Automated Sample Handling And Transfer Systems For Multi-Modal Characterization
AUG 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Automated Sample Handling Evolution and Objectives
Automated sample handling systems have evolved significantly over the past decades, transforming from simple mechanical transfer mechanisms to sophisticated robotic platforms capable of complex operations. The earliest automated sample handling systems emerged in the 1970s, primarily in clinical laboratories for basic blood and urine sample processing. These rudimentary systems offered limited functionality, focusing mainly on sample transport between fixed positions with minimal environmental control.
The 1980s and 1990s witnessed substantial advancements with the integration of programmable logic controllers and early computer systems, enabling more precise movement control and basic decision-making capabilities. This period marked the transition from purely mechanical systems to electromechanical solutions with enhanced reliability and reproducibility, particularly important for pharmaceutical research and quality control applications.
By the early 2000s, automated sample handling systems began incorporating sophisticated robotics, vision systems, and advanced sensors, allowing for real-time monitoring and adaptive control. The development of standardized laboratory automation protocols, such as SiLA (Standardization in Lab Automation) and SLAS (Society for Laboratory Automation and Screening) standards, facilitated interoperability between different instruments and systems, significantly expanding application possibilities.
The current generation of automated sample handling systems represents a convergence of multiple technologies, including artificial intelligence, machine learning, IoT connectivity, and advanced materials science. These systems can now maintain precise environmental conditions during transfer operations, handle samples of varying physical properties, and integrate seamlessly with analytical instruments across multiple characterization modalities.
The primary objectives for modern automated sample handling and transfer systems for multi-modal characterization include maximizing throughput while maintaining sample integrity, minimizing cross-contamination risks, and ensuring reproducible positioning accuracy across different analytical platforms. Additionally, these systems aim to reduce human intervention, thereby decreasing operator exposure to hazardous materials and minimizing human-induced variability in analytical results.
Future development goals focus on creating more versatile systems capable of handling increasingly diverse sample types, from traditional liquid samples to complex biological specimens, nanomaterials, and heterogeneous material composites. Enhanced environmental control during transfers, including temperature, humidity, and atmospheric composition management, represents another critical objective, particularly for sensitive biological samples and reactive materials.
The ultimate aim is to develop fully autonomous, self-calibrating systems that can adapt to changing experimental requirements, predict and prevent potential failures, and optimize transfer protocols based on sample characteristics and analytical objectives, thereby enabling truly integrated multi-modal characterization workflows with minimal human supervision.
The 1980s and 1990s witnessed substantial advancements with the integration of programmable logic controllers and early computer systems, enabling more precise movement control and basic decision-making capabilities. This period marked the transition from purely mechanical systems to electromechanical solutions with enhanced reliability and reproducibility, particularly important for pharmaceutical research and quality control applications.
By the early 2000s, automated sample handling systems began incorporating sophisticated robotics, vision systems, and advanced sensors, allowing for real-time monitoring and adaptive control. The development of standardized laboratory automation protocols, such as SiLA (Standardization in Lab Automation) and SLAS (Society for Laboratory Automation and Screening) standards, facilitated interoperability between different instruments and systems, significantly expanding application possibilities.
The current generation of automated sample handling systems represents a convergence of multiple technologies, including artificial intelligence, machine learning, IoT connectivity, and advanced materials science. These systems can now maintain precise environmental conditions during transfer operations, handle samples of varying physical properties, and integrate seamlessly with analytical instruments across multiple characterization modalities.
The primary objectives for modern automated sample handling and transfer systems for multi-modal characterization include maximizing throughput while maintaining sample integrity, minimizing cross-contamination risks, and ensuring reproducible positioning accuracy across different analytical platforms. Additionally, these systems aim to reduce human intervention, thereby decreasing operator exposure to hazardous materials and minimizing human-induced variability in analytical results.
Future development goals focus on creating more versatile systems capable of handling increasingly diverse sample types, from traditional liquid samples to complex biological specimens, nanomaterials, and heterogeneous material composites. Enhanced environmental control during transfers, including temperature, humidity, and atmospheric composition management, represents another critical objective, particularly for sensitive biological samples and reactive materials.
The ultimate aim is to develop fully autonomous, self-calibrating systems that can adapt to changing experimental requirements, predict and prevent potential failures, and optimize transfer protocols based on sample characteristics and analytical objectives, thereby enabling truly integrated multi-modal characterization workflows with minimal human supervision.
