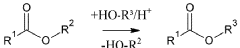How to Enhance Experimental Precision with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Research Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has been a subject of intense scientific interest since its discovery in the 1960s. This compound holds the distinction of being the strongest known superacid, with a Hammett acidity function estimated to be as low as -28. Its extreme acidity surpasses that of pure sulfuric acid by more than a trillion times, making it a powerful tool in various chemical processes and research applications.
The development of fluoroantimonic acid marked a significant milestone in the field of superacid chemistry. Its unique properties stem from the synergistic interaction between HF and SbF5, resulting in the formation of extremely acidic proton species. This superacid's ability to protonate even very weak bases has opened up new possibilities in organic synthesis, catalysis, and materials science.
Over the years, researchers have explored the potential applications of fluoroantimonic acid in diverse areas. Its exceptional protonating power has been utilized in the isomerization of alkanes, the generation of carbocations, and the activation of C-H bonds in hydrocarbons. These capabilities have significant implications for the petrochemical industry and the development of novel synthetic methodologies.
However, the extreme reactivity of fluoroantimonic acid also presents considerable challenges in its handling and application. Its corrosive nature and sensitivity to moisture necessitate specialized equipment and rigorous safety protocols. These factors have limited its widespread use in industrial settings and have driven ongoing research to develop more stable and manageable superacid systems.
The primary objective of current research involving fluoroantimonic acid is to enhance experimental precision while mitigating the inherent risks associated with its use. This goal encompasses several key areas of focus. Firstly, researchers aim to develop more accurate methods for measuring and controlling the acidity of fluoroantimonic acid solutions. This is crucial for ensuring reproducibility and reliability in experiments involving this superacid.
Secondly, there is a concerted effort to design and implement advanced containment and handling systems that can withstand the extreme corrosiveness of fluoroantimonic acid while maintaining experimental integrity. This includes the development of specialized materials for reaction vessels and analytical instruments capable of operating under highly acidic conditions.
Furthermore, researchers are exploring ways to modulate the acidity of fluoroantimonic acid systems, allowing for finer control over reaction conditions. This involves investigating the effects of varying the HF:SbF5 ratio and introducing stabilizing additives to create more manageable superacid media without significantly compromising their unique properties.
Ultimately, the overarching goal is to harness the extraordinary capabilities of fluoroantimonic acid while minimizing its associated risks and limitations. By addressing these challenges, researchers hope to expand the practical applications of this superacid and unlock new possibilities in chemical synthesis, materials processing, and analytical chemistry.
The development of fluoroantimonic acid marked a significant milestone in the field of superacid chemistry. Its unique properties stem from the synergistic interaction between HF and SbF5, resulting in the formation of extremely acidic proton species. This superacid's ability to protonate even very weak bases has opened up new possibilities in organic synthesis, catalysis, and materials science.
Over the years, researchers have explored the potential applications of fluoroantimonic acid in diverse areas. Its exceptional protonating power has been utilized in the isomerization of alkanes, the generation of carbocations, and the activation of C-H bonds in hydrocarbons. These capabilities have significant implications for the petrochemical industry and the development of novel synthetic methodologies.
However, the extreme reactivity of fluoroantimonic acid also presents considerable challenges in its handling and application. Its corrosive nature and sensitivity to moisture necessitate specialized equipment and rigorous safety protocols. These factors have limited its widespread use in industrial settings and have driven ongoing research to develop more stable and manageable superacid systems.
The primary objective of current research involving fluoroantimonic acid is to enhance experimental precision while mitigating the inherent risks associated with its use. This goal encompasses several key areas of focus. Firstly, researchers aim to develop more accurate methods for measuring and controlling the acidity of fluoroantimonic acid solutions. This is crucial for ensuring reproducibility and reliability in experiments involving this superacid.
Secondly, there is a concerted effort to design and implement advanced containment and handling systems that can withstand the extreme corrosiveness of fluoroantimonic acid while maintaining experimental integrity. This includes the development of specialized materials for reaction vessels and analytical instruments capable of operating under highly acidic conditions.
