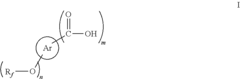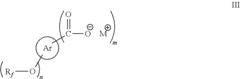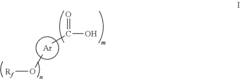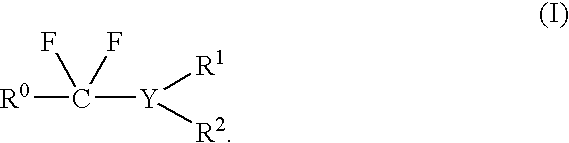Unleashing the Potential of Fluoroantimonic Acid in Technical Fields
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Evolution and Objectives
Fluoroantimonic acid, a superacid with extraordinary chemical properties, has undergone significant evolution since its discovery in the mid-20th century. Initially synthesized as a curiosity in laboratory settings, this compound has gradually emerged as a potential game-changer in various technical fields. The journey of fluoroantimonic acid from a mere scientific novelty to a substance of considerable industrial interest reflects the broader trends in advanced materials research and chemical engineering.
The development of fluoroantimonic acid can be traced through several key milestones. Early research focused on understanding its unique chemical structure and properties, particularly its extreme acidity. As analytical techniques improved, scientists gained deeper insights into its molecular behavior and reactivity. This fundamental knowledge laid the groundwork for exploring practical applications, marking a shift from purely theoretical interest to applied research.
In recent decades, the objectives surrounding fluoroantimonic acid have evolved significantly. The primary goal has transitioned from basic characterization to harnessing its exceptional properties for practical use. Researchers are now actively investigating its potential in catalysis, where its strong acidity could enable more efficient chemical reactions. The petroleum industry has shown particular interest, exploring its use in hydrocarbon cracking and isomerization processes.
Another key objective in the evolution of fluoroantimonic acid research is the development of safer handling and containment methods. Given its extreme corrosiveness, significant efforts have been directed towards creating specialized materials and protocols for its storage and use. This focus on safety and practicality is crucial for bridging the gap between laboratory curiosity and industrial application.
The current trajectory of fluoroantimonic acid research aims to unlock its full potential across various technical fields. Objectives include optimizing its synthesis for large-scale production, exploring novel applications in materials science, and investigating its role in advanced chemical processes. There is also growing interest in its potential environmental applications, such as in the breakdown of persistent pollutants.
Looking ahead, the goals for fluoroantimonic acid research are becoming increasingly interdisciplinary. Collaborations between chemists, materials scientists, and engineers are driving innovation in its application. The overarching aim is to transform this powerful superacid from a specialized laboratory tool into a versatile industrial agent, capable of revolutionizing processes across multiple sectors.
The development of fluoroantimonic acid can be traced through several key milestones. Early research focused on understanding its unique chemical structure and properties, particularly its extreme acidity. As analytical techniques improved, scientists gained deeper insights into its molecular behavior and reactivity. This fundamental knowledge laid the groundwork for exploring practical applications, marking a shift from purely theoretical interest to applied research.
In recent decades, the objectives surrounding fluoroantimonic acid have evolved significantly. The primary goal has transitioned from basic characterization to harnessing its exceptional properties for practical use. Researchers are now actively investigating its potential in catalysis, where its strong acidity could enable more efficient chemical reactions. The petroleum industry has shown particular interest, exploring its use in hydrocarbon cracking and isomerization processes.
Another key objective in the evolution of fluoroantimonic acid research is the development of safer handling and containment methods. Given its extreme corrosiveness, significant efforts have been directed towards creating specialized materials and protocols for its storage and use. This focus on safety and practicality is crucial for bridging the gap between laboratory curiosity and industrial application.
The current trajectory of fluoroantimonic acid research aims to unlock its full potential across various technical fields. Objectives include optimizing its synthesis for large-scale production, exploring novel applications in materials science, and investigating its role in advanced chemical processes. There is also growing interest in its potential environmental applications, such as in the breakdown of persistent pollutants.
Looking ahead, the goals for fluoroantimonic acid research are becoming increasingly interdisciplinary. Collaborations between chemists, materials scientists, and engineers are driving innovation in its application. The overarching aim is to transform this powerful superacid from a specialized laboratory tool into a versatile industrial agent, capable of revolutionizing processes across multiple sectors.
