Environmental And Safety Considerations For Electrochemically Produced Ammonia
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Ammonia Production Background and Objectives
Ammonia (NH3) production represents one of the most significant industrial processes globally, with the Haber-Bosch process serving as the dominant method since its development in the early 20th century. This conventional process, while effective, operates under extreme conditions of high temperature (400-500°C) and high pressure (150-300 bar), consuming approximately 1-2% of the world's total energy production and generating substantial greenhouse gas emissions. The environmental impact is considerable, with traditional ammonia production responsible for approximately 1.8% of global CO2 emissions.
Electrochemical ammonia production has emerged as a promising alternative that could revolutionize this critical industrial sector. This approach utilizes electricity to drive the reaction between nitrogen and water at ambient conditions, potentially enabling a more sustainable production pathway. The fundamental reaction involves the electrochemical reduction of nitrogen (N2) to ammonia (NH3) using water as the hydrogen source, represented by N2 + 6H2O + 6e- → 2NH3 + 6OH-.
The historical development of electrochemical ammonia synthesis dates back to the early 20th century, but significant progress has only been achieved in recent decades with advances in electrocatalysis, materials science, and renewable energy technologies. The convergence of these fields has created new opportunities to address the longstanding challenges of nitrogen activation and selectivity in electrochemical systems.
The primary technical objective of electrochemical ammonia production research is to develop efficient, selective, and stable electrocatalytic systems that can operate at ambient conditions with minimal energy input. This includes the design of advanced catalysts capable of breaking the strong N≡N triple bond, optimizing reaction pathways to enhance ammonia selectivity over competing hydrogen evolution, and developing integrated systems compatible with renewable electricity sources.
From an environmental perspective, the goal is to establish a carbon-neutral or carbon-negative ammonia production pathway that can replace the energy-intensive Haber-Bosch process. This transition could significantly reduce the carbon footprint of ammonia production, which is crucial not only for fertilizer manufacturing but also for emerging applications of ammonia as a carbon-free energy carrier and fuel.
The technological evolution in this field is increasingly focused on addressing safety considerations unique to electrochemical processes, including the management of reactive intermediates, prevention of unwanted by-product formation, and development of inherently safer system designs. These safety aspects are becoming central to research efforts as the technology advances toward practical implementation and scale-up.
Electrochemical ammonia production has emerged as a promising alternative that could revolutionize this critical industrial sector. This approach utilizes electricity to drive the reaction between nitrogen and water at ambient conditions, potentially enabling a more sustainable production pathway. The fundamental reaction involves the electrochemical reduction of nitrogen (N2) to ammonia (NH3) using water as the hydrogen source, represented by N2 + 6H2O + 6e- → 2NH3 + 6OH-.
The historical development of electrochemical ammonia synthesis dates back to the early 20th century, but significant progress has only been achieved in recent decades with advances in electrocatalysis, materials science, and renewable energy technologies. The convergence of these fields has created new opportunities to address the longstanding challenges of nitrogen activation and selectivity in electrochemical systems.
The primary technical objective of electrochemical ammonia production research is to develop efficient, selective, and stable electrocatalytic systems that can operate at ambient conditions with minimal energy input. This includes the design of advanced catalysts capable of breaking the strong N≡N triple bond, optimizing reaction pathways to enhance ammonia selectivity over competing hydrogen evolution, and developing integrated systems compatible with renewable electricity sources.
From an environmental perspective, the goal is to establish a carbon-neutral or carbon-negative ammonia production pathway that can replace the energy-intensive Haber-Bosch process. This transition could significantly reduce the carbon footprint of ammonia production, which is crucial not only for fertilizer manufacturing but also for emerging applications of ammonia as a carbon-free energy carrier and fuel.
The technological evolution in this field is increasingly focused on addressing safety considerations unique to electrochemical processes, including the management of reactive intermediates, prevention of unwanted by-product formation, and development of inherently safer system designs. These safety aspects are becoming central to research efforts as the technology advances toward practical implementation and scale-up.
