Influencing Chemical Paradigms with Fluoroantimonic Acid
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, a superacid with extraordinary chemical properties, has been a subject of intense scientific interest since its discovery in the mid-20th century. This compound, formed by combining hydrogen fluoride and antimony pentafluoride, exhibits unprecedented acidity levels, surpassing even the strongest mineral acids known to chemistry. The development of fluoroantimonic acid marks a significant milestone in the field of acid-base chemistry, pushing the boundaries of our understanding of proton transfer and molecular interactions.
The evolution of superacid chemistry can be traced back to the early 1900s, with the introduction of the Hammett acidity function. However, it was not until the 1960s that the concept of superacids gained prominence, largely due to the pioneering work of George A. Olah. The synthesis and characterization of fluoroantimonic acid in this period opened up new avenues for research in organic and inorganic chemistry, catalysis, and materials science.
The primary objective of exploring fluoroantimonic acid is to harness its extreme acidity for various applications. Its ability to protonate even exceedingly weak bases has made it an invaluable tool in studying reaction mechanisms, particularly in carbocation chemistry. Researchers aim to leverage this superacid's unique properties to develop novel synthetic routes, enhance catalytic processes, and create new materials with unprecedented characteristics.
In the realm of organic synthesis, fluoroantimonic acid holds promise for facilitating challenging transformations that are otherwise difficult or impossible with conventional acids. The goal is to exploit its protonating power to activate inert substrates, potentially leading to more efficient and selective chemical processes. This could have far-reaching implications for the pharmaceutical and fine chemical industries, where precise control over molecular transformations is crucial.
Another key objective is to investigate the fundamental nature of superacidity itself. By studying fluoroantimonic acid, scientists seek to deepen our understanding of proton behavior in extreme acidic environments, potentially redefining the limits of the pH scale. This fundamental research could lead to the discovery of even stronger acids or novel acid systems with unique properties.
The exploration of fluoroantimonic acid also aims to address significant challenges in its handling and application. Due to its extreme reactivity and corrosive nature, developing safe and practical methods for its use in both laboratory and industrial settings is a critical goal. This includes the design of specialized containment systems and the development of protocols for its controlled application in various chemical processes.
The evolution of superacid chemistry can be traced back to the early 1900s, with the introduction of the Hammett acidity function. However, it was not until the 1960s that the concept of superacids gained prominence, largely due to the pioneering work of George A. Olah. The synthesis and characterization of fluoroantimonic acid in this period opened up new avenues for research in organic and inorganic chemistry, catalysis, and materials science.
The primary objective of exploring fluoroantimonic acid is to harness its extreme acidity for various applications. Its ability to protonate even exceedingly weak bases has made it an invaluable tool in studying reaction mechanisms, particularly in carbocation chemistry. Researchers aim to leverage this superacid's unique properties to develop novel synthetic routes, enhance catalytic processes, and create new materials with unprecedented characteristics.
In the realm of organic synthesis, fluoroantimonic acid holds promise for facilitating challenging transformations that are otherwise difficult or impossible with conventional acids. The goal is to exploit its protonating power to activate inert substrates, potentially leading to more efficient and selective chemical processes. This could have far-reaching implications for the pharmaceutical and fine chemical industries, where precise control over molecular transformations is crucial.
Another key objective is to investigate the fundamental nature of superacidity itself. By studying fluoroantimonic acid, scientists seek to deepen our understanding of proton behavior in extreme acidic environments, potentially redefining the limits of the pH scale. This fundamental research could lead to the discovery of even stronger acids or novel acid systems with unique properties.
The exploration of fluoroantimonic acid also aims to address significant challenges in its handling and application. Due to its extreme reactivity and corrosive nature, developing safe and practical methods for its use in both laboratory and industrial settings is a critical goal. This includes the design of specialized containment systems and the development of protocols for its controlled application in various chemical processes.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has shown significant growth potential in recent years. This powerful superacid, known for its extreme acidity and unique chemical properties, has found increasing use across various industrial sectors. The petrochemical industry remains the largest consumer of superacids, utilizing them in catalytic processes for hydrocarbon isomerization and alkylation reactions. These applications contribute to the production of high-octane gasoline components and other valuable petrochemical products.
