrical properties.
Despite its growth potential, the market for fluoroantimonic acid faces challenges related to handling and safety concerns. The extreme corrosiveness of the acid necessitates specialized equipment and stringent safety protocols, which can increase operational costs for end-users. This has led to ongoing research into safer handling methods and alternative superacid formulations that maintain effectiveness while reducing risks.
Geographically, North America and Europe currently dominate the superacid market, with established research institutions and chemical industries driving demand. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by expanding industrial bases in countries like China and India. This regional shift is expected to reshape the global market landscape for superacid applications in the coming years.
The market is characterized by a relatively small number of specialized producers, with competition focused on product purity, technical support, and safety features. As research into new applications continues, the market is likely to see the entry of new players and the development of innovative superacid formulations tailored to specific industrial needs.
Current Challenges in Fluoroantimonic Acid Usage
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its usage and application within chemical innovation. One of the primary obstacles is its extreme reactivity, which makes handling and storage exceptionally difficult. The acid's corrosive nature necessitates specialized containment materials, as it reacts violently with most substances, including glass and many metals.
The high reactivity of fluoroantimonic acid also poses safety concerns for researchers and technicians. Exposure risks are severe, requiring stringent safety protocols and protective equipment. This limits its practical use in many laboratory and industrial settings, restricting potential applications and innovations.
Another challenge lies in the acid's instability at room temperature. Fluoroantimonic acid decomposes rapidly, releasing toxic hydrogen fluoride gas. This instability complicates long-term storage and transportation, hindering its widespread adoption in chemical processes and research.
The environmental impact of fluoroantimonic acid is a significant concern. Its production and use generate hazardous waste, and accidental releases could have severe ecological consequences. This environmental risk factor limits its application in large-scale industrial processes and green chemistry initiatives.
Controlling the acid's reactivity for selective chemical transformations remains a substantial technical challenge. While its extreme acidity can catalyze unique reactions, harnessing this power for specific, controlled outcomes is difficult. This limits its potential in fine chemical synthesis and pharmaceutical applications.
The cost associated with producing and handling fluoroantimonic acid is prohibitively high for many research institutions and industries. This economic barrier restricts its use to specialized applications and high-value processes, limiting broader exploration of its potential in chemical innovation.
Regulatory hurdles also present challenges. The extreme hazards associated with fluoroantimonic acid result in strict regulations governing its use, transport, and disposal. These regulatory constraints can impede research and development efforts, particularly in academic and small-scale industrial settings.
Lastly, the lack of standardized protocols for working with fluoroantimonic acid hampers collaborative research and knowledge sharing. The specialized nature of handling this superacid means that expertise is concentrated in a few institutions, limiting the broader scientific community's ability to contribute to innovations in this field.
The high reactivity of fluoroantimonic acid also poses safety concerns for researchers and technicians. Exposure risks are severe, requiring stringent safety protocols and protective equipment. This limits its practical use in many laboratory and industrial settings, restricting potential applications and innovations.
Another challenge lies in the acid's instability at room temperature. Fluoroantimonic acid decomposes rapidly, releasing toxic hydrogen fluoride gas. This instability complicates long-term storage and transportation, hindering its widespread adoption in chemical processes and research.
The environmental impact of fluoroantimonic acid is a significant concern. Its production and use generate hazardous waste, and accidental releases could have severe ecological consequences. This environmental risk factor limits its application in large-scale industrial processes and green chemistry initiatives.
Controlling the acid's reactivity for selective chemical transformations remains a substantial technical challenge. While its extreme acidity can catalyze unique reactions, harnessing this power for specific, controlled outcomes is difficult. This limits its potential in fine chemical synthesis and pharmaceutical applications.
The cost associated with producing and handling fluoroantimonic acid is prohibitively high for many research institutions and industries. This economic barrier restricts its use to specialized applications and high-value processes, limiting broader exploration of its potential in chemical innovation.
Regulatory hurdles also present challenges. The extreme hazards associated with fluoroantimonic acid result in strict regulations governing its use, transport, and disposal. These regulatory constraints can impede research and development efforts, particularly in academic and small-scale industrial settings.
Lastly, the lack of standardized protocols for working with fluoroantimonic acid hampers collaborative research and knowledge sharing. The specialized nature of handling this superacid means that expertise is concentrated in a few institutions, limiting the broader scientific community's ability to contribute to innovations in this field.