Market Analysis for Green Ammonia Technologies
The global green ammonia market is experiencing unprecedented growth, driven by increasing environmental concerns and the push for decarbonization across industries. Current market valuations place the green ammonia sector at approximately $72 million in 2022, with projections indicating a compound annual growth rate (CAGR) of 72.9% through 2030, potentially reaching a market value of $5.4 billion. This exponential growth trajectory reflects the mounting interest in sustainable alternatives to conventional ammonia production methods.
Electrochemically produced ammonia represents a significant segment within the broader green ammonia market. Unlike traditional Haber-Bosch processes that rely heavily on fossil fuels and generate substantial carbon emissions, electrochemical production methods utilize renewable electricity, water, and nitrogen from air to synthesize ammonia with minimal environmental impact. This technological approach aligns perfectly with global sustainability goals and carbon neutrality targets.
Demand drivers for electrochemically produced ammonia span multiple sectors. The agricultural industry, which consumes approximately 80% of global ammonia production for fertilizers, is increasingly seeking carbon-neutral inputs to reduce its environmental footprint. Additionally, the emerging hydrogen economy views ammonia as an efficient hydrogen carrier, with significant potential in energy storage and transportation applications.
Regional market analysis reveals varying adoption rates and investment patterns. Europe leads in green ammonia initiatives, supported by stringent environmental regulations and substantial government funding for clean technology development. Countries like Germany, Denmark, and the Netherlands have established pilot projects specifically focused on electrochemical ammonia production. The Asia-Pacific region, particularly Japan, South Korea, and Australia, is rapidly expanding its green ammonia infrastructure, driven by energy security concerns and export opportunities.
Investment trends indicate growing confidence in electrochemical ammonia technologies. Venture capital funding in this sector increased by 156% between 2020 and 2022, with particular interest in startups developing novel catalyst materials and membrane technologies that enhance production efficiency while reducing costs. Major industrial players are also forming strategic partnerships with technology developers to secure early access to promising electrochemical ammonia production methods.
Market barriers remain significant but are gradually being addressed. The cost differential between conventional and electrochemically produced ammonia currently stands at 2-3 times, though this gap is narrowing as renewable electricity costs decline and carbon pricing mechanisms become more widespread. Technical challenges related to catalyst durability, energy efficiency, and scale-up capabilities are being systematically addressed through collaborative research initiatives and industrial partnerships.
Electrochemically produced ammonia represents a significant segment within the broader green ammonia market. Unlike traditional Haber-Bosch processes that rely heavily on fossil fuels and generate substantial carbon emissions, electrochemical production methods utilize renewable electricity, water, and nitrogen from air to synthesize ammonia with minimal environmental impact. This technological approach aligns perfectly with global sustainability goals and carbon neutrality targets.
Demand drivers for electrochemically produced ammonia span multiple sectors. The agricultural industry, which consumes approximately 80% of global ammonia production for fertilizers, is increasingly seeking carbon-neutral inputs to reduce its environmental footprint. Additionally, the emerging hydrogen economy views ammonia as an efficient hydrogen carrier, with significant potential in energy storage and transportation applications.
Regional market analysis reveals varying adoption rates and investment patterns. Europe leads in green ammonia initiatives, supported by stringent environmental regulations and substantial government funding for clean technology development. Countries like Germany, Denmark, and the Netherlands have established pilot projects specifically focused on electrochemical ammonia production. The Asia-Pacific region, particularly Japan, South Korea, and Australia, is rapidly expanding its green ammonia infrastructure, driven by energy security concerns and export opportunities.
Investment trends indicate growing confidence in electrochemical ammonia technologies. Venture capital funding in this sector increased by 156% between 2020 and 2022, with particular interest in startups developing novel catalyst materials and membrane technologies that enhance production efficiency while reducing costs. Major industrial players are also forming strategic partnerships with technology developers to secure early access to promising electrochemical ammonia production methods.
Market barriers remain significant but are gradually being addressed. The cost differential between conventional and electrochemically produced ammonia currently stands at 2-3 times, though this gap is narrowing as renewable electricity costs decline and carbon pricing mechanisms become more widespread. Technical challenges related to catalyst durability, energy efficiency, and scale-up capabilities are being systematically addressed through collaborative research initiatives and industrial partnerships.
