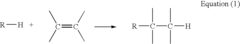
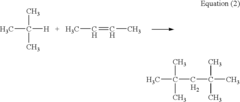
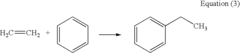
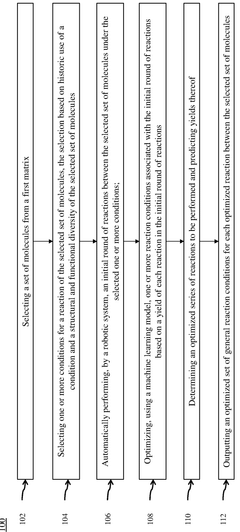
Alkyl Strategies for Optimizing Reaction Conditions
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkylation Background and Objectives
Alkylation reactions have been a cornerstone of organic synthesis for over a century, playing a crucial role in the production of various chemicals, pharmaceuticals, and materials. The process involves the transfer of an alkyl group from one molecule to another, typically through the formation of a carbon-carbon or carbon-heteroatom bond. As the chemical industry continues to evolve, there is an increasing demand for more efficient, sustainable, and selective alkylation methods.
The historical development of alkylation strategies can be traced back to the early 20th century, with pioneering work by chemists such as Friedel and Crafts. Since then, numerous advancements have been made, including the development of new catalysts, the exploration of alternative reaction media, and the application of novel activation methods. These innovations have significantly expanded the scope and utility of alkylation reactions across various fields of chemistry.
In recent years, the focus has shifted towards optimizing reaction conditions to address the challenges of modern chemical synthesis. These challenges include improving atom economy, reducing waste generation, enhancing selectivity, and developing more environmentally friendly processes. The optimization of alkylation strategies has become particularly important in the context of green chemistry and sustainable development goals.
The primary objectives of current research in alkylation strategies are multifaceted. Firstly, there is a strong emphasis on developing more efficient catalytic systems that can operate under milder conditions, thereby reducing energy consumption and improving overall process economics. Secondly, researchers are exploring novel reaction media, including ionic liquids and supercritical fluids, to enhance reactivity and selectivity while minimizing environmental impact.
Another key objective is the development of regioselective and stereoselective alkylation methods. This is particularly crucial in the pharmaceutical industry, where the precise control of molecular structure is essential for drug efficacy and safety. Additionally, there is growing interest in expanding the substrate scope of alkylation reactions, enabling the functionalization of previously unreactive or challenging compounds.
The optimization of reaction conditions also extends to the realm of flow chemistry and continuous processing. These approaches offer advantages in terms of scalability, safety, and process control, making them attractive for industrial applications. Researchers are actively investigating how to translate batch alkylation processes into continuous flow systems, addressing challenges related to mixing, heat transfer, and residence time distribution.
As we look towards the future, the field of alkylation chemistry continues to evolve, driven by the need for more sustainable and efficient synthetic methods. The integration of computational modeling and high-throughput experimentation is expected to accelerate the discovery and optimization of new alkylation strategies. Furthermore, the application of artificial intelligence and machine learning algorithms holds promise for predicting optimal reaction conditions and guiding experimental design.
The historical development of alkylation strategies can be traced back to the early 20th century, with pioneering work by chemists such as Friedel and Crafts. Since then, numerous advancements have been made, including the development of new catalysts, the exploration of alternative reaction media, and the application of novel activation methods. These innovations have significantly expanded the scope and utility of alkylation reactions across various fields of chemistry.
In recent years, the focus has shifted towards optimizing reaction conditions to address the challenges of modern chemical synthesis. These challenges include improving atom economy, reducing waste generation, enhancing selectivity, and developing more environmentally friendly processes. The optimization of alkylation strategies has become particularly important in the context of green chemistry and sustainable development goals.
The primary objectives of current research in alkylation strategies are multifaceted. Firstly, there is a strong emphasis on developing more efficient catalytic systems that can operate under milder conditions, thereby reducing energy consumption and improving overall process economics. Secondly, researchers are exploring novel reaction media, including ionic liquids and supercritical fluids, to enhance reactivity and selectivity while minimizing environmental impact.