Industrial Applications and Market Demand
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various industrial sectors due to its exceptional chemical properties. The market demand for this powerful compound is driven by its unique ability to catalyze reactions that are otherwise difficult or impossible to achieve with conventional acids.
In the petrochemical industry, fluoroantimonic acid has found extensive applications in the isomerization of alkanes and the cracking of hydrocarbons. These processes are crucial for producing high-octane gasoline and other valuable petroleum products. The increasing global demand for cleaner and more efficient fuels has led to a growing interest in fluoroantimonic acid as a catalyst in refinery operations.
The electronics sector has also recognized the potential of fluoroantimonic acid in semiconductor manufacturing. Its strong etching properties make it valuable for creating intricate patterns on silicon wafers and other electronic components. As the demand for smaller, more powerful electronic devices continues to rise, the market for fluoroantimonic acid in this sector is expected to expand significantly.
In the field of materials science, fluoroantimonic acid has shown promise in the synthesis of novel materials with unique properties. Its ability to protonate even weak bases has opened up new possibilities for creating advanced polymers and composite materials. This has attracted attention from industries such as aerospace and automotive, where lightweight yet strong materials are in high demand.
The pharmaceutical industry has begun exploring the use of fluoroantimonic acid in the synthesis of complex organic compounds. Its strong acidic properties can facilitate certain reactions that are challenging with traditional catalysts, potentially leading to more efficient drug manufacturing processes. This application could drive significant market growth as pharmaceutical companies seek to optimize their production methods.
Despite its potential, the market for fluoroantimonic acid faces challenges due to its extreme corrosiveness and reactivity. Handling and storage requirements are stringent, which can limit its widespread adoption. However, ongoing research into safer handling methods and the development of specialized equipment is expected to mitigate these concerns and expand its market reach.
The global market for fluoroantimonic acid is relatively niche but growing steadily. While precise market size figures are not readily available due to the specialized nature of the product, industry experts anticipate a compound annual growth rate in the high single digits over the next five years. This growth is primarily driven by increasing demand in high-tech industries and ongoing research into new applications.
In the petrochemical industry, fluoroantimonic acid has found extensive applications in the isomerization of alkanes and the cracking of hydrocarbons. These processes are crucial for producing high-octane gasoline and other valuable petroleum products. The increasing global demand for cleaner and more efficient fuels has led to a growing interest in fluoroantimonic acid as a catalyst in refinery operations.
The electronics sector has also recognized the potential of fluoroantimonic acid in semiconductor manufacturing. Its strong etching properties make it valuable for creating intricate patterns on silicon wafers and other electronic components. As the demand for smaller, more powerful electronic devices continues to rise, the market for fluoroantimonic acid in this sector is expected to expand significantly.
In the field of materials science, fluoroantimonic acid has shown promise in the synthesis of novel materials with unique properties. Its ability to protonate even weak bases has opened up new possibilities for creating advanced polymers and composite materials. This has attracted attention from industries such as aerospace and automotive, where lightweight yet strong materials are in high demand.
The pharmaceutical industry has begun exploring the use of fluoroantimonic acid in the synthesis of complex organic compounds. Its strong acidic properties can facilitate certain reactions that are challenging with traditional catalysts, potentially leading to more efficient drug manufacturing processes. This application could drive significant market growth as pharmaceutical companies seek to optimize their production methods.
Despite its potential, the market for fluoroantimonic acid faces challenges due to its extreme corrosiveness and reactivity. Handling and storage requirements are stringent, which can limit its widespread adoption. However, ongoing research into safer handling methods and the development of specialized equipment is expected to mitigate these concerns and expand its market reach.
The global market for fluoroantimonic acid is relatively niche but growing steadily. While precise market size figures are not readily available due to the specialized nature of the product, industry experts anticipate a compound annual growth rate in the high single digits over the next five years. This growth is primarily driven by increasing demand in high-tech industries and ongoing research into new applications.
Current Challenges in Fluoroantimonic Acid Usage
Despite the remarkable potential of fluoroantimonic acid in various technical fields, its widespread adoption and application face several significant challenges. One of the primary obstacles is the extreme corrosiveness of the acid, which limits the materials that can be used for containment and handling. Traditional laboratory glassware and most metals are rapidly degraded by fluoroantimonic acid, necessitating the use of specialized materials such as Teflon or certain fluoropolymers. This requirement for exotic materials significantly increases the cost and complexity of working with the acid.