Current Challenges in Electrochemical Ammonia Synthesis
Electrochemical ammonia synthesis faces significant technical challenges despite its promising environmental advantages over the Haber-Bosch process. The primary obstacle remains the low Faradaic efficiency, typically below 10% in most laboratory demonstrations, which severely limits commercial viability. This inefficiency stems from the competing hydrogen evolution reaction that dominates at cathode surfaces, consuming electrons that would otherwise reduce nitrogen to ammonia.
Selectivity presents another major hurdle, as nitrogen reduction must compete with hydrogen production at similar potential ranges. Researchers struggle to develop catalysts that can selectively bind nitrogen while suppressing hydrogen evolution, particularly at ambient conditions where kinetic barriers for N₂ activation are substantial.
Catalyst stability represents a persistent challenge, with many promising materials suffering from degradation during extended operation. Noble metal catalysts show higher activity but face economic barriers for large-scale implementation, while non-noble alternatives often lack sufficient durability in aqueous electrolytes.
Energy efficiency remains problematic, with current systems requiring significantly more energy input per unit of ammonia produced compared to the Haber-Bosch process. Most electrochemical systems operate at energy efficiencies below 30%, far from the theoretical minimum energy requirement of 20.3 kJ/mol NH₃.
Reaction mechanisms for electrochemical nitrogen reduction remain poorly understood, hampering rational catalyst design. The precise pathways of electron and proton transfer to nitrogen intermediates vary across different catalyst surfaces and operating conditions, creating difficulties in developing predictive models.
Scalability challenges persist as most successful demonstrations occur only at laboratory scale with carefully controlled conditions. The transition to industrial-scale production faces engineering barriers related to electrode design, electrolyte management, and system integration.
Nitrogen activation at ambient conditions represents a fundamental challenge due to the exceptional stability of the N≡N triple bond (945 kJ/mol). Breaking this bond requires significant energy input or highly specialized catalytic environments that can weaken the bond through specific electronic interactions.
Electrolyte optimization remains critical yet challenging, as the medium must facilitate nitrogen dissolution and transport while maintaining ionic conductivity and compatibility with electrode materials. Current aqueous systems suffer from limited nitrogen solubility, while non-aqueous alternatives introduce new complications regarding stability and conductivity.
Selectivity presents another major hurdle, as nitrogen reduction must compete with hydrogen production at similar potential ranges. Researchers struggle to develop catalysts that can selectively bind nitrogen while suppressing hydrogen evolution, particularly at ambient conditions where kinetic barriers for N₂ activation are substantial.
Catalyst stability represents a persistent challenge, with many promising materials suffering from degradation during extended operation. Noble metal catalysts show higher activity but face economic barriers for large-scale implementation, while non-noble alternatives often lack sufficient durability in aqueous electrolytes.
Energy efficiency remains problematic, with current systems requiring significantly more energy input per unit of ammonia produced compared to the Haber-Bosch process. Most electrochemical systems operate at energy efficiencies below 30%, far from the theoretical minimum energy requirement of 20.3 kJ/mol NH₃.
Reaction mechanisms for electrochemical nitrogen reduction remain poorly understood, hampering rational catalyst design. The precise pathways of electron and proton transfer to nitrogen intermediates vary across different catalyst surfaces and operating conditions, creating difficulties in developing predictive models.
Scalability challenges persist as most successful demonstrations occur only at laboratory scale with carefully controlled conditions. The transition to industrial-scale production faces engineering barriers related to electrode design, electrolyte management, and system integration.
Nitrogen activation at ambient conditions represents a fundamental challenge due to the exceptional stability of the N≡N triple bond (945 kJ/mol). Breaking this bond requires significant energy input or highly specialized catalytic environments that can weaken the bond through specific electronic interactions.
Electrolyte optimization remains critical yet challenging, as the medium must facilitate nitrogen dissolution and transport while maintaining ionic conductivity and compatibility with electrode materials. Current aqueous systems suffer from limited nitrogen solubility, while non-aqueous alternatives introduce new complications regarding stability and conductivity.