Another key objective is the development of regioselective and stereoselective alkylation methods. This is particularly crucial in the pharmaceutical industry, where the precise control of molecular structure is essential for drug efficacy and safety. Additionally, there is growing interest in expanding the substrate scope of alkylation reactions, enabling the functionalization of previously unreactive or challenging compounds.
The optimization of reaction conditions also extends to the realm of flow chemistry and continuous processing. These approaches offer advantages in terms of scalability, safety, and process control, making them attractive for industrial applications. Researchers are actively investigating how to translate batch alkylation processes into continuous flow systems, addressing challenges related to mixing, heat transfer, and residence time distribution.
As we look towards the future, the field of alkylation chemistry continues to evolve, driven by the need for more sustainable and efficient synthetic methods. The integration of computational modeling and high-throughput experimentation is expected to accelerate the discovery and optimization of new alkylation strategies. Furthermore, the application of artificial intelligence and machine learning algorithms holds promise for predicting optimal reaction conditions and guiding experimental design.
Industrial Demand Analysis
The demand for optimized alkyl strategies in reaction conditions has seen a significant surge across various industrial sectors. This growing interest is primarily driven by the need for more efficient and sustainable chemical processes in the manufacturing of pharmaceuticals, agrochemicals, and specialty chemicals. The global market for fine chemicals, where alkylation reactions play a crucial role, is projected to reach substantial growth in the coming years, underlining the importance of advanced alkylation techniques.
In the pharmaceutical industry, the demand for alkyl optimization strategies is particularly pronounced. Drug manufacturers are constantly seeking ways to improve yield, reduce waste, and enhance the purity of their products. Alkylation reactions are fundamental in the synthesis of many active pharmaceutical ingredients (APIs), and optimizing these reactions can lead to significant cost savings and improved product quality. The increasing focus on green chemistry and sustainable manufacturing practices has further amplified the need for efficient alkylation processes that minimize environmental impact.
The agrochemical sector also presents a substantial market for alkyl optimization techniques. As global food demand rises and arable land becomes scarce, there is an increasing pressure to develop more effective crop protection chemicals. Optimized alkylation strategies can lead to the creation of more potent and environmentally friendly pesticides and herbicides, addressing both agricultural productivity and sustainability concerns.
In the realm of specialty chemicals, industries such as electronics, cosmetics, and advanced materials are driving demand for precise and controllable alkylation processes. The production of high-performance polymers, surfactants, and electronic materials often relies on well-controlled alkylation reactions. As these industries push for innovation and product differentiation, the ability to fine-tune reaction conditions becomes a critical competitive advantage.
The petrochemical industry, while mature, continues to seek improvements in alkylation processes for the production of high-octane gasoline components and other valuable products. Optimizing reaction conditions can lead to increased yields, reduced energy consumption, and improved catalyst lifetimes, all of which translate to significant economic benefits in large-scale operations.
Environmental regulations and sustainability goals are also shaping the demand for alkyl optimization strategies. Industries are under pressure to reduce their carbon footprint and minimize the use of hazardous substances. This has led to a growing interest in developing alkylation processes that operate under milder conditions, use less toxic reagents, and produce fewer by-products. The ability to conduct efficient alkylations in aqueous media or using renewable feedstocks is becoming increasingly valuable across multiple sectors.
In the pharmaceutical industry, the demand for alkyl optimization strategies is particularly pronounced. Drug manufacturers are constantly seeking ways to improve yield, reduce waste, and enhance the purity of their products. Alkylation reactions are fundamental in the synthesis of many active pharmaceutical ingredients (APIs), and optimizing these reactions can lead to significant cost savings and improved product quality. The increasing focus on green chemistry and sustainable manufacturing practices has further amplified the need for efficient alkylation processes that minimize environmental impact.
The agrochemical sector also presents a substantial market for alkyl optimization techniques. As global food demand rises and arable land becomes scarce, there is an increasing pressure to develop more effective crop protection chemicals. Optimized alkylation strategies can lead to the creation of more potent and environmentally friendly pesticides and herbicides, addressing both agricultural productivity and sustainability concerns.