Market Demand Analysis for Multi-Modal Characterization Systems
The global market for multi-modal characterization systems is experiencing robust growth, driven primarily by increasing demands in materials science, pharmaceutical research, and advanced manufacturing sectors. Current market valuations indicate that the automated sample handling segment within analytical instrumentation reached approximately $5.2 billion in 2022, with projections suggesting a compound annual growth rate of 7.8% through 2028.
Research institutions and industrial laboratories are increasingly seeking integrated solutions that can characterize samples across multiple dimensions simultaneously, reducing analysis time and enhancing data correlation. This demand stems from the growing complexity of materials being developed, particularly in nanotechnology, semiconductor manufacturing, and biomaterials, where understanding structural, chemical, and physical properties in concert is critical.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 35% of the total demand. These industries require high-throughput screening capabilities with minimal sample contamination risks, driving innovation in automated handling systems. The ability to maintain sample integrity while transferring between different analytical instruments represents a key value proposition for end-users.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the fastest growth is observed in emerging economies, particularly China and India, where significant investments in research infrastructure and manufacturing capabilities are creating new market opportunities.
Customer surveys indicate that key purchasing factors include system reliability (cited by 87% of respondents), integration capabilities with existing laboratory information management systems (76%), and reduction in cross-contamination risks (72%). Additionally, there is growing demand for systems that can handle increasingly smaller sample volumes, reflecting the miniaturization trend in many research fields.
The COVID-19 pandemic has accelerated market growth by highlighting the need for automated systems that reduce human intervention in laboratory workflows. This trend is expected to continue as laboratories seek to improve operational efficiency and reduce labor costs through automation.
Industry analysts note that customers are increasingly seeking solutions that offer flexibility in sample types and analytical techniques, rather than dedicated systems with limited application scope. This shift is driving development of modular platforms that can be configured according to specific research needs, with seamless sample transfer between different characterization modules.
Research institutions and industrial laboratories are increasingly seeking integrated solutions that can characterize samples across multiple dimensions simultaneously, reducing analysis time and enhancing data correlation. This demand stems from the growing complexity of materials being developed, particularly in nanotechnology, semiconductor manufacturing, and biomaterials, where understanding structural, chemical, and physical properties in concert is critical.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 35% of the total demand. These industries require high-throughput screening capabilities with minimal sample contamination risks, driving innovation in automated handling systems. The ability to maintain sample integrity while transferring between different analytical instruments represents a key value proposition for end-users.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the fastest growth is observed in emerging economies, particularly China and India, where significant investments in research infrastructure and manufacturing capabilities are creating new market opportunities.
Customer surveys indicate that key purchasing factors include system reliability (cited by 87% of respondents), integration capabilities with existing laboratory information management systems (76%), and reduction in cross-contamination risks (72%). Additionally, there is growing demand for systems that can handle increasingly smaller sample volumes, reflecting the miniaturization trend in many research fields.
The COVID-19 pandemic has accelerated market growth by highlighting the need for automated systems that reduce human intervention in laboratory workflows. This trend is expected to continue as laboratories seek to improve operational efficiency and reduce labor costs through automation.
Industry analysts note that customers are increasingly seeking solutions that offer flexibility in sample types and analytical techniques, rather than dedicated systems with limited application scope. This shift is driving development of modular platforms that can be configured according to specific research needs, with seamless sample transfer between different characterization modules.
Technical Challenges in Automated Sample Transfer
Automated sample handling and transfer systems for multi-modal characterization face numerous technical challenges that impede their widespread implementation and optimal performance. One of the primary obstacles is maintaining sample integrity throughout the transfer process. Environmental exposure during transfers can lead to contamination, oxidation, or structural changes in sensitive materials, potentially compromising analytical results. This is particularly problematic for samples requiring specific atmospheric conditions or temperature control.
Precision positioning represents another significant challenge. Multi-modal characterization often requires exact sample placement across different analytical instruments, with positioning tolerances sometimes in the sub-micron range. Achieving this level of precision while maintaining high throughput remains technically demanding, especially when transferring between instruments with different coordinate systems or sample mounting requirements.