Current Environmental Safety Solutions for Ammonia Production
01 Environmental impact assessment of electrochemical ammonia production
Electrochemical ammonia production methods are being evaluated for their environmental impacts compared to traditional Haber-Bosch processes. These assessments include lifecycle analyses measuring carbon footprint, energy efficiency, and resource utilization. Studies indicate potential reductions in greenhouse gas emissions when renewable energy sources power electrochemical processes. The environmental benefits include decreased fossil fuel dependency and reduced water consumption compared to conventional ammonia production methods.- Environmental impact assessment of electrochemical ammonia production: Electrochemical ammonia production methods have significant environmental implications compared to traditional Haber-Bosch processes. These systems can reduce greenhouse gas emissions when powered by renewable energy sources. The environmental impact assessment includes analysis of carbon footprint, energy efficiency, resource utilization, and potential for integration with sustainable energy systems. Advanced lifecycle assessment methodologies help quantify the overall environmental benefits of electrochemical ammonia synthesis pathways.
- Safety protocols and risk management for electrochemical ammonia systems: Safety considerations for electrochemical ammonia production include handling of reactive materials, pressure management, and prevention of ammonia leakage. Comprehensive safety protocols involve monitoring systems, emergency shutdown procedures, and proper containment designs. Risk management frameworks address potential hazards during operation, maintenance, and transportation of electrochemically produced ammonia, with emphasis on operator training and compliance with industrial safety standards.
- Sustainable integration of electrochemical ammonia production with renewable energy: Electrochemical ammonia production can be effectively integrated with renewable energy sources to create sustainable chemical manufacturing systems. These integrated approaches utilize intermittent renewable energy for electrolysis processes, enabling energy storage in the form of ammonia. The systems can balance grid fluctuations while producing valuable chemical products, creating dual environmental benefits through clean energy utilization and reduced emissions from traditional ammonia production methods.
- Regulatory compliance and certification for electrochemical ammonia technologies: Regulatory frameworks for electrochemical ammonia production encompass environmental permits, safety certifications, and compliance with chemical manufacturing standards. These regulations vary by jurisdiction but typically address emissions limits, safety requirements, and quality control measures. Certification processes evaluate the environmental impact and safety performance of electrochemical ammonia facilities, requiring documentation of risk assessments, environmental management plans, and emergency response procedures.
- Technological innovations reducing environmental and safety risks: Recent technological innovations in electrochemical ammonia production focus on minimizing environmental impacts and enhancing safety. These include advanced catalyst designs that operate at lower temperatures and pressures, reducing explosion risks and energy consumption. Membrane technologies improve separation efficiency while minimizing waste generation. Modular system designs enable distributed production with smaller inventories of hazardous materials, while digital monitoring systems provide real-time safety oversight and environmental performance tracking.
02 Safety protocols for electrochemical ammonia systems
Safety considerations for electrochemical ammonia production include handling of catalysts, electrolytes, and the ammonia product itself. Specialized containment systems, monitoring equipment, and emergency response protocols are necessary to manage risks associated with ammonia's toxicity and flammability. Safety designs incorporate pressure relief systems, leak detection, and automated shutdown mechanisms. Personnel training requirements and protective equipment specifications are also critical components of safety management systems for these production facilities.Expand Specific Solutions03 Regulatory compliance and standards for ammonia production
Electrochemical ammonia production facilities must adhere to various regulatory frameworks governing chemical manufacturing, emissions, and workplace safety. These include environmental permits, hazardous material handling certifications, and regular compliance audits. Industry standards are being developed specifically for electrochemical ammonia production to ensure consistent safety and environmental performance. Regulatory considerations also extend to transportation and storage of produced ammonia, with requirements varying by jurisdiction.Expand Specific Solutions04 Risk mitigation strategies for electrochemical ammonia facilities
