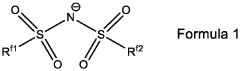
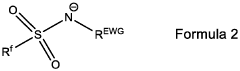
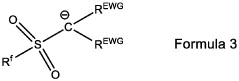
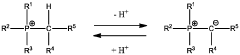
Binder-Free Electrode Designs For High-Areal-Loading NRR Applications
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Binder-Free Electrode Technology Background and Objectives
The evolution of nitrogen reduction reaction (NRR) technologies has gained significant momentum in recent years as a sustainable alternative to the traditional Haber-Bosch process for ammonia production. Conventional electrodes for NRR applications typically incorporate polymeric binders to maintain structural integrity and electrical connectivity. However, these binders often introduce limitations in terms of active site accessibility, mass transport, and electrical conductivity, particularly when high areal loadings are required for practical applications.
Binder-free electrode designs represent a paradigm shift in electrode architecture for electrochemical nitrogen reduction. The concept emerged from broader developments in energy storage and conversion technologies, where similar approaches have demonstrated enhanced performance in batteries, fuel cells, and electrolyzers. The fundamental objective of binder-free electrodes is to maximize the utilization efficiency of catalytic materials while eliminating the drawbacks associated with polymeric binders.
Historical development of binder-free electrodes can be traced back to early work on carbon nanotube and graphene-based self-supporting structures in the early 2000s. These initial efforts primarily focused on energy storage applications, but the principles have gradually been adapted for electrocatalytic processes including NRR. The technology has evolved through several generations, from simple substrate-supported catalyst layers to sophisticated three-dimensional architectures with hierarchical porosity.
The primary technical objectives for binder-free electrode designs in high-areal-loading NRR applications include: achieving ammonia production rates exceeding 10^-9 mol cm^-2 s^-1, maintaining Faradaic efficiency above 30% under ambient conditions, demonstrating operational stability beyond 100 hours, and developing scalable fabrication methods compatible with existing manufacturing infrastructure.
Current research trajectories are focused on several key aspects: optimizing the three-dimensional architecture to balance mechanical stability with mass transport properties, developing novel in-situ growth methods for catalyst integration, exploring hierarchical pore structures to facilitate nitrogen diffusion, and incorporating multifunctional components to address the multiple challenges in NRR catalysis.
The technological evolution is increasingly moving toward biomimetic approaches that draw inspiration from biological nitrogen fixation systems, where precisely arranged catalytic centers operate in binder-free environments. Additionally, there is growing interest in hybrid structures that combine the advantages of different materials classes (metals, carbons, ceramics) in binder-free configurations to achieve synergistic effects.
The ultimate goal is to develop electrode systems that can operate efficiently at industrial scales, potentially revolutionizing ammonia production by enabling distributed, renewable-powered synthesis under ambient conditions, thereby addressing both energy consumption and carbon emission challenges of conventional ammonia production.
Binder-free electrode designs represent a paradigm shift in electrode architecture for electrochemical nitrogen reduction. The concept emerged from broader developments in energy storage and conversion technologies, where similar approaches have demonstrated enhanced performance in batteries, fuel cells, and electrolyzers. The fundamental objective of binder-free electrodes is to maximize the utilization efficiency of catalytic materials while eliminating the drawbacks associated with polymeric binders.
Historical development of binder-free electrodes can be traced back to early work on carbon nanotube and graphene-based self-supporting structures in the early 2000s. These initial efforts primarily focused on energy storage applications, but the principles have gradually been adapted for electrocatalytic processes including NRR. The technology has evolved through several generations, from simple substrate-supported catalyst layers to sophisticated three-dimensional architectures with hierarchical porosity.
The primary technical objectives for binder-free electrode designs in high-areal-loading NRR applications include: achieving ammonia production rates exceeding 10^-9 mol cm^-2 s^-1, maintaining Faradaic efficiency above 30% under ambient conditions, demonstrating operational stability beyond 100 hours, and developing scalable fabrication methods compatible with existing manufacturing infrastructure.