In the realm of specialty chemicals, industries such as electronics, cosmetics, and advanced materials are driving demand for precise and controllable alkylation processes. The production of high-performance polymers, surfactants, and electronic materials often relies on well-controlled alkylation reactions. As these industries push for innovation and product differentiation, the ability to fine-tune reaction conditions becomes a critical competitive advantage.
The petrochemical industry, while mature, continues to seek improvements in alkylation processes for the production of high-octane gasoline components and other valuable products. Optimizing reaction conditions can lead to increased yields, reduced energy consumption, and improved catalyst lifetimes, all of which translate to significant economic benefits in large-scale operations.
Environmental regulations and sustainability goals are also shaping the demand for alkyl optimization strategies. Industries are under pressure to reduce their carbon footprint and minimize the use of hazardous substances. This has led to a growing interest in developing alkylation processes that operate under milder conditions, use less toxic reagents, and produce fewer by-products. The ability to conduct efficient alkylations in aqueous media or using renewable feedstocks is becoming increasingly valuable across multiple sectors.
Current Challenges in Alkylation
Alkylation reactions face several significant challenges that hinder their widespread application and efficiency in industrial processes. One of the primary issues is the lack of selectivity in many alkylation reactions. This often results in the formation of unwanted side products, reducing the overall yield and purity of the desired compound. The challenge of controlling regioselectivity and stereoselectivity in complex molecular structures remains a persistent problem for chemists.
Another major hurdle is the use of harsh reaction conditions, including high temperatures and pressures, which can lead to increased energy consumption and safety concerns in large-scale operations. These conditions also limit the applicability of alkylation reactions to sensitive substrates that may degrade under such extreme environments. The need for milder, more sustainable reaction conditions is a key area of focus for researchers in this field.
The reliance on stoichiometric amounts of strong bases or acids as catalysts in many alkylation reactions presents additional challenges. These reagents can be corrosive, environmentally harmful, and difficult to handle on an industrial scale. Furthermore, the disposal of these reagents after use poses environmental concerns and increases the overall cost of the process. Developing catalytic systems that can operate under milder conditions and with lower catalyst loadings is crucial for improving the sustainability and economic viability of alkylation reactions.
Substrate scope limitations also present a significant challenge in alkylation chemistry. Many alkylation methods work well for simple, unfunctionalized molecules but struggle when applied to more complex, highly functionalized substrates. This limitation restricts the applicability of alkylation reactions in the synthesis of complex natural products and pharmaceuticals, where selective functionalization of specific sites is often required.
The use of toxic or environmentally harmful alkylating agents, such as alkyl halides or sulfates, is another concern. These reagents not only pose health and safety risks but also contribute to the generation of hazardous waste. Finding greener alternatives or developing methods that use more benign alkylating agents is an important area of research in sustainable chemistry.
Lastly, the scalability of alkylation reactions from laboratory to industrial scale remains challenging. Issues such as heat and mass transfer limitations, mixing efficiency, and reaction kinetics can significantly impact the performance of alkylation reactions when scaled up. Developing robust and scalable alkylation processes that maintain high efficiency and selectivity at larger scales is crucial for the successful implementation of these reactions in industrial settings.
Another major hurdle is the use of harsh reaction conditions, including high temperatures and pressures, which can lead to increased energy consumption and safety concerns in large-scale operations. These conditions also limit the applicability of alkylation reactions to sensitive substrates that may degrade under such extreme environments. The need for milder, more sustainable reaction conditions is a key area of focus for researchers in this field.
The reliance on stoichiometric amounts of strong bases or acids as catalysts in many alkylation reactions presents additional challenges. These reagents can be corrosive, environmentally harmful, and difficult to handle on an industrial scale. Furthermore, the disposal of these reagents after use poses environmental concerns and increases the overall cost of the process. Developing catalytic systems that can operate under milder conditions and with lower catalyst loadings is crucial for improving the sustainability and economic viability of alkylation reactions.