Risk assessment and mitigation strategies are essential for electrochemical ammonia production facilities. These include engineering controls such as ventilation systems, containment structures, and process automation to minimize exposure risks. Comprehensive risk management approaches incorporate failure mode analysis, emergency response planning, and regular safety drills. Advanced monitoring technologies enable real-time detection of potential hazards, allowing for proactive intervention before incidents occur.Expand Specific Solutions05 Sustainable integration of electrochemical ammonia production
Electrochemical ammonia production can be integrated into sustainable energy systems, particularly when coupled with renewable power sources. This integration enables energy storage capabilities, grid balancing, and carbon-neutral fertilizer production. Sustainable implementation models include distributed production facilities that reduce transportation emissions and enhance local agricultural sustainability. Research focuses on optimizing system efficiency, reducing catalyst requirements, and minimizing waste generation throughout the production lifecycle.Expand Specific Solutions
Leading Organizations in Electrochemical Ammonia Research
The electrochemically produced ammonia market is in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market potential is substantial, driven by decarbonization initiatives and the $70+ billion conventional ammonia market. Technical maturity varies across players: academic institutions (Technical University of Denmark, Delft University, Massachusetts Institute of Technology) focus on fundamental research, while companies demonstrate different development stages. Established industrial players like Siemens AG and Yara International are leveraging their manufacturing expertise, while specialized startups such as GenCell, Atmonia, and Battolyser are developing innovative technologies. Chinese institutions (South China University of Technology, Zhejiang University) are increasingly active, indicating growing global competition in this emerging clean technology field.
Technical University of Denmark
Technical Solution: The Technical University of Denmark has developed an advanced electrochemical ammonia synthesis platform that operates at atmospheric pressure and temperatures below 100°C. Their system employs specialized proton-conducting ceramic electrolytes that enable selective nitrogen reduction while minimizing competing hydrogen evolution reactions. Safety features include distributed production architecture that limits the quantity of ammonia present at any location, reducing catastrophic release risks. Environmental considerations are addressed through comprehensive lifecycle assessment methodologies that have identified optimal operating parameters to minimize overall environmental impact. Their research has demonstrated ammonia production with energy efficiencies approaching 60% of theoretical limits, significantly higher than previous electrochemical approaches. The system incorporates real-time monitoring of electrolyte composition and ammonia concentration, with automated controls to prevent formation of explosive mixtures. Their catalyst design minimizes use of precious metals while maintaining high activity and selectivity for nitrogen reduction.
Strengths: Cutting-edge research with multiple peer-reviewed publications demonstrating concept viability; comprehensive understanding of reaction mechanisms enabling targeted improvements; strong focus on sustainable materials selection. Weaknesses: Technology remains primarily at laboratory scale; catalyst stability issues under long-term operation conditions; higher energy consumption compared to theoretical minimums.
Yara International ASA
Technical Solution: Yara has developed a comprehensive electrochemical ammonia production system that integrates renewable energy sources with advanced electrolysis technology. Their approach utilizes specialized catalysts to enable nitrogen reduction at lower temperatures and pressures compared to conventional Haber-Bosch processes. The system incorporates multiple safety mechanisms including real-time monitoring of ammonia concentrations, automated shutdown protocols, and advanced ventilation systems to prevent hazardous accumulations. Environmental considerations are addressed through closed-loop water recycling systems that minimize wastewater discharge and reduce water consumption by approximately 30% compared to conventional methods. Yara's technology also features modular design principles allowing for scalable implementation from distributed agricultural applications to industrial-scale production facilities.
Strengths: Industry-leading experience in ammonia production and distribution networks; established safety protocols that exceed regulatory requirements; significant reduction in carbon footprint compared to conventional methods. Weaknesses: Higher initial capital costs compared to traditional ammonia production; technology still requires further optimization for maximum energy efficiency.