Furthermore, researchers are exploring ways to modulate the acidity of fluoroantimonic acid systems, allowing for finer control over reaction conditions. This involves investigating the effects of varying the HF:SbF5 ratio and introducing stabilizing additives to create more manageable superacid media without significantly compromising their unique properties.
Ultimately, the overarching goal is to harness the extraordinary capabilities of fluoroantimonic acid while minimizing its associated risks and limitations. By addressing these challenges, researchers hope to expand the practical applications of this superacid and unlock new possibilities in chemical synthesis, materials processing, and analytical chemistry.
Market Demand for High-Precision Experimental Techniques
The demand for high-precision experimental techniques in the field of fluoroantimonic acid research has been steadily increasing over the past decade. This surge in interest is primarily driven by the unique properties of fluoroantimonic acid, which is known as one of the strongest superacids. Its ability to protonate even very weak bases makes it an invaluable tool in various industrial and research applications.
In the chemical industry, there is a growing need for more precise and controlled reactions, especially in the production of high-value specialty chemicals. Fluoroantimonic acid's extreme acidity allows for catalysis of reactions that are otherwise difficult or impossible to achieve. This has led to increased demand for techniques that can harness its power while maintaining experimental precision.
The pharmaceutical sector has also shown significant interest in high-precision techniques involving fluoroantimonic acid. Drug discovery and development processes often require the synthesis of complex organic compounds, where traditional methods may fall short. The use of fluoroantimonic acid in these processes can potentially open new pathways for molecule creation, but requires extremely precise control to avoid unwanted side reactions or degradation of target compounds.
In the field of materials science, researchers are exploring the use of fluoroantimonic acid for surface modifications and the creation of novel materials. The demand for precise experimental techniques in this area is driven by the need to control acid-substrate interactions at the molecular level, which can lead to the development of materials with unique properties.
The petrochemical industry has also expressed interest in high-precision techniques involving fluoroantimonic acid, particularly for isomerization reactions and the cracking of hydrocarbons. The ability to precisely control these processes could lead to more efficient fuel production and the development of new petrochemical products.
Academic research institutions are another significant driver of demand for high-precision experimental techniques with fluoroantimonic acid. As researchers delve deeper into understanding chemical reactivity and developing new synthetic methodologies, the need for tools that can provide unprecedented levels of control and precision continues to grow.
The market for analytical instruments and laboratory equipment capable of handling fluoroantimonic acid with high precision is expanding. Manufacturers are developing specialized containment systems, reaction vessels, and measurement tools to meet the stringent requirements of working with this superacid. This has created a niche market for high-end, corrosion-resistant equipment designed specifically for superacid experiments.
As environmental concerns become more prominent, there is also a growing demand for techniques that can minimize the use of fluoroantimonic acid while maximizing its effectiveness. This has led to research into microreactor technologies and flow chemistry systems that can precisely control the acid's application in small-scale, highly efficient processes.
In the chemical industry, there is a growing need for more precise and controlled reactions, especially in the production of high-value specialty chemicals. Fluoroantimonic acid's extreme acidity allows for catalysis of reactions that are otherwise difficult or impossible to achieve. This has led to increased demand for techniques that can harness its power while maintaining experimental precision.
The pharmaceutical sector has also shown significant interest in high-precision techniques involving fluoroantimonic acid. Drug discovery and development processes often require the synthesis of complex organic compounds, where traditional methods may fall short. The use of fluoroantimonic acid in these processes can potentially open new pathways for molecule creation, but requires extremely precise control to avoid unwanted side reactions or degradation of target compounds.
In the field of materials science, researchers are exploring the use of fluoroantimonic acid for surface modifications and the creation of novel materials. The demand for precise experimental techniques in this area is driven by the need to control acid-substrate interactions at the molecular level, which can lead to the development of materials with unique properties.
The petrochemical industry has also expressed interest in high-precision techniques involving fluoroantimonic acid, particularly for isomerization reactions and the cracking of hydrocarbons. The ability to precisely control these processes could lead to more efficient fuel production and the development of new petrochemical products.