Existing Applications of Fluoroantimonic Acid
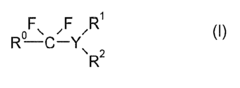
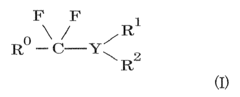
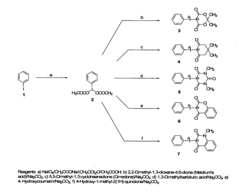
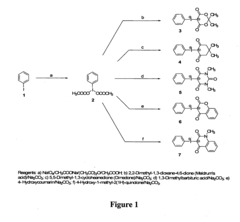
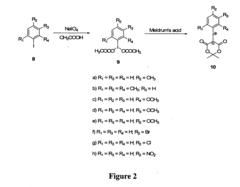
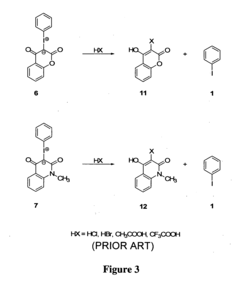
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized by combining hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of these highly reactive compounds under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the resulting superacid.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in the production of certain chemicals and materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The acid's unique properties make it suitable for generating highly reactive surfaces or interfaces in advanced materials.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and controlled environments. Proper neutralization and disposal methods are crucial to prevent environmental contamination and ensure worker safety.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analyses, and specialized titration procedures. Such techniques are essential for understanding the acid's behavior, determining its purity, and monitoring reactions in which it is involved.
02 Applications in chemical reactions and catalysis
Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions due to its extremely high acidity. It is particularly effective in promoting reactions such as isomerization, alkylation, and polymerization of hydrocarbons. The superacid's catalytic properties are exploited in industrial processes and organic synthesis.Expand Specific Solutions03 Material compatibility and storage
Due to its highly corrosive nature, fluoroantimonic acid requires specialized materials for handling and storage. Research has been conducted on developing compatible materials, such as fluoropolymers and certain alloys, that can withstand the acid's aggressive properties. Proper containment and storage techniques are crucial for safe handling and preventing environmental contamination.Expand Specific Solutions04 Safety measures and environmental considerations
Handling fluoroantimonic acid requires strict safety protocols due to its extreme reactivity and corrosiveness. Research has focused on developing improved safety measures, including personal protective equipment, containment systems, and neutralization techniques. Environmental impact assessments and waste management strategies have also been studied to minimize potential hazards associated with its use and disposal.Expand Specific Solutions05 Analytical applications and detection methods
Fluoroantimonic acid has found use in analytical chemistry and materials characterization. Research has been conducted on developing methods for detecting and quantifying the acid, as well as utilizing its unique properties for analyzing complex chemical mixtures. Spectroscopic and electrochemical techniques have been explored for these purposes.Expand Specific Solutions
Key Players in Superacid Research and Industry
The chemical innovation landscape utilizing fluoroantimonic acid is in a mature yet evolving stage, with a growing market driven by its unique superacid properties. The technology's maturity is evident from the involvement of established industry leaders like 3M Innovative Properties Co. and DuPont de Nemours, Inc., alongside academic institutions such as the University of California and Massachusetts Institute of Technology. The competitive field is diverse, spanning multinational corporations, specialized chemical companies, and research institutions, indicating a robust ecosystem for innovation. Companies like Mitsubishi Gas Chemical Co., Inc. and DAIKIN INDUSTRIES Ltd. are leveraging their expertise to explore new applications, while universities contribute to fundamental research, potentially leading to breakthrough developments in this niche but significant area of chemical technology.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the safe handling and application of fluoroantimonic acid in chemical synthesis. Their method involves using specialized containment systems made of fluoropolymer materials resistant to the acid's corrosive nature. The process incorporates advanced safety protocols, including remote handling techniques and real-time monitoring systems, to minimize risks associated with this superacid. DuPont's approach allows for controlled reactions in small-scale, high-value chemical production, particularly in the synthesis of novel fluorinated compounds for various industries.
Strengths: Expertise in handling hazardous materials, advanced safety protocols, potential for high-value chemical synthesis. Weaknesses: Limited large-scale applicability, high costs associated with specialized equipment and safety measures.
Mitsubishi Gas Chemical Co., Inc.