Key Innovations in Electrochemical Ammonia Safety Systems
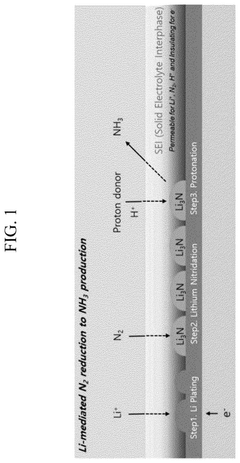
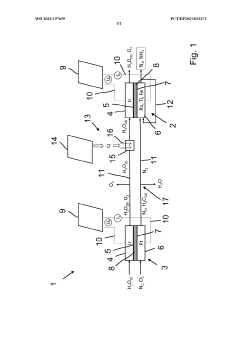
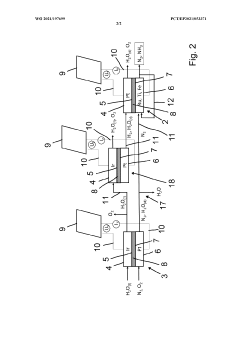
ELECTROLYTE AND MANUFACTURING METHOD OF GREEN AMMONIA FROM Li-MEDIATRED NITROGEN REDUCTION USING THE SAME
PatentPendingUS20250171914A1
Innovation
- An electrolyte comprising a lithium compound, a proton donor, and a sulfur compound is used in an electrochemical reactor to facilitate the production of ammonia through a Li-mediated nitrogen reduction reaction, which forms lithium nitride and subsequently ammonia.
Process and apparatus for synthesis of ammonia
PatentWO2021197699A1
Innovation
- An electrochemical ammonia synthesis method using a pre-cell and main cell with cation exchange membranes, where nitrogen is supplied through an additional electrochemical cell, eliminating the need for cryogenic air separation, and utilizing catalysts like iridium and platinum, with renewable energy sources for voltage, to produce ammonia from air and water.
Regulatory Framework for Electrochemical Ammonia Production
The regulatory landscape for electrochemical ammonia production is evolving rapidly as this emerging technology approaches commercial viability. Currently, regulations governing this sector exist within a patchwork of frameworks originally designed for conventional ammonia production, industrial chemicals, and renewable energy technologies. In the United States, the Environmental Protection Agency (EPA) oversees chemical manufacturing processes through the Toxic Substances Control Act (TSCA) and Clean Air Act provisions, while the Occupational Safety and Health Administration (OSHA) establishes workplace safety standards for handling ammonia and related compounds.
The European Union has implemented more comprehensive regulations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, which requires extensive safety documentation and risk assessments for new chemical production processes. Additionally, the EU's Industrial Emissions Directive (IED) sets stringent standards for emissions control that would apply to scaled electrochemical ammonia facilities.
In Asia, regulatory approaches vary significantly, with Japan and South Korea adopting frameworks similar to European models, while China has recently strengthened its environmental regulations through the revised Environmental Protection Law, which could impact future electrochemical ammonia production facilities.
A critical regulatory gap exists in the classification of electrochemically produced ammonia. Unlike conventional Haber-Bosch ammonia, electrochemical production may involve different precursors, catalysts, and potential contaminants. Regulatory bodies have not yet established specific guidelines addressing these distinctions, creating uncertainty for technology developers and potential investors.
Carbon accounting frameworks represent another important regulatory consideration. As electrochemical ammonia production is often positioned as a green alternative to conventional methods, carbon credits and renewable energy certificates may apply depending on the electricity source. The EU's Carbon Border Adjustment Mechanism and similar emerging policies in other regions could significantly impact the economic viability of different production approaches.
Safety regulations present particular challenges, as existing frameworks for ammonia handling were developed primarily for large-scale industrial facilities rather than the potentially distributed production model that electrochemical methods might enable. This regulatory mismatch could either hinder deployment through overregulation or create safety risks through inadequate oversight.
International harmonization efforts are underway through organizations like the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC), which are developing standards specifically for green hydrogen and derivative products including electrochemically produced ammonia. These standards will likely form the foundation for future regulatory frameworks as the technology matures toward widespread commercial implementation.
The European Union has implemented more comprehensive regulations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, which requires extensive safety documentation and risk assessments for new chemical production processes. Additionally, the EU's Industrial Emissions Directive (IED) sets stringent standards for emissions control that would apply to scaled electrochemical ammonia facilities.
In Asia, regulatory approaches vary significantly, with Japan and South Korea adopting frameworks similar to European models, while China has recently strengthened its environmental regulations through the revised Environmental Protection Law, which could impact future electrochemical ammonia production facilities.