Compatibility issues between diverse analytical platforms further complicate automated transfer systems. Each characterization technique typically has unique sample preparation requirements, mounting configurations, and environmental conditions. Designing universal sample holders and transfer mechanisms that accommodate these variations without compromising analytical performance requires sophisticated engineering solutions.
Speed-integrity trade-offs present ongoing challenges for system designers. While rapid sample transfer is desirable for throughput optimization, faster movements can introduce vibrations, thermal fluctuations, or mechanical stresses that affect sample quality or positioning accuracy. Balancing these competing requirements necessitates advanced motion control algorithms and mechanical designs.
Vacuum compatibility presents particular difficulties in systems involving techniques like electron microscopy or surface analysis. Maintaining vacuum integrity during transfers between chambers requires complex lock systems, specialized seals, and careful consideration of materials to minimize outgassing. The mechanical complexity of vacuum-compatible robotics also increases system cost and maintenance requirements.
Software integration challenges are equally significant. Coordinating multiple analytical instruments with different control systems, data formats, and communication protocols requires sophisticated middleware solutions. Developing unified control architectures that can orchestrate complex sample workflows while providing error recovery capabilities remains technically challenging.
Miniaturization pressures further complicate system design as laboratories seek to maximize space efficiency. Creating compact transfer mechanisms that maintain precision while fitting within constrained laboratory environments often requires custom engineering solutions that may sacrifice some functionality or increase system complexity.
AI and machine learning integration represents the newest frontier in addressing these challenges, with potential for adaptive positioning systems, predictive maintenance, and automated workflow optimization. However, implementing these technologies requires significant computational resources and specialized expertise that may not be readily available in many research environments.
Precision positioning represents another significant challenge. Multi-modal characterization often requires exact sample placement across different analytical instruments, with positioning tolerances sometimes in the sub-micron range. Achieving this level of precision while maintaining high throughput remains technically demanding, especially when transferring between instruments with different coordinate systems or sample mounting requirements.
Compatibility issues between diverse analytical platforms further complicate automated transfer systems. Each characterization technique typically has unique sample preparation requirements, mounting configurations, and environmental conditions. Designing universal sample holders and transfer mechanisms that accommodate these variations without compromising analytical performance requires sophisticated engineering solutions.
Speed-integrity trade-offs present ongoing challenges for system designers. While rapid sample transfer is desirable for throughput optimization, faster movements can introduce vibrations, thermal fluctuations, or mechanical stresses that affect sample quality or positioning accuracy. Balancing these competing requirements necessitates advanced motion control algorithms and mechanical designs.
Vacuum compatibility presents particular difficulties in systems involving techniques like electron microscopy or surface analysis. Maintaining vacuum integrity during transfers between chambers requires complex lock systems, specialized seals, and careful consideration of materials to minimize outgassing. The mechanical complexity of vacuum-compatible robotics also increases system cost and maintenance requirements.
Software integration challenges are equally significant. Coordinating multiple analytical instruments with different control systems, data formats, and communication protocols requires sophisticated middleware solutions. Developing unified control architectures that can orchestrate complex sample workflows while providing error recovery capabilities remains technically challenging.
Miniaturization pressures further complicate system design as laboratories seek to maximize space efficiency. Creating compact transfer mechanisms that maintain precision while fitting within constrained laboratory environments often requires custom engineering solutions that may sacrifice some functionality or increase system complexity.
AI and machine learning integration represents the newest frontier in addressing these challenges, with potential for adaptive positioning systems, predictive maintenance, and automated workflow optimization. However, implementing these technologies requires significant computational resources and specialized expertise that may not be readily available in many research environments.
Current Automation Solutions for Multi-Modal Analysis
01 Robotic sample handling systems
Automated robotic systems designed for handling and transferring laboratory samples with precision. These systems typically include robotic arms, grippers, and positioning mechanisms that can pick up, move, and place samples in various laboratory equipment. They are programmed to perform repetitive tasks with high accuracy, reducing human error and increasing throughput in laboratory workflows.- Robotic sample handling systems: Automated robotic systems designed for handling and transferring laboratory samples with high precision and efficiency. These systems typically include robotic arms, grippers, and positioning mechanisms that can pick up, move, and place samples in various laboratory equipment. They are programmed to perform repetitive tasks with consistency, reducing human error and increasing throughput in laboratory workflows.