Substrate scope limitations also present a significant challenge in alkylation chemistry. Many alkylation methods work well for simple, unfunctionalized molecules but struggle when applied to more complex, highly functionalized substrates. This limitation restricts the applicability of alkylation reactions in the synthesis of complex natural products and pharmaceuticals, where selective functionalization of specific sites is often required.
The use of toxic or environmentally harmful alkylating agents, such as alkyl halides or sulfates, is another concern. These reagents not only pose health and safety risks but also contribute to the generation of hazardous waste. Finding greener alternatives or developing methods that use more benign alkylating agents is an important area of research in sustainable chemistry.
Lastly, the scalability of alkylation reactions from laboratory to industrial scale remains challenging. Issues such as heat and mass transfer limitations, mixing efficiency, and reaction kinetics can significantly impact the performance of alkylation reactions when scaled up. Developing robust and scalable alkylation processes that maintain high efficiency and selectivity at larger scales is crucial for the successful implementation of these reactions in industrial settings.
Existing Alkylation Strategies
01 Alkylation reaction conditions
Specific reaction conditions are crucial for successful alkylation strategies. These conditions may include temperature control, pressure regulation, catalyst selection, and solvent choice. Optimizing these parameters can significantly improve the yield and selectivity of alkylation reactions.- Alkylation reaction conditions: Specific reaction conditions are crucial for successful alkylation strategies. These conditions may include temperature control, pressure regulation, catalyst selection, and solvent choice. Optimizing these parameters can significantly improve the yield and selectivity of alkylation reactions.
- Catalyst systems for alkylation: Various catalyst systems are employed in alkylation reactions to enhance reaction rates and selectivity. These may include heterogeneous catalysts, homogeneous catalysts, or enzyme-based catalysts. The choice of catalyst can greatly influence the reaction pathway and product distribution.
- Solvent effects on alkylation: The choice of solvent can have a significant impact on alkylation reactions. Factors such as polarity, miscibility, and ability to stabilize intermediates or transition states are considered when selecting appropriate solvents. Some reactions may benefit from solvent-free conditions or the use of ionic liquids.
- Regioselective and stereoselective alkylation: Strategies for controlling the regioselectivity and stereoselectivity of alkylation reactions are important in organic synthesis. These may involve the use of chiral catalysts, directing groups, or specific reaction conditions to favor the formation of desired isomers or enantiomers.
- Green chemistry approaches to alkylation: Environmentally friendly alkylation strategies are being developed to reduce waste and improve sustainability. These may include the use of renewable feedstocks, recyclable catalysts, or alternative reaction media such as supercritical fluids or water. Process intensification and continuous flow methods are also explored to enhance efficiency.
02 Catalyst systems for alkylation
Various catalyst systems are employed in alkylation reactions to enhance reaction rates and selectivity. These may include heterogeneous catalysts, homogeneous catalysts, or biocatalysts. The choice of catalyst can greatly influence the reaction outcome and efficiency.Expand Specific Solutions03 Solvent effects on alkylation
The choice of solvent plays a crucial role in alkylation reactions. Different solvents can affect reaction kinetics, product distribution, and overall yield. Polar aprotic solvents, non-polar solvents, or green solvents may be used depending on the specific alkylation strategy.Expand Specific Solutions04 Temperature and pressure control
Precise control of temperature and pressure is essential for many alkylation reactions. These parameters can affect reaction rates, equilibrium, and product selectivity. Strategies may involve low-temperature conditions, high-pressure environments, or carefully controlled heating and cooling cycles.Expand Specific Solutions05 Green chemistry approaches in alkylation
Environmentally friendly approaches to alkylation are gaining importance. These may include the use of renewable feedstocks, solvent-free conditions, or recyclable catalysts. Such strategies aim to reduce waste, improve atom economy, and minimize environmental impact while maintaining reaction efficiency.Expand Specific Solutions
Key Players in Alkylation Industry
The competitive landscape for "Alkyl Strategies for Optimizing Reaction Conditions" is characterized by a mature market with significant industry players. The global petrochemical and chemical sectors dominate this field, with major corporations like China Petroleum & Chemical Corp., ExxonMobil Chemical Patents, Inc., and BASF Corp. leading the way. The market size is substantial, driven by the widespread application of alkyl strategies in various industries. Technologically, the field is well-developed, with companies like Dow Global Technologies LLC and Merck Patent GmbH contributing to ongoing advancements. Academic institutions such as Fudan University and California Institute of Technology also play crucial roles in research and innovation, indicating a collaborative industry-academia approach to optimizing reaction conditions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced alkyl strategies for optimizing reaction conditions in petrochemical processes. Their approach involves the use of novel alkylation catalysts and innovative reactor designs to enhance product yield and selectivity. Sinopec's research focuses on improving the efficiency of alkylation reactions in gasoline production, utilizing a combination of solid acid catalysts and optimized reaction parameters. They have implemented a proprietary alkylation technology that operates at lower temperatures and pressures, resulting in reduced energy consumption and improved product quality[1][3]. Additionally, Sinopec has developed a continuous flow alkylation process that allows for better control of reaction conditions and increased throughput[5].