Safety concerns pose another major challenge in the utilization of fluoroantimonic acid. Its highly reactive nature and ability to produce toxic fumes upon contact with moisture make it exceptionally dangerous to handle. Stringent safety protocols and specialized equipment are essential, which can be prohibitively expensive for many research institutions and industrial applications. The need for extensive safety measures also limits the scalability of processes involving fluoroantimonic acid.
The environmental impact of fluoroantimonic acid usage is a growing concern. Its production and disposal can lead to the release of harmful fluorine compounds, contributing to environmental pollution and potential health hazards. Developing environmentally friendly methods for synthesizing and disposing of the acid remains a significant challenge for researchers and industry professionals.
Storage and transportation of fluoroantimonic acid present additional hurdles. The acid's extreme reactivity and sensitivity to moisture require specialized containers and controlled environments. This complicates logistics and increases costs associated with its use, particularly for applications requiring large quantities or long-distance transport.
Another challenge lies in the limited understanding of fluoroantimonic acid's full range of applications. While its superacidity properties are well-known, exploring and developing novel applications in catalysis, materials science, and other fields is hindered by the difficulties in handling and experimenting with the acid. This knowledge gap slows down potential innovations and technological advancements.
The regulatory landscape surrounding fluoroantimonic acid usage adds another layer of complexity. Strict regulations govern its production, transportation, and use due to its hazardous nature. Navigating these regulatory requirements can be time-consuming and costly, deterring some researchers and industries from exploring its potential applications.
Lastly, the lack of standardized protocols for working with fluoroantimonic acid poses challenges in research reproducibility and industrial process optimization. Developing consistent methodologies for its use across different applications and scales remains an ongoing challenge in the scientific and industrial communities.
Safety concerns pose another major challenge in the utilization of fluoroantimonic acid. Its highly reactive nature and ability to produce toxic fumes upon contact with moisture make it exceptionally dangerous to handle. Stringent safety protocols and specialized equipment are essential, which can be prohibitively expensive for many research institutions and industrial applications. The need for extensive safety measures also limits the scalability of processes involving fluoroantimonic acid.
The environmental impact of fluoroantimonic acid usage is a growing concern. Its production and disposal can lead to the release of harmful fluorine compounds, contributing to environmental pollution and potential health hazards. Developing environmentally friendly methods for synthesizing and disposing of the acid remains a significant challenge for researchers and industry professionals.
Storage and transportation of fluoroantimonic acid present additional hurdles. The acid's extreme reactivity and sensitivity to moisture require specialized containers and controlled environments. This complicates logistics and increases costs associated with its use, particularly for applications requiring large quantities or long-distance transport.
Another challenge lies in the limited understanding of fluoroantimonic acid's full range of applications. While its superacidity properties are well-known, exploring and developing novel applications in catalysis, materials science, and other fields is hindered by the difficulties in handling and experimenting with the acid. This knowledge gap slows down potential innovations and technological advancements.
The regulatory landscape surrounding fluoroantimonic acid usage adds another layer of complexity. Strict regulations govern its production, transportation, and use due to its hazardous nature. Navigating these regulatory requirements can be time-consuming and costly, deterring some researchers and industries from exploring its potential applications.
Lastly, the lack of standardized protocols for working with fluoroantimonic acid poses challenges in research reproducibility and industrial process optimization. Developing consistent methodologies for its use across different applications and scales remains an ongoing challenge in the scientific and industrial communities.
Existing Handling and Application Techniques
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and preparation methods: Fluoroantimonic acid is a superacid that can be synthesized through various methods. These methods often involve the combination of antimony pentafluoride and hydrogen fluoride under specific conditions. The synthesis process may require careful handling due to the highly corrosive and reactive nature of the components.
- Applications in catalysis: Fluoroantimonic acid finds applications as a powerful catalyst in various chemical reactions. Its strong acidity makes it effective for promoting reactions such as isomerization, alkylation, and polymerization. It is particularly useful in the petrochemical industry for processes involving hydrocarbons.