Current research trajectories are focused on several key aspects: optimizing the three-dimensional architecture to balance mechanical stability with mass transport properties, developing novel in-situ growth methods for catalyst integration, exploring hierarchical pore structures to facilitate nitrogen diffusion, and incorporating multifunctional components to address the multiple challenges in NRR catalysis.
The technological evolution is increasingly moving toward biomimetic approaches that draw inspiration from biological nitrogen fixation systems, where precisely arranged catalytic centers operate in binder-free environments. Additionally, there is growing interest in hybrid structures that combine the advantages of different materials classes (metals, carbons, ceramics) in binder-free configurations to achieve synergistic effects.
The ultimate goal is to develop electrode systems that can operate efficiently at industrial scales, potentially revolutionizing ammonia production by enabling distributed, renewable-powered synthesis under ambient conditions, thereby addressing both energy consumption and carbon emission challenges of conventional ammonia production.
Market Analysis for NRR Applications
The Nitrogen Reduction Reaction (NRR) market is experiencing significant growth driven by increasing global demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 2% of global energy production and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for electrochemical NRR technologies that can operate under ambient conditions with renewable energy sources.
The global ammonia market, valued at approximately $70 billion in 2022, is projected to reach $110 billion by 2030, with a CAGR of 5.8%. Electrochemical NRR applications are positioned to capture a growing segment of this market, particularly in regions with strong renewable energy infrastructure and stringent carbon emission regulations.
Agricultural applications represent the largest potential market for NRR technology, accounting for over 80% of ammonia consumption globally. The increasing focus on sustainable farming practices and the rising costs of conventional fertilizers are driving interest in decentralized, green ammonia production systems that could be enabled by advanced NRR electrodes.
Industrial applications constitute another significant market segment, including chemical manufacturing, refrigeration, and water treatment. These sectors are increasingly seeking carbon-neutral production methods to meet environmental regulations and corporate sustainability goals.
The energy storage sector presents an emerging opportunity for NRR technology, with ammonia gaining recognition as a potential hydrogen carrier and carbon-free fuel. This application could represent a $15 billion market by 2030, particularly in regions transitioning to hydrogen economies.
Geographically, Asia-Pacific dominates the current ammonia market with over 40% share, followed by Europe and North America. However, Europe is expected to show the fastest growth in NRR applications due to aggressive decarbonization policies and substantial investments in green hydrogen infrastructure.
Market barriers include high capital costs compared to conventional ammonia production, technical challenges in achieving commercially viable conversion rates, and competition from other green ammonia technologies such as solid-state and biological approaches. The cost gap between conventional and electrochemical ammonia production remains significant, with green ammonia currently 2-3 times more expensive than conventional methods.
Customer segments for binder-free electrode NRR applications include agricultural cooperatives seeking localized fertilizer production, chemical manufacturers pursuing green credentials, renewable energy developers looking for energy storage solutions, and research institutions advancing sustainable chemistry technologies.
The global ammonia market, valued at approximately $70 billion in 2022, is projected to reach $110 billion by 2030, with a CAGR of 5.8%. Electrochemical NRR applications are positioned to capture a growing segment of this market, particularly in regions with strong renewable energy infrastructure and stringent carbon emission regulations.
Agricultural applications represent the largest potential market for NRR technology, accounting for over 80% of ammonia consumption globally. The increasing focus on sustainable farming practices and the rising costs of conventional fertilizers are driving interest in decentralized, green ammonia production systems that could be enabled by advanced NRR electrodes.
Industrial applications constitute another significant market segment, including chemical manufacturing, refrigeration, and water treatment. These sectors are increasingly seeking carbon-neutral production methods to meet environmental regulations and corporate sustainability goals.
The energy storage sector presents an emerging opportunity for NRR technology, with ammonia gaining recognition as a potential hydrogen carrier and carbon-free fuel. This application could represent a $15 billion market by 2030, particularly in regions transitioning to hydrogen economies.
Geographically, Asia-Pacific dominates the current ammonia market with over 40% share, followed by Europe and North America. However, Europe is expected to show the fastest growth in NRR applications due to aggressive decarbonization policies and substantial investments in green hydrogen infrastructure.