In the electronics sector, superacids play a crucial role in the etching and cleaning of semiconductor materials. As the demand for smaller and more efficient electronic devices continues to rise, the need for precise etching agents like fluoroantimonic acid is expected to grow correspondingly. This trend is further supported by the ongoing expansion of 5G networks and the Internet of Things (IoT), which require advanced semiconductor components.
The pharmaceutical industry has also begun to explore the potential of superacids in drug synthesis and purification processes. Fluoroantimonic acid's ability to catalyze certain reactions under milder conditions than traditional acids makes it an attractive option for developing more efficient and cost-effective manufacturing processes for complex pharmaceutical compounds.
Emerging applications in materials science, particularly in the development of advanced polymers and composites, are opening new avenues for superacid use. Researchers are investigating the potential of fluoroantimonic acid in creating novel materials with enhanced properties, such as improved thermal stability or chemical resistance.
The global market for superacids is projected to experience steady growth over the next decade. While exact figures vary among different market research reports, the consensus indicates a compound annual growth rate (CAGR) in the mid-single digits. This growth is primarily driven by increasing demand from established industries and the emergence of new applications in cutting-edge technologies.
However, the market for superacids, especially fluoroantimonic acid, faces certain challenges. Environmental and safety concerns associated with the handling and disposal of these highly corrosive substances pose significant regulatory hurdles. Additionally, the high cost of production and specialized handling requirements limit widespread adoption in some potential application areas.
Despite these challenges, ongoing research and development efforts are focused on addressing safety concerns and expanding the range of applications for superacids. As industries continue to seek more efficient and effective chemical processes, the demand for powerful catalysts and reagents like fluoroantimonic acid is likely to persist, driving further innovation and market growth in the superacid sector.
In the electronics sector, superacids play a crucial role in the etching and cleaning of semiconductor materials. As the demand for smaller and more efficient electronic devices continues to rise, the need for precise etching agents like fluoroantimonic acid is expected to grow correspondingly. This trend is further supported by the ongoing expansion of 5G networks and the Internet of Things (IoT), which require advanced semiconductor components.
The pharmaceutical industry has also begun to explore the potential of superacids in drug synthesis and purification processes. Fluoroantimonic acid's ability to catalyze certain reactions under milder conditions than traditional acids makes it an attractive option for developing more efficient and cost-effective manufacturing processes for complex pharmaceutical compounds.
Emerging applications in materials science, particularly in the development of advanced polymers and composites, are opening new avenues for superacid use. Researchers are investigating the potential of fluoroantimonic acid in creating novel materials with enhanced properties, such as improved thermal stability or chemical resistance.
The global market for superacids is projected to experience steady growth over the next decade. While exact figures vary among different market research reports, the consensus indicates a compound annual growth rate (CAGR) in the mid-single digits. This growth is primarily driven by increasing demand from established industries and the emergence of new applications in cutting-edge technologies.
However, the market for superacids, especially fluoroantimonic acid, faces certain challenges. Environmental and safety concerns associated with the handling and disposal of these highly corrosive substances pose significant regulatory hurdles. Additionally, the high cost of production and specialized handling requirements limit widespread adoption in some potential application areas.
Despite these challenges, ongoing research and development efforts are focused on addressing safety concerns and expanding the range of applications for superacids. As industries continue to seek more efficient and effective chemical processes, the demand for powerful catalysts and reagents like fluoroantimonic acid is likely to persist, driving further innovation and market growth in the superacid sector.