Technical Solution: Mitsubishi Gas Chemical has developed a novel catalytic system utilizing fluoroantimonic acid for the production of high-performance polymers. Their approach involves using the superacid as a catalyst in controlled polymerization reactions, resulting in materials with enhanced thermal and chemical resistance. The company has engineered a closed-loop system that allows for the efficient recycling and reuse of the acid, minimizing waste and environmental impact. This technology enables the production of specialized polymers for use in extreme environments, such as aerospace and deep-sea applications.
Strengths: Innovative polymer production, efficient acid recycling, applications in high-performance materials. Weaknesses: Specialized market focus, potential regulatory challenges due to the use of highly hazardous materials.
Breakthrough Innovations in Superacid Chemistry
Method of fluorination by microwaves
PatentInactiveEP2189466A2
Innovation
- A method involving fluorination of saccharides using specific fluorinating agents under thermal conditions or irradiation with microwave or electromagnetic waves, allowing for selective fluorination at specific positions within a wide temperature range, thereby overcoming the limitations of conventional methods.
No-Carrier-Added Nucleophilic [F-18] Fluorination of Aromatic Compounds
PatentInactiveUS20120123120A1
Innovation
- A novel aromatic nucleophilic halogenation reaction is developed without the addition of an ion carrier, using no-carrier-added [F-18] fluoride ion reacted with phenyliodonium ylide derivatives, which are synthesized through specific procedures, to achieve regiospecific F-18 labeled aromatic compounds suitable for PET.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that must be carefully addressed in any chemical innovation process. The extreme corrosiveness and reactivity of this compound necessitate stringent safety protocols and specialized handling equipment. Personal protective equipment (PPE) for workers must include fully encapsulating chemical-resistant suits, gloves, and respiratory protection to prevent any exposure.
Containment systems for fluoroantimonic acid require materials that can withstand its highly corrosive nature, such as fluoropolymers or certain alloys. Any storage or reaction vessels must be designed to prevent leaks and maintain the acid's anhydrous conditions. Emergency response plans and specialized neutralization procedures are essential in case of accidental releases.
Environmental considerations are equally critical when working with fluoroantimonic acid. Its potential for severe environmental damage if released necessitates robust containment and disposal protocols. Waste management systems must be designed to neutralize and safely dispose of any acid residues or byproducts. Air and water quality monitoring in the vicinity of facilities using this superacid is crucial to detect any potential contamination.
The use of fluoroantimonic acid in chemical innovations must also consider its lifecycle environmental impact. This includes the energy-intensive production process and the environmental footprint of its precursor materials, particularly hydrofluoric acid and antimony pentafluoride. Researchers should explore alternatives or methods to minimize the quantity of fluoroantimonic acid required in reactions to reduce overall environmental risks.
Regulatory compliance is another key aspect of safety and environmental considerations. Facilities working with fluoroantimonic acid must adhere to strict chemical safety regulations, including proper labeling, storage, and transportation guidelines. Environmental impact assessments and regular audits should be conducted to ensure compliance with local and international environmental protection standards.
In the context of chemical innovation, researchers must balance the unique catalytic properties of fluoroantimonic acid against these safety and environmental challenges. Developing safer handling techniques, exploring less hazardous alternatives, or creating novel applications that require minimal quantities of the acid are potential areas for innovation that could enhance both safety and environmental sustainability in chemical processes involving this powerful superacid.
Containment systems for fluoroantimonic acid require materials that can withstand its highly corrosive nature, such as fluoropolymers or certain alloys. Any storage or reaction vessels must be designed to prevent leaks and maintain the acid's anhydrous conditions. Emergency response plans and specialized neutralization procedures are essential in case of accidental releases.
Environmental considerations are equally critical when working with fluoroantimonic acid. Its potential for severe environmental damage if released necessitates robust containment and disposal protocols. Waste management systems must be designed to neutralize and safely dispose of any acid residues or byproducts. Air and water quality monitoring in the vicinity of facilities using this superacid is crucial to detect any potential contamination.
The use of fluoroantimonic acid in chemical innovations must also consider its lifecycle environmental impact. This includes the energy-intensive production process and the environmental footprint of its precursor materials, particularly hydrofluoric acid and antimony pentafluoride. Researchers should explore alternatives or methods to minimize the quantity of fluoroantimonic acid required in reactions to reduce overall environmental risks.
Regulatory compliance is another key aspect of safety and environmental considerations. Facilities working with fluoroantimonic acid must adhere to strict chemical safety regulations, including proper labeling, storage, and transportation guidelines. Environmental impact assessments and regular audits should be conducted to ensure compliance with local and international environmental protection standards.