Academic research institutions are another significant driver of demand for high-precision experimental techniques with fluoroantimonic acid. As researchers delve deeper into understanding chemical reactivity and developing new synthetic methodologies, the need for tools that can provide unprecedented levels of control and precision continues to grow.
The market for analytical instruments and laboratory equipment capable of handling fluoroantimonic acid with high precision is expanding. Manufacturers are developing specialized containment systems, reaction vessels, and measurement tools to meet the stringent requirements of working with this superacid. This has created a niche market for high-end, corrosion-resistant equipment designed specifically for superacid experiments.
As environmental concerns become more prominent, there is also a growing demand for techniques that can minimize the use of fluoroantimonic acid while maximizing its effectiveness. This has led to research into microreactor technologies and flow chemistry systems that can precisely control the acid's application in small-scale, highly efficient processes.
Current Challenges in Fluoroantimonic Acid Utilization
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its utilization for enhancing experimental precision. The primary obstacle lies in its extreme reactivity, which makes handling and containment exceptionally difficult. Standard laboratory glassware and most conventional materials are rapidly corroded or dissolved by this powerful acid, necessitating specialized equipment and protocols.
The volatility of fluoroantimonic acid poses another major challenge. Its tendency to release highly toxic hydrogen fluoride gas requires stringent safety measures and specialized ventilation systems. This not only complicates experimental setups but also introduces potential sources of error and contamination, which can compromise precision.
Maintaining the purity and stability of fluoroantimonic acid is crucial for experimental accuracy, yet it remains a significant hurdle. The acid's hygroscopic nature means it readily absorbs moisture from the air, altering its composition and potentially leading to side reactions. This necessitates storage and handling under strictly anhydrous conditions, which can be technically demanding and resource-intensive.
The extreme acidity of fluoroantimonic acid also presents challenges in measurement and standardization. Traditional pH scales and measurement techniques are inadequate for such strong acids, requiring the development and use of alternative acidity functions like the Hammett acidity function. This complicates the comparison and reproducibility of experiments across different laboratories.
Temperature control during experiments with fluoroantimonic acid is another critical challenge. The acid's highly exothermic reactions can lead to rapid and significant temperature fluctuations, potentially affecting reaction kinetics and experimental outcomes. Precise temperature regulation systems are essential but can be difficult to implement given the acid's corrosive nature.
The disposal and neutralization of fluoroantimonic acid after experiments pose environmental and safety challenges. Its extreme reactivity makes it hazardous to neutralize, and improper disposal can lead to severe environmental contamination. Developing safe, efficient, and environmentally friendly disposal methods remains an ongoing challenge in the field.
Lastly, the limited availability and high cost of high-purity fluoroantimonic acid restrict its widespread use in research and industry. This scarcity can lead to compromises in experimental design or the use of lower-quality alternatives, potentially impacting the precision and reliability of results. Overcoming these challenges requires innovative approaches in acid synthesis, handling, and experimental design to fully harness the potential of fluoroantimonic acid in enhancing experimental precision.
The volatility of fluoroantimonic acid poses another major challenge. Its tendency to release highly toxic hydrogen fluoride gas requires stringent safety measures and specialized ventilation systems. This not only complicates experimental setups but also introduces potential sources of error and contamination, which can compromise precision.
Maintaining the purity and stability of fluoroantimonic acid is crucial for experimental accuracy, yet it remains a significant hurdle. The acid's hygroscopic nature means it readily absorbs moisture from the air, altering its composition and potentially leading to side reactions. This necessitates storage and handling under strictly anhydrous conditions, which can be technically demanding and resource-intensive.
The extreme acidity of fluoroantimonic acid also presents challenges in measurement and standardization. Traditional pH scales and measurement techniques are inadequate for such strong acids, requiring the development and use of alternative acidity functions like the Hammett acidity function. This complicates the comparison and reproducibility of experiments across different laboratories.