- Integrated laboratory automation platforms: Comprehensive automation platforms that integrate multiple laboratory processes including sample preparation, analysis, and storage. These systems connect various instruments and modules through automated transfer mechanisms, creating a seamless workflow. They often incorporate tracking software, scheduling algorithms, and quality control measures to ensure efficient sample processing and data management across the entire laboratory operation.
- Microfluidic sample handling technologies: Systems that manipulate and transfer small volumes of liquid samples through microchannels and chambers. These technologies enable precise control over sample movement, mixing, and analysis at microscale levels. Microfluidic platforms often incorporate pumps, valves, and sensors to automate the handling of minute sample quantities, making them particularly valuable for applications requiring minimal sample consumption and high-throughput screening.
- AI and machine learning in sample management: Advanced systems that incorporate artificial intelligence and machine learning algorithms to optimize sample handling workflows. These technologies enable predictive maintenance, adaptive scheduling, and intelligent decision-making in automated laboratory environments. By analyzing operational data, these systems can identify patterns, predict bottlenecks, and automatically adjust parameters to improve efficiency and reliability in sample processing and transfer operations.
- Clinical and diagnostic sample automation: Specialized automated systems designed for handling biological and clinical samples in diagnostic laboratories and healthcare settings. These systems incorporate features for maintaining sample integrity, preventing cross-contamination, and ensuring traceability throughout the testing process. They often include temperature control, barcode tracking, and chain-of-custody documentation to meet regulatory requirements while improving throughput and accuracy in diagnostic testing workflows.
02 Integrated laboratory automation platforms
Comprehensive automation platforms that integrate multiple laboratory processes including sample preparation, analysis, and storage. These systems coordinate various instruments and modules through centralized control software, enabling seamless sample transfer between different analytical steps. They often incorporate tracking mechanisms to monitor sample location and status throughout the workflow.Expand Specific Solutions03 Microfluidic sample handling technologies
Systems that manipulate and transfer small volumes of liquid samples through microchannels and chambers. These technologies enable precise control over sample movement, mixing, and processing at microscale levels. They often utilize pressure differentials, capillary forces, or electrokinetic mechanisms to facilitate sample transfer with minimal volume loss and contamination risk.Expand Specific Solutions04 AI and machine learning in sample management
Implementation of artificial intelligence and machine learning algorithms to optimize sample handling workflows. These systems can predict optimal sample routing, identify potential bottlenecks, and adapt to changing laboratory conditions. They analyze operational data to continuously improve efficiency, reduce processing time, and enhance decision-making in complex sample handling environments.Expand Specific Solutions05 Modular and scalable transfer systems
Flexible sample handling architectures that can be reconfigured or expanded based on changing laboratory needs. These systems feature standardized interfaces and communication protocols that allow different modules to be connected and operated as a unified system. The modular approach enables laboratories to start with basic automation and gradually add capabilities without replacing the entire infrastructure.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The automated sample handling and transfer systems for multi-modal characterization market is currently in a growth phase, with increasing adoption across pharmaceutical and diagnostic sectors. The market size is expanding rapidly due to rising demand for high-throughput screening and multi-modal analysis capabilities. Technologically, the field shows varying maturity levels, with established players like Roche Diagnostics, Abbott Laboratories, and Hologic offering integrated solutions, while companies such as Biomeme and TissueVision are introducing innovative portable and specialized systems. Hitachi, ABB Group, and Waters Technology are leveraging their automation expertise to develop advanced handling systems. The competitive landscape is diversifying as diagnostic specialists (Cepheid, bioMérieux) compete with laboratory automation experts (Elemental Scientific, STRATEC) to address the growing need for seamless sample workflows across multiple analytical platforms.
Roche Diagnostics Operations, Inc.
Technical Solution: Roche Diagnostics has developed the cobas® connection modules (CCM) system, an advanced automated sample handling and transfer solution that integrates with their analytical platforms. This modular system enables seamless sample transportation between pre-analytical, analytical, and post-analytical processes. The technology employs robotic arms and conveyor belts in a track-based architecture to transport samples between different diagnostic instruments. Their system incorporates barcode tracking technology for real-time sample monitoring and intelligent routing algorithms that optimize sample workflow based on test priority and instrument availability. The cobas® system also features automated cap removal and replacement capabilities, reducing manual handling and contamination risks. Roche's solution emphasizes connectivity between diverse analytical platforms, allowing for multi-modal characterization across chemistry, immunoassay, molecular, and hematology testing from a single sample.