Market barriers include high capital costs compared to conventional ammonia production, technical challenges in achieving commercially viable conversion rates, and competition from other green ammonia technologies such as solid-state and biological approaches. The cost gap between conventional and electrochemical ammonia production remains significant, with green ammonia currently 2-3 times more expensive than conventional methods.
Customer segments for binder-free electrode NRR applications include agricultural cooperatives seeking localized fertilizer production, chemical manufacturers pursuing green credentials, renewable energy developers looking for energy storage solutions, and research institutions advancing sustainable chemistry technologies.
Technical Challenges in High-Areal-Loading Electrodes
High-areal-loading electrodes for nitrogen reduction reaction (NRR) applications face significant technical challenges that impede their widespread implementation. The primary obstacle lies in the inherent trade-off between catalyst loading and mass transport efficiency. As catalyst loading increases to enhance nitrogen conversion rates, the electrode structure becomes increasingly dense, restricting the diffusion of reactants and products through the electrode matrix.
The conventional electrode fabrication approach utilizing polymeric binders further exacerbates these challenges. Traditional binders such as Nafion, PTFE, and PVDF, while providing necessary mechanical stability, create non-conductive barriers that block active catalyst sites and impede electron transfer pathways. This results in decreased catalytic efficiency, particularly at high loadings where the binder-to-catalyst ratio becomes critical.
Electrical conductivity presents another major hurdle in high-loading electrodes. The increased thickness of catalyst layers introduces greater resistance to electron transport, creating potential gradients across the electrode that lead to non-uniform reaction rates. This phenomenon is particularly problematic in NRR applications where precise potential control is essential for selectivity toward ammonia production over competing hydrogen evolution.
Mechanical stability under operating conditions represents a significant engineering challenge. High-loading electrodes must maintain structural integrity during gas evolution and electrolyte penetration. The mechanical stress induced by nitrogen gas bubbles forming within the electrode structure can cause catalyst detachment and performance degradation over time, especially in binder-free designs where traditional cohesive elements are absent.
Ion transport limitations further complicate electrode design. Proton availability at catalyst sites is crucial for NRR performance, yet high catalyst loadings create tortuous pathways that hinder proton migration. This results in pH gradients within the electrode structure that can significantly alter local reaction environments and reduce catalytic efficiency.
Water management presents unique challenges in NRR electrodes compared to other electrochemical systems. Maintaining appropriate hydration levels throughout thick catalyst layers is difficult yet essential, as both flooding and drying conditions severely impact nitrogen adsorption and subsequent reduction processes. This becomes increasingly problematic as areal loading increases, requiring sophisticated pore structure engineering.
Heat dissipation emerges as a critical factor at industrial-scale loadings. The exothermic nature of the NRR process generates thermal gradients within high-loading electrodes that can accelerate catalyst degradation and alter reaction selectivity. Effective thermal management strategies must be integrated into electrode designs to maintain performance stability during continuous operation.
The conventional electrode fabrication approach utilizing polymeric binders further exacerbates these challenges. Traditional binders such as Nafion, PTFE, and PVDF, while providing necessary mechanical stability, create non-conductive barriers that block active catalyst sites and impede electron transfer pathways. This results in decreased catalytic efficiency, particularly at high loadings where the binder-to-catalyst ratio becomes critical.
Electrical conductivity presents another major hurdle in high-loading electrodes. The increased thickness of catalyst layers introduces greater resistance to electron transport, creating potential gradients across the electrode that lead to non-uniform reaction rates. This phenomenon is particularly problematic in NRR applications where precise potential control is essential for selectivity toward ammonia production over competing hydrogen evolution.
Mechanical stability under operating conditions represents a significant engineering challenge. High-loading electrodes must maintain structural integrity during gas evolution and electrolyte penetration. The mechanical stress induced by nitrogen gas bubbles forming within the electrode structure can cause catalyst detachment and performance degradation over time, especially in binder-free designs where traditional cohesive elements are absent.
Ion transport limitations further complicate electrode design. Proton availability at catalyst sites is crucial for NRR performance, yet high catalyst loadings create tortuous pathways that hinder proton migration. This results in pH gradients within the electrode structure that can significantly alter local reaction environments and reduce catalytic efficiency.