Current Challenges in Fluoroantimonic Acid Research
Fluoroantimonic acid, known as the world's strongest superacid, presents several significant challenges in current research. One of the primary obstacles is its extreme reactivity, which makes handling and storage exceptionally difficult. The acid's corrosive nature limits the materials that can be used for containment, with only a few fluoropolymers like Teflon being resistant to its effects.
The synthesis and purification of fluoroantimonic acid pose considerable challenges. The process requires precise control of reaction conditions and the use of highly pure precursors. Any impurities can significantly affect the acid's properties and reactivity, making consistent production at scale a formidable task.
Another major hurdle is the characterization of fluoroantimonic acid and its reactions. Traditional analytical techniques are often inadequate due to the acid's extreme reactivity. Spectroscopic methods are limited by the acid's tendency to react with or destroy conventional probe materials. This complicates efforts to study its structure and behavior in solution, hindering a deeper understanding of its chemical properties.
The environmental and safety concerns associated with fluoroantimonic acid present additional challenges. Its highly corrosive and toxic nature necessitates stringent safety protocols and specialized equipment for handling. Disposal of the acid and its byproducts requires careful consideration to prevent environmental contamination.
Researchers face difficulties in exploring the full potential of fluoroantimonic acid in organic synthesis and catalysis. While its superacidity offers unique opportunities for activating unreactive substrates, controlling its reactivity to achieve selective transformations remains a significant challenge. The acid's tendency to protonate and decompose many organic compounds limits its applicability in complex molecule synthesis.
The development of practical applications for fluoroantimonic acid is hindered by its extreme properties. While it shows promise in areas such as hydrocarbon cracking and isomerization, translating laboratory findings into industrial processes presents numerous engineering and safety challenges. The acid's incompatibility with most materials complicates the design of large-scale reactors and processing equipment.
Theoretical understanding of fluoroantimonic acid's behavior at the molecular level is another area of ongoing research. Computational studies are challenging due to the complex electronic structures involved and the limitations of current models in accurately representing superacidic systems. This gap in theoretical knowledge hampers efforts to predict and optimize the acid's reactivity in various chemical transformations.
The synthesis and purification of fluoroantimonic acid pose considerable challenges. The process requires precise control of reaction conditions and the use of highly pure precursors. Any impurities can significantly affect the acid's properties and reactivity, making consistent production at scale a formidable task.
Another major hurdle is the characterization of fluoroantimonic acid and its reactions. Traditional analytical techniques are often inadequate due to the acid's extreme reactivity. Spectroscopic methods are limited by the acid's tendency to react with or destroy conventional probe materials. This complicates efforts to study its structure and behavior in solution, hindering a deeper understanding of its chemical properties.
The environmental and safety concerns associated with fluoroantimonic acid present additional challenges. Its highly corrosive and toxic nature necessitates stringent safety protocols and specialized equipment for handling. Disposal of the acid and its byproducts requires careful consideration to prevent environmental contamination.
Researchers face difficulties in exploring the full potential of fluoroantimonic acid in organic synthesis and catalysis. While its superacidity offers unique opportunities for activating unreactive substrates, controlling its reactivity to achieve selective transformations remains a significant challenge. The acid's tendency to protonate and decompose many organic compounds limits its applicability in complex molecule synthesis.
The development of practical applications for fluoroantimonic acid is hindered by its extreme properties. While it shows promise in areas such as hydrocarbon cracking and isomerization, translating laboratory findings into industrial processes presents numerous engineering and safety challenges. The acid's incompatibility with most materials complicates the design of large-scale reactors and processing equipment.
Theoretical understanding of fluoroantimonic acid's behavior at the molecular level is another area of ongoing research. Computational studies are challenging due to the complex electronic structures involved and the limitations of current models in accurately representing superacidic systems. This gap in theoretical knowledge hampers efforts to predict and optimize the acid's reactivity in various chemical transformations.