Temperature control during experiments with fluoroantimonic acid is another critical challenge. The acid's highly exothermic reactions can lead to rapid and significant temperature fluctuations, potentially affecting reaction kinetics and experimental outcomes. Precise temperature regulation systems are essential but can be difficult to implement given the acid's corrosive nature.
The disposal and neutralization of fluoroantimonic acid after experiments pose environmental and safety challenges. Its extreme reactivity makes it hazardous to neutralize, and improper disposal can lead to severe environmental contamination. Developing safe, efficient, and environmentally friendly disposal methods remains an ongoing challenge in the field.
Lastly, the limited availability and high cost of high-purity fluoroantimonic acid restrict its widespread use in research and industry. This scarcity can lead to compromises in experimental design or the use of lower-quality alternatives, potentially impacting the precision and reliability of results. Overcoming these challenges requires innovative approaches in acid synthesis, handling, and experimental design to fully harness the potential of fluoroantimonic acid in enhancing experimental precision.
Existing Precision Enhancement Methods with Fluoroantimonic Acid
01 Precision measurement techniques for fluoroantimonic acid
Advanced measurement techniques are employed to enhance the precision of fluoroantimonic acid experiments. These methods may include spectroscopic analysis, high-precision titration, and specialized analytical instruments designed to handle highly corrosive substances. The focus is on improving accuracy and reproducibility in quantitative analysis of this superacid.- Precision measurement techniques for fluoroantimonic acid: Advanced measurement techniques are employed to enhance the experimental precision when working with fluoroantimonic acid. These methods may include spectroscopic analysis, high-precision titration, and specialized electrochemical sensors designed to withstand the highly corrosive nature of the acid.
- Specialized equipment for handling fluoroantimonic acid: Due to the extreme reactivity of fluoroantimonic acid, specialized equipment is required for its handling and experimentation. This may include corrosion-resistant containers, advanced fume hoods, and precision dispensing systems designed to maintain the integrity of the acid and ensure accurate measurements.
- Calibration methods for fluoroantimonic acid experiments: Precise calibration methods are crucial for maintaining experimental accuracy when working with fluoroantimonic acid. These may involve the use of reference standards, regular equipment checks, and specialized calibration procedures tailored to the unique properties of this superacid.
- Safety protocols for fluoroantimonic acid precision experiments: Stringent safety protocols are essential when conducting precision experiments with fluoroantimonic acid. These may include advanced personal protective equipment, specialized containment systems, and emergency response procedures designed to mitigate the risks associated with this highly corrosive substance.
- Data analysis and error minimization in fluoroantimonic acid experiments: Advanced data analysis techniques and error minimization strategies are employed to ensure the highest level of precision in fluoroantimonic acid experiments. This may involve the use of sophisticated statistical methods, computer modeling, and machine learning algorithms to process and interpret experimental results.
02 Specialized equipment for handling fluoroantimonic acid
Due to the extreme corrosiveness of fluoroantimonic acid, specialized equipment is crucial for experimental precision. This includes corrosion-resistant containers, precise dispensing systems, and safety apparatus. The equipment is designed to minimize contamination and ensure accurate handling of the superacid during experiments.Expand Specific Solutions03 Environmental control for fluoroantimonic acid experiments
Maintaining a controlled environment is essential for precise experiments with fluoroantimonic acid. This involves regulating temperature, humidity, and atmospheric conditions to prevent unwanted reactions or degradation of the acid. Specialized chambers or gloveboxes may be used to create an inert atmosphere for handling this highly reactive substance.Expand Specific Solutions04 Calibration and standardization methods for fluoroantimonic acid analysis
To ensure experimental precision, rigorous calibration and standardization methods are developed for fluoroantimonic acid analysis. This includes the use of reference materials, standardized protocols for instrument calibration, and inter-laboratory comparisons to validate results and minimize systematic errors in measurements.Expand Specific Solutions05 Data processing and error analysis in fluoroantimonic acid experiments
Advanced data processing techniques and error analysis methods are employed to enhance the precision of fluoroantimonic acid experiments. This involves statistical analysis of experimental data, uncertainty quantification, and the use of computational models to interpret results and identify potential sources of error in measurements.Expand Specific Solutions
Key Players in Superacid Research and Development
The enhancement of experimental precision with fluoroantimonic acid is a highly specialized field in advanced chemical research. The market is currently in a growth phase, with increasing demand for ultra-precise measurements in various industries. Key players like Siemens Corp., Roche Diagnostics GmbH, and Koninklijke Philips NV are investing heavily in R&D to develop cutting-edge applications. The technology's maturity varies, with companies like Battelle Memorial Institute and Tsinghua University leading in fundamental research, while QuantaRed Technologies GmbH and Hamamatsu Photonics KK focus on practical implementations. The market size is expanding, driven by applications in materials science, pharmaceuticals, and advanced manufacturing, with potential for significant growth in the coming years.