Strengths: Extensive experience in large-scale petrochemical processes, strong R&D capabilities, and access to significant resources. Weaknesses: Potential environmental concerns associated with traditional petrochemical processes and dependence on fossil fuel feedstocks.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil Chemical Patents, Inc. has pioneered several innovative alkyl strategies for optimizing reaction conditions. Their approach includes the development of highly selective zeolite catalysts for alkylation processes, which offer improved activity and longer catalyst lifetimes[2]. ExxonMobil has also introduced a novel fixed-bed alkylation technology that utilizes ionic liquids as catalysts, allowing for more efficient and environmentally friendly alkylation reactions[4]. This technology operates at milder conditions compared to traditional sulfuric acid-based processes, resulting in reduced corrosion and improved safety. Furthermore, ExxonMobil has implemented advanced process control systems that utilize real-time monitoring and machine learning algorithms to continuously optimize reaction conditions, maximizing product yield and quality[6].
Strengths: Strong intellectual property portfolio, extensive global presence, and significant financial resources for R&D. Weaknesses: Reliance on fossil fuel-based feedstocks and potential public perception issues related to environmental impact.
Innovative Alkylation Catalysts
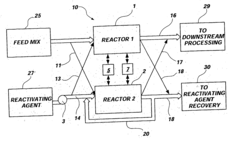
Method and system for reactivating catalysts and a method and system for recycling supercritical fluids used to reactivate the catalysts
PatentInactiveUS20040063567A1
Innovation
- A method involving a fluid reactivating agent that reacts with and dissolves fouling agents, allowing for catalyst reactivation at or above its critical point and sufficient density to remove impurities, which can be recycled and reused, independent of optimal alkylation conditions.
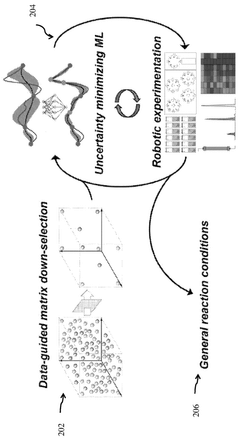
Closed-loop optimization of general reaction conditions for heteroaryl suzuki-miyaura coupling
PatentPendingAU2023366930A1
Innovation
- A closed-loop workflow combining data-guided matrix down-selection, uncertainty-minimizing machine learning, and robotic experimentation to efficiently navigate vast substrate-condition space and discover general reaction conditions.
Green Chemistry Considerations
Green chemistry considerations play a crucial role in optimizing reaction conditions for alkyl strategies. The principles of green chemistry emphasize the need for sustainable and environmentally friendly processes in chemical synthesis. When developing alkyl strategies, it is essential to focus on reducing waste, improving energy efficiency, and minimizing the use of hazardous substances.
One key aspect of green chemistry in alkyl reactions is the selection of solvents. Traditional organic solvents often pose environmental and health risks. Researchers are increasingly exploring the use of green solvents, such as water, supercritical CO2, and ionic liquids, which offer reduced toxicity and improved recyclability. These alternative solvents can significantly reduce the environmental impact of alkyl reactions while maintaining or even enhancing reaction efficiency.