In the context of chemical innovation, researchers must balance the unique catalytic properties of fluoroantimonic acid against these safety and environmental challenges. Developing safer handling techniques, exploring less hazardous alternatives, or creating novel applications that require minimal quantities of the acid are potential areas for innovation that could enhance both safety and environmental sustainability in chemical processes involving this powerful superacid.
Economic Impact of Fluoroantimonic Acid Innovations
The economic impact of fluoroantimonic acid innovations extends far beyond the realm of chemistry, influencing various sectors of the global economy. As the strongest known superacid, fluoroantimonic acid has the potential to revolutionize chemical processes, leading to significant cost reductions and efficiency improvements in industries such as petrochemicals, pharmaceuticals, and materials science.
In the petrochemical industry, fluoroantimonic acid catalysts could enhance the efficiency of oil refining processes, potentially reducing energy consumption and increasing yield. This could lead to lower fuel prices and decreased dependency on fossil fuels, impacting global energy markets and geopolitical dynamics.
The pharmaceutical sector stands to benefit from fluoroantimonic acid innovations through the development of novel synthetic routes for complex drug molecules. This could accelerate drug discovery processes, reduce production costs, and ultimately lead to more affordable medications for consumers. The economic implications of such advancements could be substantial, potentially reshaping the healthcare industry and improving access to life-saving treatments.
Materials science innovations utilizing fluoroantimonic acid may result in the creation of new super-strong or highly resistant materials. These materials could find applications in aerospace, automotive, and construction industries, driving technological advancements and economic growth in these sectors. The development of more durable and lightweight materials could lead to significant energy savings and reduced maintenance costs across various industries.
The environmental impact of fluoroantimonic acid innovations should also be considered from an economic perspective. While the acid itself is highly corrosive and dangerous, its application in green chemistry processes could lead to more sustainable industrial practices. This could result in reduced waste generation, lower emissions, and improved resource efficiency, potentially saving billions in environmental remediation costs and aligning with global sustainability goals.
However, the economic impact of fluoroantimonic acid innovations is not without challenges. The high cost of production and handling of this superacid may initially limit its widespread adoption. Additionally, safety concerns and regulatory requirements could pose significant barriers to entry for some industries, potentially slowing down the realization of its economic benefits.
In conclusion, the economic impact of fluoroantimonic acid innovations has the potential to be far-reaching and transformative. From reducing production costs in key industries to enabling the development of new materials and processes, the ripple effects of these innovations could significantly influence global economic trends and industrial practices in the coming years.
In the petrochemical industry, fluoroantimonic acid catalysts could enhance the efficiency of oil refining processes, potentially reducing energy consumption and increasing yield. This could lead to lower fuel prices and decreased dependency on fossil fuels, impacting global energy markets and geopolitical dynamics.
The pharmaceutical sector stands to benefit from fluoroantimonic acid innovations through the development of novel synthetic routes for complex drug molecules. This could accelerate drug discovery processes, reduce production costs, and ultimately lead to more affordable medications for consumers. The economic implications of such advancements could be substantial, potentially reshaping the healthcare industry and improving access to life-saving treatments.
Materials science innovations utilizing fluoroantimonic acid may result in the creation of new super-strong or highly resistant materials. These materials could find applications in aerospace, automotive, and construction industries, driving technological advancements and economic growth in these sectors. The development of more durable and lightweight materials could lead to significant energy savings and reduced maintenance costs across various industries.
The environmental impact of fluoroantimonic acid innovations should also be considered from an economic perspective. While the acid itself is highly corrosive and dangerous, its application in green chemistry processes could lead to more sustainable industrial practices. This could result in reduced waste generation, lower emissions, and improved resource efficiency, potentially saving billions in environmental remediation costs and aligning with global sustainability goals.
However, the economic impact of fluoroantimonic acid innovations is not without challenges. The high cost of production and handling of this superacid may initially limit its widespread adoption. Additionally, safety concerns and regulatory requirements could pose significant barriers to entry for some industries, potentially slowing down the realization of its economic benefits.
In conclusion, the economic impact of fluoroantimonic acid innovations has the potential to be far-reaching and transformative. From reducing production costs in key industries to enabling the development of new materials and processes, the ripple effects of these innovations could significantly influence global economic trends and industrial practices in the coming years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!