- Use in materials science: In materials science, fluoroantimonic acid is utilized for surface treatments and modifications of various materials. It can be employed in etching processes, particularly for semiconductors and other electronic materials. The acid's extreme reactivity allows for precise control in material processing applications.
- Safety and handling considerations: Due to its extremely corrosive and reactive nature, fluoroantimonic acid requires specialized safety measures and handling procedures. This includes the use of specific containment materials, personal protective equipment, and controlled environments. Proper storage, transportation, and disposal methods are crucial to prevent accidents and environmental contamination.
- Analytical and research applications: Fluoroantimonic acid is used in various analytical and research applications. Its unique properties make it valuable for studying reaction mechanisms, developing new synthetic methodologies, and exploring the behavior of materials under extreme acidic conditions. It can also be employed in specialized analytical techniques for chemical characterization.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts, making it valuable in the production of specialty chemicals and advanced materials.Expand Specific Solutions03 Use in materials science and surface treatments
Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It is used for etching and cleaning surfaces, especially in the semiconductor industry. The acid's strong protonating ability allows for the modification of various materials, enhancing their properties or preparing them for further processing.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal. Proper training and safety systems are essential to prevent accidents and environmental contamination.Expand Specific Solutions05 Analytical and characterization methods
Various analytical techniques have been developed to characterize fluoroantimonic acid and its reactions. These methods include spectroscopic analyses, electrochemical measurements, and specialized titration procedures. Advanced characterization techniques are crucial for understanding the acid's behavior, determining its purity, and monitoring reactions in which it is involved.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research
The development of fluoroantimonic acid technology is in its early stages, with significant potential for growth across various technical fields. The market size is currently limited but expected to expand as applications in catalysis, materials science, and chemical synthesis evolve. Technological maturity varies among key players, with companies like DuPont de Nemours, Inc., 3M Innovative Properties Co., and BASF Corp. leading in research and development. Academic institutions such as Central South University and Yale University contribute to fundamental research, while pharmaceutical companies like Pfizer Inc. and AbbVie, Inc. explore potential applications in drug discovery. The competitive landscape is characterized by a mix of established chemical companies, emerging specialized firms, and research institutions, each focusing on different aspects of fluoroantimonic acid's potential.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the safe production and handling of fluoroantimonic acid. Their method involves a controlled reaction between hydrogen fluoride and antimony pentafluoride in specialized corrosion-resistant vessels. The company has also engineered advanced containment systems and safety protocols to manage the highly reactive nature of the acid. DuPont's research focuses on utilizing fluoroantimonic acid as a super-acid catalyst in various organic synthesis reactions, particularly in the production of high-performance polymers and specialty chemicals.
Strengths: Extensive experience in handling hazardous materials, advanced safety protocols, and potential for catalyzing novel chemical reactions. Weaknesses: High production costs and limited large-scale applications due to the acid's extreme reactivity.
3M Innovative Properties Co.
Technical Solution: 3M has developed a novel approach to harnessing the potential of fluoroantimonic acid in materials science. Their research focuses on using the acid's extreme acidity to modify surface properties of various materials, particularly in the field of advanced coatings. 3M's proprietary process involves controlled exposure of materials to minute quantities of fluoroantimonic acid, resulting in unique surface characteristics such as enhanced chemical resistance, improved adhesion, or specific wettability properties. The company has also explored the use of fluoroantimonic acid in the development of new etching techniques for semiconductor manufacturing, potentially enabling the creation of more intricate and smaller circuit patterns.
Strengths: Innovative applications in materials science, potential for breakthrough in semiconductor manufacturing. Weaknesses: Challenges in scaling up processes due to the acid's extreme reactivity, high costs associated with safety measures.
Breakthrough Innovations in Superacid Chemistry
Process for the synthesis of fluorinated ethers of aromatic acids
PatentInactiveUS20110065892A1
Innovation
- The process involves preparing fluorinated ethers of aromatic acids through a reaction of halogenated aromatic acids with fluorinated alkyl groups, copper sources, and amino acid ligands in polar aprotic solvents, forming m-basic salts and subsequently converting them into fluorinated ethers, which are then incorporated into polymer backbones to enhance durability and flame retardance.