Zhonghao Chenguang Research Institute of Chemical Ind Co Ltd
Technical Solution: Zhonghao Chenguang Research Institute has developed a proprietary method for synthesizing high-purity fluoroantimonic acid using advanced fluorination techniques. Their process involves carefully controlled reactions between antimony pentafluoride and hydrogen fluoride under precise temperature and pressure conditions. The resulting fluoroantimonic acid is then purified using specialized distillation and crystallization methods to achieve ultra-high purity levels exceeding 99.99%. The institute has also engineered custom-designed reaction vessels and handling equipment made from materials resistant to this extremely corrosive superacid.
Strengths: Expertise in fluorine chemistry, advanced purification techniques, and specialized equipment for handling superacids. Weaknesses: High production costs and limited scalability due to the hazardous nature of the materials involved.
Hubei Sinophorus Electronic Materials Co., Ltd.
Technical Solution: Hubei Sinophorus has developed a novel approach to enhance the experimental precision of fluoroantimonic acid-based reactions. Their method involves using specially designed microreactors with precise temperature control and inert gas purging systems. These microreactors allow for extremely small-scale reactions, minimizing the amount of hazardous materials used while maintaining high precision. The company has also created a unique online monitoring system that uses spectroscopic techniques to analyze the reaction in real-time, allowing for immediate adjustments to reaction conditions to optimize yield and purity.
Strengths: Innovative microreactor technology, real-time monitoring capabilities, and improved safety due to small-scale reactions. Weaknesses: Limited applicability to large-scale industrial processes and high initial investment costs for specialized equipment.
Innovative Approaches in Fluoroantimonic Acid Handling
Process for quantitative determination of fatty acid esters in fuels
PatentWO2019048124A1
Innovation
- A selective chemical reaction is applied to the analyte, altering the carbonyl band intensity, allowing for more precise measurement using FTIR spectroscopy with quantum cascade lasers, which reduces interference and enhances selectivity, enabling robust and accurate on-site analysis.
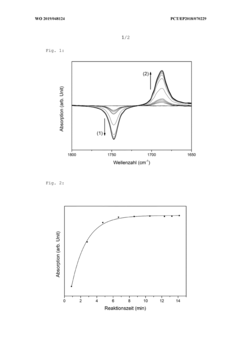
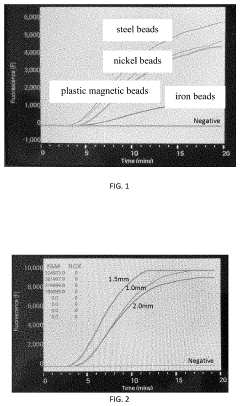
Method of enhancing isothermal amplification sensitivity of nucleic acid and reagents thereof
PatentInactiveUS20200362399A1
Innovation
- The method involves using magnetic beads with specific materials, diameters, and mixing times within the RAA reaction system to enhance isothermal amplification sensitivity, including the use of steel beads with a 1.5 mm diameter and a 20-second mixing time, combined with agarose gel and fluorescence detection.