Strengths: Highly integrated ecosystem that connects multiple analytical platforms; exceptional sample tracking capabilities; proven scalability for high-throughput environments. Weaknesses: Proprietary system architecture limits compatibility with non-Roche instruments; significant initial capital investment required; complex implementation process requiring specialized technical support.
Abbott Laboratories
Technical Solution: Abbott Laboratories has developed the Accelerator a3600 system, an advanced automated sample handling and transfer solution designed for high-volume laboratories. This technology employs a continuous-flow track system with intelligent routing capabilities that can process up to 3,600 samples per hour. The system features Abbott's proprietary Adaptive Sample Handler (ASH) technology, which dynamically adjusts sample routing based on real-time instrument availability and test priority. Their solution incorporates robotic sample manipulation modules for centrifugation, decapping, aliquoting, and recapping, minimizing manual handling requirements. Abbott's system emphasizes connectivity between their diverse analytical platforms, including chemistry, immunoassay, hematology, and molecular diagnostics, enabling comprehensive multi-modal characterization from a single sample tube. The technology also includes advanced sample integrity checks that detect issues such as clots, bubbles, or insufficient volume before samples reach analytical instruments.
Strengths: Exceptional throughput capacity ideal for high-volume laboratories; comprehensive connectivity across multiple testing modalities; advanced sample integrity verification systems. Weaknesses: Substantial physical footprint requiring significant laboratory space; complex implementation and validation process; higher initial investment compared to smaller-scale automation solutions.
Key Patents in Sample Transfer Technology
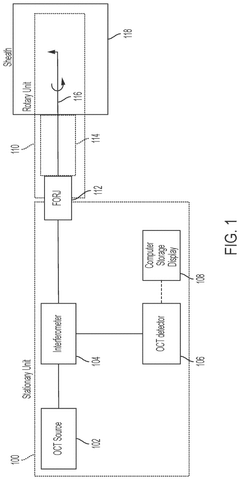
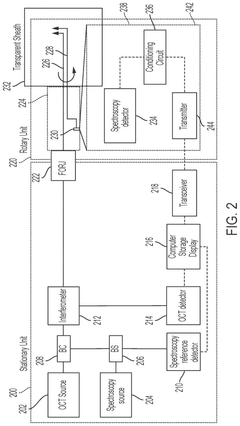
Multi modality rotary optical systems and methods of their use
PatentPendingUS20240350015A1
Innovation
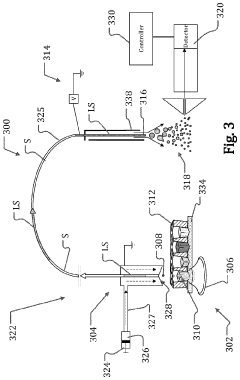
- The system employs direct detection of electromagnetic radiation within the rotary unit before the FORJ, converting the signal to other forms of energy, such as electrical, and transmitting it wirelessly or through electrical rotary junctions, allowing for high-fidelity multimodal characterization by separating modalities prior to fiber-optic junctions, thereby reducing system complexity and increasing signal-to-noise ratio.
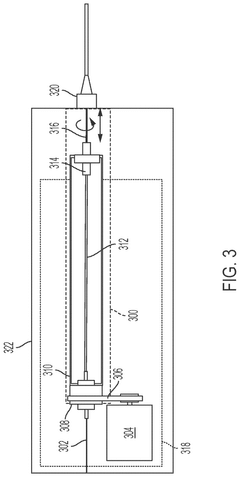
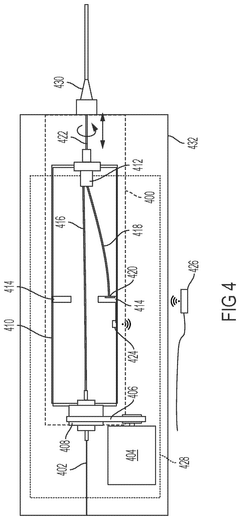
Mass analysis
PatentPendingUS20230238230A1
Innovation
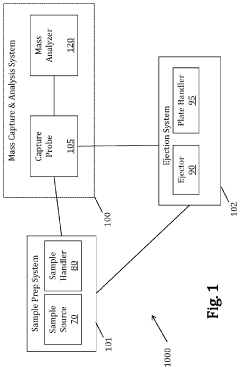
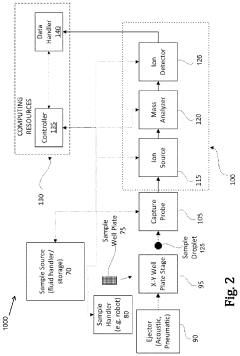
- A centralized control system orchestrates sample handling, preparation, and analysis by integrating robotics and mechanical devices to synchronize operations across subsystems, enabling efficient retrieval, capture, dilution, and mass analysis of multiple samples using a sample handler, capture probe, and mass analysis instrument.