Catalysis is another critical area where green chemistry principles can be applied to alkyl strategies. The development of highly selective and efficient catalysts can lead to reduced energy consumption and waste generation. Biocatalysts, in particular, have gained attention for their ability to operate under mild conditions and their inherent biodegradability. Enzyme-catalyzed alkyl reactions offer the potential for increased selectivity and reduced by-product formation, aligning well with green chemistry goals.
The use of renewable feedstocks is a fundamental principle of green chemistry that can be incorporated into alkyl strategies. Biomass-derived starting materials can replace petroleum-based reagents, contributing to the sustainability of the chemical industry. Researchers are exploring various bio-based alkyl sources, such as those derived from plant oils or agricultural waste, to develop more environmentally friendly alkylation processes.
Energy efficiency is another crucial consideration in green chemistry approaches to alkyl reactions. Optimizing reaction conditions to operate at lower temperatures and pressures can significantly reduce energy consumption. Additionally, the use of alternative energy sources, such as microwave irradiation or photochemical reactions, can offer more energy-efficient pathways for alkyl transformations.
Waste reduction and atom economy are essential principles in green alkyl strategies. Developing reactions with high atom economy ensures that a maximum amount of starting materials is incorporated into the final product, minimizing waste generation. Additionally, designing processes that generate easily separable and recyclable by-products can further enhance the sustainability of alkyl reactions.
In conclusion, integrating green chemistry considerations into alkyl strategies for optimizing reaction conditions is crucial for developing sustainable and environmentally friendly chemical processes. By focusing on green solvents, efficient catalysts, renewable feedstocks, energy efficiency, and waste reduction, researchers can significantly improve the environmental profile of alkyl reactions while maintaining or enhancing their synthetic utility.
One key aspect of green chemistry in alkyl reactions is the selection of solvents. Traditional organic solvents often pose environmental and health risks. Researchers are increasingly exploring the use of green solvents, such as water, supercritical CO2, and ionic liquids, which offer reduced toxicity and improved recyclability. These alternative solvents can significantly reduce the environmental impact of alkyl reactions while maintaining or even enhancing reaction efficiency.
Catalysis is another critical area where green chemistry principles can be applied to alkyl strategies. The development of highly selective and efficient catalysts can lead to reduced energy consumption and waste generation. Biocatalysts, in particular, have gained attention for their ability to operate under mild conditions and their inherent biodegradability. Enzyme-catalyzed alkyl reactions offer the potential for increased selectivity and reduced by-product formation, aligning well with green chemistry goals.
The use of renewable feedstocks is a fundamental principle of green chemistry that can be incorporated into alkyl strategies. Biomass-derived starting materials can replace petroleum-based reagents, contributing to the sustainability of the chemical industry. Researchers are exploring various bio-based alkyl sources, such as those derived from plant oils or agricultural waste, to develop more environmentally friendly alkylation processes.
Energy efficiency is another crucial consideration in green chemistry approaches to alkyl reactions. Optimizing reaction conditions to operate at lower temperatures and pressures can significantly reduce energy consumption. Additionally, the use of alternative energy sources, such as microwave irradiation or photochemical reactions, can offer more energy-efficient pathways for alkyl transformations.
Waste reduction and atom economy are essential principles in green alkyl strategies. Developing reactions with high atom economy ensures that a maximum amount of starting materials is incorporated into the final product, minimizing waste generation. Additionally, designing processes that generate easily separable and recyclable by-products can further enhance the sustainability of alkyl reactions.
In conclusion, integrating green chemistry considerations into alkyl strategies for optimizing reaction conditions is crucial for developing sustainable and environmentally friendly chemical processes. By focusing on green solvents, efficient catalysts, renewable feedstocks, energy efficiency, and waste reduction, researchers can significantly improve the environmental profile of alkyl reactions while maintaining or enhancing their synthetic utility.
Alkylation Process Modeling
Alkylation process modeling plays a crucial role in optimizing reaction conditions for alkyl strategies. This advanced computational approach enables researchers and engineers to simulate and predict the behavior of complex alkylation reactions under various conditions. By leveraging mathematical models and algorithms, process modeling provides valuable insights into the intricate relationships between reaction parameters and outcomes.