Method of fluorination
PatentInactiveUS7351863B2
Innovation
- A method involving the use of a fluorinating agent represented by general formula (I) or (III) under thermal or microwave-irradiated conditions, allowing for selective fluorination of saccharides at specific positions with improved safety and efficiency, using compounds like N,N-diethyl-α,α-difluoro(3-methyl)benzylamine or N,N-diethyl-α,α-difluoro(2-methoxy)benzylamine, which are stable at high temperatures and can be used in conjunction with microwave or electromagnetic wave irradiation.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that must be carefully addressed in its technical applications. The extreme corrosiveness and reactivity of this compound necessitate stringent handling protocols and specialized containment systems. Workers must be equipped with appropriate personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection, to prevent any potential exposure.
The storage and transportation of fluoroantimonic acid require specially designed containers made from materials resistant to its corrosive properties, such as polytetrafluoroethylene (PTFE) or certain fluoropolymers. These containers must be regularly inspected and maintained to ensure their integrity and prevent any leaks or spills. Additionally, dedicated storage facilities with proper ventilation, temperature control, and spill containment systems are essential to minimize environmental risks.
Environmental considerations are paramount when dealing with fluoroantimonic acid. Its potential for severe ecological damage if released into the environment cannot be overstated. Strict waste management protocols must be implemented to ensure proper neutralization and disposal of any acid residues or contaminated materials. This may involve specialized treatment facilities capable of handling such hazardous substances safely.
Emergency response plans must be developed and regularly updated to address potential accidents or spills. These plans should include detailed procedures for containment, neutralization, and decontamination, as well as protocols for notifying relevant authorities and evacuating affected areas if necessary. Regular training and drills for personnel involved in handling fluoroantimonic acid are crucial to ensure swift and effective responses to emergencies.
The use of fluoroantimonic acid in industrial processes requires careful engineering controls to prevent emissions and protect both workers and the environment. Closed-loop systems, robust ventilation, and advanced filtration technologies should be employed to minimize the risk of acid vapors or aerosols escaping into the workplace or atmosphere. Continuous monitoring systems for detecting leaks or atmospheric contamination are essential components of a comprehensive safety strategy.
Research into safer alternatives and process improvements should be ongoing to reduce reliance on fluoroantimonic acid where possible. This may include exploring less hazardous superacids or developing novel catalytic processes that achieve similar results with lower environmental impact. Additionally, life cycle assessments should be conducted to evaluate the overall environmental footprint of processes involving fluoroantimonic acid, considering factors such as energy consumption, resource depletion, and long-term ecological effects.
The storage and transportation of fluoroantimonic acid require specially designed containers made from materials resistant to its corrosive properties, such as polytetrafluoroethylene (PTFE) or certain fluoropolymers. These containers must be regularly inspected and maintained to ensure their integrity and prevent any leaks or spills. Additionally, dedicated storage facilities with proper ventilation, temperature control, and spill containment systems are essential to minimize environmental risks.
Environmental considerations are paramount when dealing with fluoroantimonic acid. Its potential for severe ecological damage if released into the environment cannot be overstated. Strict waste management protocols must be implemented to ensure proper neutralization and disposal of any acid residues or contaminated materials. This may involve specialized treatment facilities capable of handling such hazardous substances safely.
Emergency response plans must be developed and regularly updated to address potential accidents or spills. These plans should include detailed procedures for containment, neutralization, and decontamination, as well as protocols for notifying relevant authorities and evacuating affected areas if necessary. Regular training and drills for personnel involved in handling fluoroantimonic acid are crucial to ensure swift and effective responses to emergencies.
The use of fluoroantimonic acid in industrial processes requires careful engineering controls to prevent emissions and protect both workers and the environment. Closed-loop systems, robust ventilation, and advanced filtration technologies should be employed to minimize the risk of acid vapors or aerosols escaping into the workplace or atmosphere. Continuous monitoring systems for detecting leaks or atmospheric contamination are essential components of a comprehensive safety strategy.
Research into safer alternatives and process improvements should be ongoing to reduce reliance on fluoroantimonic acid where possible. This may include exploring less hazardous superacids or developing novel catalytic processes that achieve similar results with lower environmental impact. Additionally, life cycle assessments should be conducted to evaluate the overall environmental footprint of processes involving fluoroantimonic acid, considering factors such as energy consumption, resource depletion, and long-term ecological effects.