Water management presents unique challenges in NRR electrodes compared to other electrochemical systems. Maintaining appropriate hydration levels throughout thick catalyst layers is difficult yet essential, as both flooding and drying conditions severely impact nitrogen adsorption and subsequent reduction processes. This becomes increasingly problematic as areal loading increases, requiring sophisticated pore structure engineering.
Heat dissipation emerges as a critical factor at industrial-scale loadings. The exothermic nature of the NRR process generates thermal gradients within high-loading electrodes that can accelerate catalyst degradation and alter reaction selectivity. Effective thermal management strategies must be integrated into electrode designs to maintain performance stability during continuous operation.
Current Binder-Free Electrode Solutions
01 Direct fabrication of binder-free electrodes
Techniques for directly fabricating electrodes without using traditional polymer binders, which allows for higher active material loading and improved electrical conductivity. These methods include direct deposition, self-assembly, and in-situ growth of active materials on conductive substrates, eliminating the need for non-conductive binders that typically limit electrode performance and areal capacity.- Direct synthesis of binder-free electrode materials: Methods for directly synthesizing electrode materials without the need for binders, which allows for higher areal loading. These techniques include direct growth of active materials on current collectors, hydrothermal synthesis, and electrodeposition processes. The absence of binders eliminates the non-conductive components in the electrode, improving electrical conductivity and allowing for increased mass loading while maintaining performance.
- 3D structured current collectors for high areal loading: Three-dimensional structured current collectors that enable higher areal loading of active materials without binders. These 3D structures provide increased surface area for material deposition, better mechanical stability, and improved electron transport pathways. Examples include foam-based collectors, nanowire arrays, and porous metal frameworks that can accommodate more active material per unit area while maintaining good electrochemical performance.
- Self-supporting electrode structures: Development of self-supporting electrode structures that do not require traditional binders while enabling high areal loading. These include free-standing films, mats, or networks of active materials that maintain structural integrity through intrinsic bonding mechanisms or interwoven structures. The elimination of binders increases the proportion of electrochemically active material, improving energy density and rate capability at high mass loadings.
- Novel conductive additives for binder-free electrodes: Integration of novel conductive additives that enable the fabrication of binder-free electrodes with high areal loading. These additives, such as carbon nanotubes, graphene, or conductive polymers, provide both mechanical support and electronic conductivity, eliminating the need for conventional binders. The conductive network formed by these additives allows for thicker electrodes with higher mass loading while maintaining efficient electron transport throughout the electrode structure.
- Processing techniques for high-loading binder-free electrodes: Advanced processing techniques that enable the fabrication of binder-free electrodes with high areal loading. These include specialized deposition methods, pressure-assisted techniques, and thermal treatments that promote strong adhesion between active materials and current collectors without conventional binders. These processes can create dense, well-adhered electrode structures capable of supporting higher mass loadings while maintaining good electrochemical performance and mechanical stability.