Standardization and Interoperability Frameworks
The standardization and interoperability frameworks for automated sample handling and transfer systems represent a critical foundation for enabling seamless multi-modal characterization workflows. Currently, several industry standards are emerging to address the integration challenges between diverse analytical instruments and sample handling systems. The SLAS (Society for Laboratory Automation and Screening) microplate standards have become fundamental reference points, defining physical dimensions, well positions, and labeling conventions that facilitate compatibility across vendor platforms.
Beyond physical standardization, data exchange protocols such as AnIML (Analytical Information Markup Language) and SiLA (Standardization in Lab Automation) are gaining traction for enabling communication between automated sample handlers and analytical instruments. These frameworks provide structured approaches for command execution, status reporting, and analytical data transfer, reducing integration complexity and implementation costs.
The OPC UA (Open Platform Communications Unified Architecture) framework has emerged as a promising solution for industrial automation integration, offering secure, reliable communication between sample handling systems and characterization instruments. Its vendor-independent approach allows for consistent implementation across diverse hardware ecosystems while maintaining robust security protocols.
Recent collaborative efforts between instrument manufacturers have resulted in the development of FAIR (Findable, Accessible, Interoperable, Reusable) principles applied specifically to automated characterization workflows. These principles ensure that sample metadata remains consistently associated with analytical results throughout the characterization process, preserving data provenance and enabling more effective data mining.
Regulatory bodies including ASTM International and ISO have established working groups focused on developing consensus standards for automated sample transfer systems, particularly addressing safety interlocks, error handling, and validation methodologies. These standards are increasingly important as multi-modal characterization systems move from research environments into regulated manufacturing settings.
The emergence of digital twins for sample handling systems represents another significant advancement in standardization efforts. These virtual representations enable simulation and validation of complex sample transfer operations before physical implementation, reducing integration risks and accelerating deployment timelines. The Digital Twin Consortium has recently published reference architectures specifically addressing laboratory automation systems.
Despite these advances, significant challenges remain in achieving true plug-and-play interoperability across different vendors and analytical techniques. Proprietary interfaces continue to fragment the ecosystem, though industry consortia are actively working to harmonize approaches through open-source reference implementations and certification programs that verify compliance with established standards.
Beyond physical standardization, data exchange protocols such as AnIML (Analytical Information Markup Language) and SiLA (Standardization in Lab Automation) are gaining traction for enabling communication between automated sample handlers and analytical instruments. These frameworks provide structured approaches for command execution, status reporting, and analytical data transfer, reducing integration complexity and implementation costs.
The OPC UA (Open Platform Communications Unified Architecture) framework has emerged as a promising solution for industrial automation integration, offering secure, reliable communication between sample handling systems and characterization instruments. Its vendor-independent approach allows for consistent implementation across diverse hardware ecosystems while maintaining robust security protocols.
Recent collaborative efforts between instrument manufacturers have resulted in the development of FAIR (Findable, Accessible, Interoperable, Reusable) principles applied specifically to automated characterization workflows. These principles ensure that sample metadata remains consistently associated with analytical results throughout the characterization process, preserving data provenance and enabling more effective data mining.
Regulatory bodies including ASTM International and ISO have established working groups focused on developing consensus standards for automated sample transfer systems, particularly addressing safety interlocks, error handling, and validation methodologies. These standards are increasingly important as multi-modal characterization systems move from research environments into regulated manufacturing settings.
The emergence of digital twins for sample handling systems represents another significant advancement in standardization efforts. These virtual representations enable simulation and validation of complex sample transfer operations before physical implementation, reducing integration risks and accelerating deployment timelines. The Digital Twin Consortium has recently published reference architectures specifically addressing laboratory automation systems.