One of the primary advantages of alkylation process modeling is its ability to explore a wide range of reaction conditions without the need for extensive experimental work. This virtual experimentation allows for rapid screening of potential optimization strategies, significantly reducing the time and resources required for process development. Researchers can investigate the effects of temperature, pressure, catalyst composition, and reactant ratios on product yield, selectivity, and overall process efficiency.
Advanced modeling techniques incorporate kinetic and thermodynamic data to create accurate representations of the alkylation reaction system. These models often include detailed descriptions of reaction mechanisms, mass transfer phenomena, and heat transfer processes. By integrating these various aspects, process models can provide a comprehensive understanding of the entire alkylation system, from reactant mixing to product separation.
Machine learning and artificial intelligence techniques have further enhanced the capabilities of alkylation process modeling. These advanced algorithms can analyze large datasets from historical experiments and identify complex patterns that may not be apparent through traditional analysis methods. This data-driven approach enables more accurate predictions of reaction outcomes and can suggest novel optimization strategies that might otherwise be overlooked.
Real-time process monitoring and control systems can be developed based on these models, allowing for dynamic adjustments to reaction conditions. This adaptive approach ensures that the alkylation process remains at optimal performance even in the face of changing feedstock qualities or environmental conditions. Such systems can significantly improve product consistency and overall process efficiency.
Furthermore, alkylation process modeling facilitates scale-up efforts by providing insights into how reaction dynamics may change with increasing reactor size. This predictive capability helps identify potential issues before they arise in full-scale production, saving time and resources during the commercialization phase. Additionally, these models can be used to evaluate the economic feasibility of different process configurations, aiding in decision-making for industrial implementation.
As sustainability becomes an increasingly important consideration in chemical processes, alkylation process modeling also plays a vital role in optimizing energy consumption and minimizing waste generation. By simulating various process scenarios, engineers can identify opportunities for heat integration, solvent recycling, and byproduct valorization, leading to more environmentally friendly alkylation processes.
One of the primary advantages of alkylation process modeling is its ability to explore a wide range of reaction conditions without the need for extensive experimental work. This virtual experimentation allows for rapid screening of potential optimization strategies, significantly reducing the time and resources required for process development. Researchers can investigate the effects of temperature, pressure, catalyst composition, and reactant ratios on product yield, selectivity, and overall process efficiency.
Advanced modeling techniques incorporate kinetic and thermodynamic data to create accurate representations of the alkylation reaction system. These models often include detailed descriptions of reaction mechanisms, mass transfer phenomena, and heat transfer processes. By integrating these various aspects, process models can provide a comprehensive understanding of the entire alkylation system, from reactant mixing to product separation.
Machine learning and artificial intelligence techniques have further enhanced the capabilities of alkylation process modeling. These advanced algorithms can analyze large datasets from historical experiments and identify complex patterns that may not be apparent through traditional analysis methods. This data-driven approach enables more accurate predictions of reaction outcomes and can suggest novel optimization strategies that might otherwise be overlooked.
Real-time process monitoring and control systems can be developed based on these models, allowing for dynamic adjustments to reaction conditions. This adaptive approach ensures that the alkylation process remains at optimal performance even in the face of changing feedstock qualities or environmental conditions. Such systems can significantly improve product consistency and overall process efficiency.
Furthermore, alkylation process modeling facilitates scale-up efforts by providing insights into how reaction dynamics may change with increasing reactor size. This predictive capability helps identify potential issues before they arise in full-scale production, saving time and resources during the commercialization phase. Additionally, these models can be used to evaluate the economic feasibility of different process configurations, aiding in decision-making for industrial implementation.
As sustainability becomes an increasingly important consideration in chemical processes, alkylation process modeling also plays a vital role in optimizing energy consumption and minimizing waste generation. By simulating various process scenarios, engineers can identify opportunities for heat integration, solvent recycling, and byproduct valorization, leading to more environmentally friendly alkylation processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!