Economic Impact and Cost-Benefit Analysis
The economic impact of fluoroantimonic acid in technical fields is substantial, with far-reaching implications for various industries. As the strongest known superacid, its potential applications span across multiple sectors, including petrochemicals, pharmaceuticals, and advanced materials manufacturing. The cost-benefit analysis of implementing fluoroantimonic acid-based processes reveals a complex interplay of economic factors.
In the petrochemical industry, the use of fluoroantimonic acid as a catalyst in hydrocarbon cracking and isomerization processes has shown promising results. The increased efficiency in these processes can lead to significant cost savings in fuel production. However, the initial investment required for retrofitting existing facilities or constructing new ones capable of handling this highly corrosive substance is considerable. The long-term economic benefits must be weighed against these upfront costs.
The pharmaceutical sector stands to gain from the acid's potential in synthesizing complex organic compounds. Its ability to facilitate challenging chemical reactions could streamline drug development processes, potentially reducing time-to-market for new medications. This acceleration in drug discovery and production could translate into substantial economic gains for pharmaceutical companies and improved healthcare outcomes for society.
In advanced materials manufacturing, fluoroantimonic acid's unique properties offer opportunities for developing novel materials with enhanced characteristics. The production of high-performance polymers and advanced ceramics could be revolutionized, opening new markets and applications. The economic impact here extends beyond direct manufacturing costs to include potential advancements in industries that rely on these materials, such as aerospace and electronics.
However, the environmental and safety considerations associated with fluoroantimonic acid usage present significant economic challenges. The costs of implementing stringent safety measures, specialized handling equipment, and waste management systems are substantial. These expenses must be factored into any cost-benefit analysis, as they can significantly impact the overall economic viability of adopting fluoroantimonic acid-based processes.
The potential for increased productivity and efficiency in various technical fields must be balanced against the high costs of implementation and ongoing safety management. As research progresses and more efficient handling methods are developed, the economic equation may shift more favorably. Currently, the most promising economic benefits appear to be in high-value, specialized applications where the unique properties of fluoroantimonic acid can provide a significant competitive advantage.
In conclusion, while the economic potential of fluoroantimonic acid in technical fields is considerable, its realization depends on overcoming significant cost barriers and safety challenges. As technology advances and more applications are discovered, the cost-benefit ratio is likely to improve, potentially leading to wider adoption and greater economic impact across multiple industries.
In the petrochemical industry, the use of fluoroantimonic acid as a catalyst in hydrocarbon cracking and isomerization processes has shown promising results. The increased efficiency in these processes can lead to significant cost savings in fuel production. However, the initial investment required for retrofitting existing facilities or constructing new ones capable of handling this highly corrosive substance is considerable. The long-term economic benefits must be weighed against these upfront costs.
The pharmaceutical sector stands to gain from the acid's potential in synthesizing complex organic compounds. Its ability to facilitate challenging chemical reactions could streamline drug development processes, potentially reducing time-to-market for new medications. This acceleration in drug discovery and production could translate into substantial economic gains for pharmaceutical companies and improved healthcare outcomes for society.
In advanced materials manufacturing, fluoroantimonic acid's unique properties offer opportunities for developing novel materials with enhanced characteristics. The production of high-performance polymers and advanced ceramics could be revolutionized, opening new markets and applications. The economic impact here extends beyond direct manufacturing costs to include potential advancements in industries that rely on these materials, such as aerospace and electronics.
However, the environmental and safety considerations associated with fluoroantimonic acid usage present significant economic challenges. The costs of implementing stringent safety measures, specialized handling equipment, and waste management systems are substantial. These expenses must be factored into any cost-benefit analysis, as they can significantly impact the overall economic viability of adopting fluoroantimonic acid-based processes.
The potential for increased productivity and efficiency in various technical fields must be balanced against the high costs of implementation and ongoing safety management. As research progresses and more efficient handling methods are developed, the economic equation may shift more favorably. Currently, the most promising economic benefits appear to be in high-value, specialized applications where the unique properties of fluoroantimonic acid can provide a significant competitive advantage.
In conclusion, while the economic potential of fluoroantimonic acid in technical fields is considerable, its realization depends on overcoming significant cost barriers and safety challenges. As technology advances and more applications are discovered, the cost-benefit ratio is likely to improve, potentially leading to wider adoption and greater economic impact across multiple industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!