Despite these advances, significant challenges remain in achieving true plug-and-play interoperability across different vendors and analytical techniques. Proprietary interfaces continue to fragment the ecosystem, though industry consortia are actively working to harmonize approaches through open-source reference implementations and certification programs that verify compliance with established standards.
Risk Assessment and Quality Control Measures
Automated sample handling systems for multi-modal characterization inherently involve various risks that must be systematically assessed and mitigated. The primary risks include sample contamination, mechanical failures, software errors, and human interface complications. Each risk category requires specific control measures to ensure system reliability and data integrity.
Sample contamination represents a critical risk factor that can compromise experimental results. Implementation of closed-system designs with minimal exposure points significantly reduces contamination probability. Regular cleaning protocols using validated methods and materials compatible with both samples and analytical instruments must be established. Environmental monitoring systems that continuously track particulate levels, temperature, and humidity provide real-time alerts when conditions deviate from acceptable parameters.
Mechanical failures in robotic components pose substantial operational risks. Preventive maintenance schedules based on component lifecycle analysis rather than fixed time intervals optimize system availability. Redundant critical components and fail-safe mechanisms ensure that system failures result in controlled shutdowns rather than sample damage. Vibration monitoring systems can detect early signs of mechanical wear before catastrophic failures occur.
Software validation represents another crucial quality control domain. Comprehensive validation protocols must include boundary testing, error recovery procedures, and data integrity verification. Automated logging systems that record all sample movements, environmental conditions, and system interventions create audit trails essential for troubleshooting and regulatory compliance. Regular software updates must undergo regression testing to verify that new features do not compromise existing functionality.
Calibration verification procedures ensure measurement accuracy across multiple characterization modalities. Cross-validation between different analytical techniques helps identify systematic errors that might not be apparent within a single measurement system. Statistical process control methods applied to reference samples can detect subtle shifts in system performance before they affect experimental results.
Emergency response protocols must address power failures, environmental control disruptions, and mechanical malfunctions. Uninterruptible power supplies and automated sample recovery procedures minimize risk during unexpected events. Regular simulation of failure scenarios trains operators to respond effectively to emergencies while providing opportunities to refine recovery procedures.
Documentation and training form the foundation of quality control implementation. Comprehensive standard operating procedures must cover normal operations, maintenance activities, and emergency responses. Operator certification programs ensure that all personnel possess the necessary skills to interact safely with automated systems. Regular competency assessments verify continued compliance with established procedures.
Sample contamination represents a critical risk factor that can compromise experimental results. Implementation of closed-system designs with minimal exposure points significantly reduces contamination probability. Regular cleaning protocols using validated methods and materials compatible with both samples and analytical instruments must be established. Environmental monitoring systems that continuously track particulate levels, temperature, and humidity provide real-time alerts when conditions deviate from acceptable parameters.
Mechanical failures in robotic components pose substantial operational risks. Preventive maintenance schedules based on component lifecycle analysis rather than fixed time intervals optimize system availability. Redundant critical components and fail-safe mechanisms ensure that system failures result in controlled shutdowns rather than sample damage. Vibration monitoring systems can detect early signs of mechanical wear before catastrophic failures occur.
Software validation represents another crucial quality control domain. Comprehensive validation protocols must include boundary testing, error recovery procedures, and data integrity verification. Automated logging systems that record all sample movements, environmental conditions, and system interventions create audit trails essential for troubleshooting and regulatory compliance. Regular software updates must undergo regression testing to verify that new features do not compromise existing functionality.
Calibration verification procedures ensure measurement accuracy across multiple characterization modalities. Cross-validation between different analytical techniques helps identify systematic errors that might not be apparent within a single measurement system. Statistical process control methods applied to reference samples can detect subtle shifts in system performance before they affect experimental results.
Emergency response protocols must address power failures, environmental control disruptions, and mechanical malfunctions. Uninterruptible power supplies and automated sample recovery procedures minimize risk during unexpected events. Regular simulation of failure scenarios trains operators to respond effectively to emergencies while providing opportunities to refine recovery procedures.
Documentation and training form the foundation of quality control implementation. Comprehensive standard operating procedures must cover normal operations, maintenance activities, and emergency responses. Operator certification programs ensure that all personnel possess the necessary skills to interact safely with automated systems. Regular competency assessments verify continued compliance with established procedures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!