Existing Applications of Fluoroantimonic Acid
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are challenging or impossible with conventional acids, making it valuable in the production of specialty chemicals and advanced materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It is used for etching, cleaning, and activating surfaces of metals, semiconductors, and other materials. The acid's unique properties allow for precise control of surface characteristics, enhancing material performance in specific applications.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and controlled environments. Proper disposal and neutralization techniques are essential to prevent environmental contamination and ensure worker safety.
- Analytical and characterization methods: Various analytical techniques have been developed to characterize fluoroantimonic acid and its derivatives. These methods include spectroscopic analysis, electrochemical measurements, and specialized titration procedures. Advanced characterization techniques are crucial for determining the acid's purity, composition, and properties, which are essential for its effective use in research and industrial applications.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates reactions such as alkylation, isomerization, and polymerization of hydrocarbons. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials such as polymers and ceramics.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These methods include spectroscopic techniques, electrochemical analysis, and specialized apparatus designed to handle and analyze this highly reactive superacid under controlled conditions.Expand Specific Solutions
Key Players in Superacid Industry
The field of fluoroantimonic acid research is in its early developmental stage, with a growing market driven by its potential applications in various industries. The global market size for superacids, including fluoroantimonic acid, is expanding, albeit still relatively niche. Technologically, the field is moderately mature, with ongoing research to enhance its applications and safety. Key players like DuPont de Nemours, Inc., BASF Corp., and Central Glass Co., Ltd. are at the forefront of development, leveraging their expertise in chemical engineering. Academic institutions such as Harvard College and Yale University are contributing significant research, while companies like 3M Innovative Properties Co. are exploring novel applications. The competitive landscape is characterized by a mix of established chemical companies and specialized research institutions, each contributing to the advancement of fluoroantimonic acid technology.
Pfizer Inc.
Technical Solution: Pfizer has explored the use of fluoroantimonic acid in pharmaceutical synthesis, particularly for the creation of complex organic molecules. Their approach involves using fluoroantimonic acid as a powerful activating agent in multi-step synthesis processes. This allows for the formation of challenging chemical bonds and the generation of novel molecular structures with potential therapeutic applications. Pfizer's method also incorporates advanced purification techniques to ensure the removal of any acid residues from the final products, meeting strict pharmaceutical quality standards.
Strengths: Enables synthesis of complex pharmaceutical compounds, potential for new drug discoveries. Weaknesses: High cost of acid handling in pharmaceutical settings, regulatory challenges.
Central Glass Co., Ltd.
Technical Solution: Central Glass has developed an innovative application of fluoroantimonic acid in the field of surface treatment for advanced materials. Their technique involves using highly diluted fluoroantimonic acid solutions to create nanoscale etching patterns on various substrates, including metals and semiconductors. This process allows for precise control over surface properties, enhancing the performance of materials in applications such as electronics and catalysis. Central Glass has also implemented a closed-loop system for acid management, minimizing waste and environmental impact.
Strengths: Enables precise nanoscale surface modifications, applicable to various materials. Weaknesses: Requires stringent safety measures, limited to specialized applications.
Innovations in Fluoroantimonic Acid Synthesis
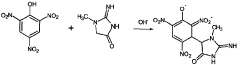
Method for the production of [<18>f] fluoride-marked aromatic l-amino acids
PatentWO2005037737A1
Innovation
- A method involving nucleophilic substitution of a negatively charged 18F fluoride ion with a suitable L-enantiomeric compound, followed by cleavage of protective groups, to produce 18F fluorine-labeled aromatic L-amino acids in a few steps, ensuring high reproducibility and stereochemical purity.
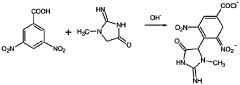
Fluorimetric analytical method for the determination of creatinine in clinically significant biological samples and a fluorimetric reagent for use in this method
PatentWO2021086209A1
Innovation
- A fluorimetric method using a multi-component reagent containing 3,5-dinitrobenzoate anions, hydrogen peroxide, and an organic solvent in a strongly alkaline medium, which reacts with creatinine to produce a fluorophore detectable at specific wavelengths, offering improved selectivity and precision.