Safety Protocols and Environmental Considerations
The use of fluoroantimonic acid in experimental procedures necessitates stringent safety protocols and environmental considerations due to its extreme corrosiveness and reactivity. Proper handling and storage of this superacid are paramount to ensure the safety of laboratory personnel and prevent environmental contamination.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. Researchers must wear chemical-resistant suits, gloves, and face shields or goggles. Respiratory protection, such as a self-contained breathing apparatus, is essential to prevent inhalation of toxic fumes. All PPE should be thoroughly inspected before use and replaced immediately if any signs of degradation are observed.
Specialized containment systems are required for the storage and handling of fluoroantimonic acid. These systems should be constructed from materials resistant to superacids, such as fluoropolymers or certain grades of stainless steel. Double containment is recommended to prevent accidental spills or leaks. Ventilation systems must be designed to effectively remove any vapors or fumes generated during experiments.
Emergency response procedures must be established and regularly practiced. This includes the availability of appropriate spill kits, neutralizing agents, and decontamination equipment. Personnel should be trained in proper evacuation procedures and the use of emergency eyewash stations and safety showers.
Environmental considerations are equally critical when working with fluoroantimonic acid. Strict waste management protocols must be implemented to prevent the release of this hazardous substance into the environment. Neutralization of waste should be performed using appropriate bases, followed by proper disposal through licensed hazardous waste management facilities.
Monitoring systems should be in place to detect any potential leaks or emissions. Regular environmental assessments should be conducted to ensure that laboratory activities do not impact surrounding ecosystems or groundwater. Compliance with local, national, and international regulations regarding the use and disposal of superacids is essential.
Research institutions should establish clear guidelines for the transportation of fluoroantimonic acid, including proper packaging, labeling, and documentation. Only trained personnel should be authorized to handle and transport this substance, and all movements should be carefully tracked and recorded.
Continuous training and education of laboratory staff are vital to maintain a culture of safety and environmental responsibility. Regular safety audits and reviews of protocols should be conducted to identify and address any potential risks or areas for improvement in handling fluoroantimonic acid.
By implementing these comprehensive safety protocols and environmental considerations, researchers can minimize the risks associated with using fluoroantimonic acid while maximizing experimental precision and reliability.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. Researchers must wear chemical-resistant suits, gloves, and face shields or goggles. Respiratory protection, such as a self-contained breathing apparatus, is essential to prevent inhalation of toxic fumes. All PPE should be thoroughly inspected before use and replaced immediately if any signs of degradation are observed.
Specialized containment systems are required for the storage and handling of fluoroantimonic acid. These systems should be constructed from materials resistant to superacids, such as fluoropolymers or certain grades of stainless steel. Double containment is recommended to prevent accidental spills or leaks. Ventilation systems must be designed to effectively remove any vapors or fumes generated during experiments.
Emergency response procedures must be established and regularly practiced. This includes the availability of appropriate spill kits, neutralizing agents, and decontamination equipment. Personnel should be trained in proper evacuation procedures and the use of emergency eyewash stations and safety showers.
Environmental considerations are equally critical when working with fluoroantimonic acid. Strict waste management protocols must be implemented to prevent the release of this hazardous substance into the environment. Neutralization of waste should be performed using appropriate bases, followed by proper disposal through licensed hazardous waste management facilities.
Monitoring systems should be in place to detect any potential leaks or emissions. Regular environmental assessments should be conducted to ensure that laboratory activities do not impact surrounding ecosystems or groundwater. Compliance with local, national, and international regulations regarding the use and disposal of superacids is essential.
Research institutions should establish clear guidelines for the transportation of fluoroantimonic acid, including proper packaging, labeling, and documentation. Only trained personnel should be authorized to handle and transport this substance, and all movements should be carefully tracked and recorded.
Continuous training and education of laboratory staff are vital to maintain a culture of safety and environmental responsibility. Regular safety audits and reviews of protocols should be conducted to identify and address any potential risks or areas for improvement in handling fluoroantimonic acid.
By implementing these comprehensive safety protocols and environmental considerations, researchers can minimize the risks associated with using fluoroantimonic acid while maximizing experimental precision and reliability.