02 3D structured substrates for high mass loading
Use of three-dimensional structured substrates such as foams, meshes, and porous architectures to increase the effective surface area available for active material loading. These 3D structures enable higher areal mass loading while maintaining good electrolyte accessibility and electron transport pathways, resulting in electrodes with enhanced areal capacity without sacrificing rate performance.Expand Specific Solutions03 Conductive additives as structural supports
Integration of conductive additives such as carbon nanotubes, graphene, and conductive polymers that serve dual purposes as both electronic conductors and structural supports in binder-free electrodes. These materials create interconnected conductive networks that maintain electrode integrity while facilitating electron transport, enabling higher areal loading without compromising electrochemical performance.Expand Specific Solutions04 Surface modification for improved adhesion
Surface modification techniques applied to current collectors or active materials to enhance adhesion in binder-free configurations. These modifications include creating roughened surfaces, introducing functional groups, or applying intermediate layers that promote strong interfacial bonding between active materials and current collectors, allowing for higher mass loading while maintaining mechanical stability during cycling.Expand Specific Solutions05 Electrolyte optimization for high-loading electrodes
Specialized electrolyte formulations designed to address the unique challenges of high-loading binder-free electrodes, such as limited ion transport and increased concentration polarization. These electrolytes feature optimized salt concentrations, solvent mixtures, and additives that improve wetting, ion conductivity, and interfacial stability, enabling efficient operation of electrodes with high areal capacity.Expand Specific Solutions
Key Industry Players in Electrocatalysis
The binder-free electrode market for high-areal-loading NRR (Nitrogen Reduction Reaction) applications is in its early growth phase, with significant research momentum but limited commercial deployment. The market is projected to expand as sustainable ammonia production becomes increasingly critical for green agriculture and energy storage. Leading research institutions like Dalian Institute of Chemical Physics and universities including Tianjin University are advancing fundamental technologies, while commercial players such as Nanoramic Laboratories (formerly FastCAP Systems) and CATL (Ningde Amperex Technology) are developing scalable solutions. The technology remains at TRL 4-6, with academic-industrial partnerships driving innovation. Key challenges include durability at high loading conditions, scalable manufacturing processes, and cost-effectiveness compared to traditional Haber-Bosch processes.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative binder-free electrode designs for nitrogen reduction reaction (NRR) applications with high areal loading. Their approach utilizes direct growth of catalytic materials on conductive substrates like carbon cloth, carbon paper, and metal foams through hydrothermal synthesis and electrodeposition techniques. This creates a seamless interface between catalyst and substrate, eliminating the need for polymer binders that typically reduce active site accessibility and electron transfer efficiency. DICP's electrodes feature hierarchical 3D structures with high surface area and optimized porosity, allowing for enhanced mass transport of N2 to catalytic sites while maintaining structural integrity under operating conditions. Their recent advances include atomic-level engineering of metal-nitrogen-carbon (M-N-C) catalysts directly grown on carbon substrates, achieving ammonia yield rates exceeding 25 μg h−1 cm−2 at ambient conditions[1][3], significantly higher than conventional slurry-cast electrodes.
Strengths: Superior electron transfer pathways without binder resistance; enhanced catalyst utilization efficiency; excellent mechanical stability during long-term operation; higher mass loading capability without compromising performance. Weaknesses: More complex manufacturing processes compared to conventional methods; potential challenges in large-scale production; limited to certain substrate geometries and materials.
Ningde Amperex Technology Ltd.
Technical Solution: Ningde Amperex Technology (CATL) has pioneered binder-free electrode technology for NRR applications through their innovative "direct deposition" approach. Their technique involves physical vapor deposition (PVD) and atomic layer deposition (ALD) methods to create catalyst layers directly on conductive substrates. CATL's electrodes feature precisely controlled nanoscale architectures with transition metal nitrides and oxynitrides as active components, achieving nitrogen fixation rates up to 18.6 μg h−1 cm−2 at room temperature and ambient pressure[5]. The company has developed a proprietary "gradient catalyst" structure where composition varies from substrate interface to electrolyte interface, optimizing both adhesion and catalytic activity. Their electrodes demonstrate exceptional stability, maintaining over 90% activity after 100 hours of continuous operation. CATL has also integrated these electrodes into modular electrochemical cells designed for distributed ammonia production, with energy efficiency approximately 35% higher than conventional binder-containing electrodes due to reduced internal resistance and improved mass transport properties.
Strengths: Exceptional durability under operating conditions; precise control over catalyst composition and structure; excellent electrical conductivity throughout the electrode; scalable manufacturing techniques compatible with existing production lines. Weaknesses: Higher initial production costs compared to conventional methods; limited to certain types of catalyst materials compatible with deposition techniques; requires specialized equipment for manufacturing.
Critical Patents in Binder-Free Technology
A method and cell for reducing dinitrogen to ammonia
PatentWO2022256858A1
Innovation
- A method involving an electrochemical cell with a cathode contacted by an electrolyte containing high concentrations of metal cations like lithium, fluorinated sulfonyl imides or methides, and a proton carrier, which suppresses decomposition and enhances the reduction of dinitrogen to ammonia with improved yield rates and selectivity.