Safety and Handling Protocols
Fluoroantimonic acid, recognized as one of the strongest superacids known, demands exceptionally stringent safety and handling protocols due to its extreme corrosiveness and reactivity. The primary concern when working with this compound is its ability to react violently with water and most organic materials, necessitating specialized containment and personal protective equipment.
Proper storage of fluoroantimonic acid requires the use of fluoropolymer containers, such as those made from polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). These materials resist the acid's corrosive nature and prevent degradation of the storage vessel. The storage area must be kept cool, dry, and well-ventilated to minimize the risk of accidental reactions or vapor accumulation.
Personal protective equipment (PPE) for handling fluoroantimonic acid includes fully encapsulating chemical-resistant suits, preferably made from fluoropolymer materials. Respiratory protection is crucial, with the use of self-contained breathing apparatus (SCBA) recommended for any potential exposure. Double-layered gloves, with an inner layer of fluoroelastomer and an outer layer of neoprene or butyl rubber, provide the necessary hand protection.
Handling procedures must be conducted in a designated area with restricted access, preferably within a fume hood equipped with acid-resistant ductwork. All equipment used in the handling process, including pipettes and transfer vessels, must be constructed from fluoropolymer materials. Strict protocols for the addition of reagents and the order of mixing must be followed to prevent uncontrolled reactions.
Emergency response plans are critical when working with fluoroantimonic acid. Specialized spill kits containing neutralizing agents compatible with superacids, such as sodium bicarbonate or calcium oxide, should be readily available. Personnel must be trained in proper evacuation procedures and the use of emergency showers and eyewash stations.
Disposal of fluoroantimonic acid and contaminated materials requires careful consideration. Neutralization should be performed under controlled conditions, typically involving slow addition to a large excess of crushed ice or very cold water, followed by neutralization with a base. The resulting solution can then be treated as hazardous waste and disposed of according to local regulations.
Regular safety audits and equipment inspections are essential to maintain the integrity of containment systems and PPE. Documentation of all handling procedures, including risk assessments and safety data sheets, must be kept up-to-date and readily accessible to all personnel working with or in proximity to fluoroantimonic acid.
Proper storage of fluoroantimonic acid requires the use of fluoropolymer containers, such as those made from polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). These materials resist the acid's corrosive nature and prevent degradation of the storage vessel. The storage area must be kept cool, dry, and well-ventilated to minimize the risk of accidental reactions or vapor accumulation.
Personal protective equipment (PPE) for handling fluoroantimonic acid includes fully encapsulating chemical-resistant suits, preferably made from fluoropolymer materials. Respiratory protection is crucial, with the use of self-contained breathing apparatus (SCBA) recommended for any potential exposure. Double-layered gloves, with an inner layer of fluoroelastomer and an outer layer of neoprene or butyl rubber, provide the necessary hand protection.
Handling procedures must be conducted in a designated area with restricted access, preferably within a fume hood equipped with acid-resistant ductwork. All equipment used in the handling process, including pipettes and transfer vessels, must be constructed from fluoropolymer materials. Strict protocols for the addition of reagents and the order of mixing must be followed to prevent uncontrolled reactions.
Emergency response plans are critical when working with fluoroantimonic acid. Specialized spill kits containing neutralizing agents compatible with superacids, such as sodium bicarbonate or calcium oxide, should be readily available. Personnel must be trained in proper evacuation procedures and the use of emergency showers and eyewash stations.
Disposal of fluoroantimonic acid and contaminated materials requires careful consideration. Neutralization should be performed under controlled conditions, typically involving slow addition to a large excess of crushed ice or very cold water, followed by neutralization with a base. The resulting solution can then be treated as hazardous waste and disposed of according to local regulations.