Interdisciplinary Applications of Fluoroantimonic Acid
Fluoroantimonic acid, known as the strongest superacid, has found applications beyond its traditional use in organic synthesis and catalysis. Its unique properties have led to interdisciplinary applications across various scientific and industrial fields, showcasing its versatility and potential for enhancing experimental precision.
In materials science, fluoroantimonic acid has been employed to create novel surface modifications on metals and semiconductors. By carefully controlling the acid's concentration and exposure time, researchers have achieved unprecedented levels of surface etching precision, enabling the fabrication of nanoscale structures with enhanced properties. This has significant implications for the development of advanced electronic devices and high-performance materials.
The field of analytical chemistry has also benefited from fluoroantimonic acid's extreme acidity. It has been utilized in the development of highly sensitive detection methods for trace elements and compounds. By leveraging its ability to protonate even weakly basic species, researchers have improved the detection limits of various analytical techniques, including mass spectrometry and chromatography.
In the realm of energy research, fluoroantimonic acid has shown promise in the development of next-generation battery technologies. Its strong oxidizing properties have been exploited to create novel electrode materials with improved stability and conductivity. This application has the potential to significantly enhance the performance and longevity of high-capacity energy storage systems.
The pharmaceutical industry has explored the use of fluoroantimonic acid in drug discovery and synthesis. Its ability to catalyze challenging chemical transformations has led to the development of new synthetic routes for complex drug molecules. This has not only improved the efficiency of drug production but also opened up possibilities for creating previously inaccessible pharmaceutical compounds.
Environmental science has found applications for fluoroantimonic acid in the treatment of persistent organic pollutants. Its extreme acidity allows for the breakdown of highly stable chemical compounds that are resistant to conventional treatment methods. This approach has shown promise in the remediation of contaminated soil and water, offering a potential solution to challenging environmental issues.
In the field of nanotechnology, fluoroantimonic acid has been employed in the synthesis and modification of nanoparticles. Its strong protonating ability has been used to control the size, shape, and surface properties of nanostructures, leading to the development of materials with tailored characteristics for specific applications in areas such as catalysis, sensing, and drug delivery.
In materials science, fluoroantimonic acid has been employed to create novel surface modifications on metals and semiconductors. By carefully controlling the acid's concentration and exposure time, researchers have achieved unprecedented levels of surface etching precision, enabling the fabrication of nanoscale structures with enhanced properties. This has significant implications for the development of advanced electronic devices and high-performance materials.
The field of analytical chemistry has also benefited from fluoroantimonic acid's extreme acidity. It has been utilized in the development of highly sensitive detection methods for trace elements and compounds. By leveraging its ability to protonate even weakly basic species, researchers have improved the detection limits of various analytical techniques, including mass spectrometry and chromatography.
In the realm of energy research, fluoroantimonic acid has shown promise in the development of next-generation battery technologies. Its strong oxidizing properties have been exploited to create novel electrode materials with improved stability and conductivity. This application has the potential to significantly enhance the performance and longevity of high-capacity energy storage systems.
The pharmaceutical industry has explored the use of fluoroantimonic acid in drug discovery and synthesis. Its ability to catalyze challenging chemical transformations has led to the development of new synthetic routes for complex drug molecules. This has not only improved the efficiency of drug production but also opened up possibilities for creating previously inaccessible pharmaceutical compounds.
Environmental science has found applications for fluoroantimonic acid in the treatment of persistent organic pollutants. Its extreme acidity allows for the breakdown of highly stable chemical compounds that are resistant to conventional treatment methods. This approach has shown promise in the remediation of contaminated soil and water, offering a potential solution to challenging environmental issues.
In the field of nanotechnology, fluoroantimonic acid has been employed in the synthesis and modification of nanoparticles. Its strong protonating ability has been used to control the size, shape, and surface properties of nanostructures, leading to the development of materials with tailored characteristics for specific applications in areas such as catalysis, sensing, and drug delivery.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!