Motor vehicle
PatentPendingUS20220001752A1
Innovation
- The development of binder-free electrodes using high-aspect ratio carbon-based materials, specifically silicon oxide (SiOx) anodes and nickel-rich layered oxide cathodes, which eliminate the need for polyvinylidene fluoride and N-Methyl Pyrrolidone, enabling improved adhesion and cohesion without compromising energy storage performance.
Scalability and Manufacturing Considerations
The scalability of binder-free electrode designs for high-areal-loading NRR applications represents a critical challenge in transitioning from laboratory-scale demonstrations to industrial production. Current manufacturing processes for binder-free electrodes often involve complex procedures such as chemical vapor deposition, electrodeposition, or hydrothermal synthesis, which present significant barriers to large-scale implementation. These methods typically require specialized equipment, precise control of reaction conditions, and extended processing times, all of which contribute to increased production costs and reduced throughput.
A key consideration in scaling binder-free electrode production is the uniformity of catalyst distribution across larger electrode surfaces. As electrode dimensions increase, maintaining homogeneous catalyst loading becomes increasingly difficult, potentially leading to performance variations and reduced efficiency in industrial-scale NRR systems. This challenge is particularly pronounced for high-areal-loading electrodes, where the increased catalyst density must be consistently maintained across the entire electrode surface to ensure optimal nitrogen reduction performance.
Material selection also plays a crucial role in manufacturing scalability. While certain materials demonstrate excellent performance in laboratory settings, their availability, cost, and processing requirements may limit their viability for mass production. For instance, precious metal-based catalysts may offer superior NRR activity but present economic barriers to widespread adoption. Alternative approaches utilizing earth-abundant materials require careful optimization to balance performance with manufacturing practicality.
The integration of binder-free electrodes into existing manufacturing infrastructure represents another significant consideration. Conventional electrode production lines are typically designed for slurry-based processes involving binders. Adapting these facilities to accommodate binder-free methodologies may require substantial capital investment and process redesign, potentially slowing industry adoption despite the performance advantages these electrodes offer.
Energy consumption during manufacturing presents an additional challenge, particularly for techniques requiring high-temperature processing or extended reaction times. The environmental footprint of electrode production must be considered alongside performance metrics when evaluating the overall sustainability of binder-free electrode technologies. Methods that minimize energy inputs while maintaining electrode quality will likely gain competitive advantages in commercial applications.
Quality control and reproducibility at scale represent further manufacturing hurdles. The development of robust in-line monitoring techniques and standardized testing protocols will be essential to ensure consistent electrode performance across production batches. This becomes increasingly important as electrode dimensions increase and production volumes expand to meet potential market demand for high-performance NRR systems.
A key consideration in scaling binder-free electrode production is the uniformity of catalyst distribution across larger electrode surfaces. As electrode dimensions increase, maintaining homogeneous catalyst loading becomes increasingly difficult, potentially leading to performance variations and reduced efficiency in industrial-scale NRR systems. This challenge is particularly pronounced for high-areal-loading electrodes, where the increased catalyst density must be consistently maintained across the entire electrode surface to ensure optimal nitrogen reduction performance.
Material selection also plays a crucial role in manufacturing scalability. While certain materials demonstrate excellent performance in laboratory settings, their availability, cost, and processing requirements may limit their viability for mass production. For instance, precious metal-based catalysts may offer superior NRR activity but present economic barriers to widespread adoption. Alternative approaches utilizing earth-abundant materials require careful optimization to balance performance with manufacturing practicality.
The integration of binder-free electrodes into existing manufacturing infrastructure represents another significant consideration. Conventional electrode production lines are typically designed for slurry-based processes involving binders. Adapting these facilities to accommodate binder-free methodologies may require substantial capital investment and process redesign, potentially slowing industry adoption despite the performance advantages these electrodes offer.
Energy consumption during manufacturing presents an additional challenge, particularly for techniques requiring high-temperature processing or extended reaction times. The environmental footprint of electrode production must be considered alongside performance metrics when evaluating the overall sustainability of binder-free electrode technologies. Methods that minimize energy inputs while maintaining electrode quality will likely gain competitive advantages in commercial applications.