Regular safety audits and equipment inspections are essential to maintain the integrity of containment systems and PPE. Documentation of all handling procedures, including risk assessments and safety data sheets, must be kept up-to-date and readily accessible to all personnel working with or in proximity to fluoroantimonic acid.
Environmental Impact Assessment
The environmental impact of fluoroantimonic acid, the world's strongest superacid, is a critical concern that demands thorough assessment. This highly corrosive substance poses significant risks to ecosystems and human health if not properly managed. When released into the environment, fluoroantimonic acid can cause severe damage to soil and water systems, leading to long-term ecological disruptions.
In aquatic environments, even small quantities of fluoroantimonic acid can dramatically alter pH levels, potentially decimating fish populations and aquatic vegetation. The acid's extreme reactivity with water produces toxic fumes, including hydrogen fluoride, which can harm both terrestrial and aquatic organisms. Soil contamination by fluoroantimonic acid can render large areas infertile, disrupting plant growth and microbial communities essential for ecosystem balance.
The production and use of fluoroantimonic acid also raise air quality concerns. Accidental releases or improper handling can result in the emission of hazardous gases, contributing to air pollution and potentially affecting human respiratory health in surrounding areas. The acid's ability to react with many substances, including glass and metals, complicates containment efforts and increases the risk of environmental exposure.
Long-term environmental effects of fluoroantimonic acid contamination may include bioaccumulation of fluoride compounds in food chains, potentially impacting wildlife and human populations. The persistence of fluoride in the environment could lead to chronic exposure issues, affecting bone development and thyroid function in various species.
Mitigation strategies for environmental risks associated with fluoroantimonic acid must be robust and multifaceted. These should include stringent containment protocols, advanced treatment technologies for waste streams, and comprehensive emergency response plans. Continuous environmental monitoring in areas where the acid is produced or used is essential to detect and address potential leaks or spills promptly.
Research into less hazardous alternatives and the development of more environmentally friendly synthesis methods for superacids should be prioritized. Additionally, regulatory frameworks must evolve to ensure proper handling, storage, and disposal practices are enforced across industries utilizing fluoroantimonic acid.
In conclusion, while fluoroantimonic acid offers unique capabilities in chemical processes, its potential environmental impact necessitates careful management and ongoing research to minimize risks and ensure sustainable use.
In aquatic environments, even small quantities of fluoroantimonic acid can dramatically alter pH levels, potentially decimating fish populations and aquatic vegetation. The acid's extreme reactivity with water produces toxic fumes, including hydrogen fluoride, which can harm both terrestrial and aquatic organisms. Soil contamination by fluoroantimonic acid can render large areas infertile, disrupting plant growth and microbial communities essential for ecosystem balance.
The production and use of fluoroantimonic acid also raise air quality concerns. Accidental releases or improper handling can result in the emission of hazardous gases, contributing to air pollution and potentially affecting human respiratory health in surrounding areas. The acid's ability to react with many substances, including glass and metals, complicates containment efforts and increases the risk of environmental exposure.
Long-term environmental effects of fluoroantimonic acid contamination may include bioaccumulation of fluoride compounds in food chains, potentially impacting wildlife and human populations. The persistence of fluoride in the environment could lead to chronic exposure issues, affecting bone development and thyroid function in various species.
Mitigation strategies for environmental risks associated with fluoroantimonic acid must be robust and multifaceted. These should include stringent containment protocols, advanced treatment technologies for waste streams, and comprehensive emergency response plans. Continuous environmental monitoring in areas where the acid is produced or used is essential to detect and address potential leaks or spills promptly.
Research into less hazardous alternatives and the development of more environmentally friendly synthesis methods for superacids should be prioritized. Additionally, regulatory frameworks must evolve to ensure proper handling, storage, and disposal practices are enforced across industries utilizing fluoroantimonic acid.
In conclusion, while fluoroantimonic acid offers unique capabilities in chemical processes, its potential environmental impact necessitates careful management and ongoing research to minimize risks and ensure sustainable use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!