Quality control and reproducibility at scale represent further manufacturing hurdles. The development of robust in-line monitoring techniques and standardized testing protocols will be essential to ensure consistent electrode performance across production batches. This becomes increasingly important as electrode dimensions increase and production volumes expand to meet potential market demand for high-performance NRR systems.
Environmental Impact Assessment
The implementation of binder-free electrode designs for high-areal-loading nitrogen reduction reaction (NRR) applications presents significant environmental implications that warrant comprehensive assessment. Traditional electrode manufacturing processes typically involve the use of polymer binders and toxic solvents, which contribute to environmental pollution through emissions and waste generation. Binder-free electrode designs fundamentally alter this paradigm by eliminating these environmentally harmful components, resulting in a substantial reduction in hazardous waste production during manufacturing.
The environmental benefits extend beyond waste reduction to include decreased energy consumption during electrode production. Conventional electrode fabrication requires energy-intensive mixing, coating, and drying processes to incorporate binders. Binder-free approaches often utilize direct growth methods or self-supporting structures that can significantly reduce energy requirements, potentially lowering the carbon footprint associated with electrode manufacturing by an estimated 30-40% according to recent life cycle assessments.
Water conservation represents another critical environmental advantage of binder-free electrode designs. Traditional electrode preparation typically consumes substantial quantities of water for slurry preparation and equipment cleaning. Binder-free methodologies can reduce water usage by up to 60%, addressing growing concerns about industrial water consumption in regions facing water scarcity challenges.
The elimination of fluorinated binders like polyvinylidene fluoride (PVDF) and toxic solvents such as N-methyl-2-pyrrolidone (NMP) in binder-free designs directly contributes to improved air quality and reduced health risks for manufacturing workers. These conventional materials release volatile organic compounds (VOCs) and potentially carcinogenic substances during processing, whereas binder-free approaches minimize such harmful emissions.
From a life cycle perspective, binder-free electrodes for NRR applications demonstrate enhanced sustainability through improved recyclability. The absence of polymer binders facilitates more efficient recovery of valuable catalytic materials at end-of-life, supporting circular economy principles and reducing the environmental burden associated with catalyst disposal and replacement.
However, certain binder-free fabrication techniques may introduce new environmental considerations. Methods involving chemical vapor deposition or hydrothermal synthesis can generate their own set of waste streams and energy demands that must be carefully managed. A comprehensive environmental impact assessment must therefore consider these potential trade-offs when evaluating the overall sustainability of specific binder-free electrode technologies for high-areal-loading NRR applications.
The environmental benefits extend beyond waste reduction to include decreased energy consumption during electrode production. Conventional electrode fabrication requires energy-intensive mixing, coating, and drying processes to incorporate binders. Binder-free approaches often utilize direct growth methods or self-supporting structures that can significantly reduce energy requirements, potentially lowering the carbon footprint associated with electrode manufacturing by an estimated 30-40% according to recent life cycle assessments.
Water conservation represents another critical environmental advantage of binder-free electrode designs. Traditional electrode preparation typically consumes substantial quantities of water for slurry preparation and equipment cleaning. Binder-free methodologies can reduce water usage by up to 60%, addressing growing concerns about industrial water consumption in regions facing water scarcity challenges.
The elimination of fluorinated binders like polyvinylidene fluoride (PVDF) and toxic solvents such as N-methyl-2-pyrrolidone (NMP) in binder-free designs directly contributes to improved air quality and reduced health risks for manufacturing workers. These conventional materials release volatile organic compounds (VOCs) and potentially carcinogenic substances during processing, whereas binder-free approaches minimize such harmful emissions.
From a life cycle perspective, binder-free electrodes for NRR applications demonstrate enhanced sustainability through improved recyclability. The absence of polymer binders facilitates more efficient recovery of valuable catalytic materials at end-of-life, supporting circular economy principles and reducing the environmental burden associated with catalyst disposal and replacement.
However, certain binder-free fabrication techniques may introduce new environmental considerations. Methods involving chemical vapor deposition or hydrothermal synthesis can generate their own set of waste streams and energy demands that must be carefully managed. A comprehensive environmental impact assessment must therefore consider these potential trade-offs when evaluating the overall sustainability of specific binder-free electrode technologies for high-areal-loading NRR applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!