A critical regulatory gap exists in the classification of electrochemically produced ammonia. Unlike conventional Haber-Bosch ammonia, electrochemical production may involve different precursors, catalysts, and potential contaminants. Regulatory bodies have not yet established specific guidelines addressing these distinctions, creating uncertainty for technology developers and potential investors.
Carbon accounting frameworks represent another important regulatory consideration. As electrochemical ammonia production is often positioned as a green alternative to conventional methods, carbon credits and renewable energy certificates may apply depending on the electricity source. The EU's Carbon Border Adjustment Mechanism and similar emerging policies in other regions could significantly impact the economic viability of different production approaches.
Safety regulations present particular challenges, as existing frameworks for ammonia handling were developed primarily for large-scale industrial facilities rather than the potentially distributed production model that electrochemical methods might enable. This regulatory mismatch could either hinder deployment through overregulation or create safety risks through inadequate oversight.
International harmonization efforts are underway through organizations like the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC), which are developing standards specifically for green hydrogen and derivative products including electrochemically produced ammonia. These standards will likely form the foundation for future regulatory frameworks as the technology matures toward widespread commercial implementation.
Life Cycle Assessment of Electrochemical Ammonia Systems
Life Cycle Assessment (LCA) of electrochemical ammonia production systems provides a comprehensive framework for evaluating environmental impacts across the entire production chain. This assessment methodology examines impacts from raw material extraction through manufacturing, operation, and end-of-life disposal, offering crucial insights into the sustainability of these emerging technologies.
The system boundaries for electrochemical ammonia LCA typically encompass electricity generation, electrode material production, electrolyte preparation, system assembly, operation, maintenance, and decommissioning phases. Recent studies indicate that the environmental footprint of electrochemical ammonia production is heavily dependent on the electricity source, with renewable energy integration showing potential to reduce greenhouse gas emissions by 70-90% compared to conventional Haber-Bosch processes.
Energy efficiency remains a critical factor in LCA outcomes. Current electrochemical systems demonstrate energy efficiencies ranging from 30-65%, significantly affecting overall environmental performance. Research indicates that improving catalyst efficiency and optimizing cell design could potentially increase these values to 70-80%, substantially enhancing sustainability metrics.
Water consumption represents another important consideration, with electrochemical processes requiring 1.5-3 times less water than conventional methods when accounting for cooling requirements. However, water quality impacts from electrolyte disposal require careful management to prevent localized environmental degradation.
Material intensity analysis reveals that electrochemical systems generally require more specialized materials per unit of ammonia produced compared to conventional plants, particularly regarding catalyst materials containing precious metals. This creates potential resource constraints that must be addressed through recycling strategies and alternative material development.
Comparative LCA studies demonstrate that electrochemical ammonia production can achieve carbon intensity reductions of 40-95% versus conventional methods when powered by low-carbon electricity. However, these systems may show increased impacts in categories such as resource depletion and potential toxicity, highlighting the importance of holistic assessment approaches.
Sensitivity analysis of LCA results identifies electricity source, system lifetime, and catalyst loading as the most influential parameters affecting environmental outcomes. This underscores the need for policy frameworks that incentivize renewable energy integration and extended producer responsibility to maximize sustainability benefits of electrochemical ammonia technologies.
The system boundaries for electrochemical ammonia LCA typically encompass electricity generation, electrode material production, electrolyte preparation, system assembly, operation, maintenance, and decommissioning phases. Recent studies indicate that the environmental footprint of electrochemical ammonia production is heavily dependent on the electricity source, with renewable energy integration showing potential to reduce greenhouse gas emissions by 70-90% compared to conventional Haber-Bosch processes.
Energy efficiency remains a critical factor in LCA outcomes. Current electrochemical systems demonstrate energy efficiencies ranging from 30-65%, significantly affecting overall environmental performance. Research indicates that improving catalyst efficiency and optimizing cell design could potentially increase these values to 70-80%, substantially enhancing sustainability metrics.
Water consumption represents another important consideration, with electrochemical processes requiring 1.5-3 times less water than conventional methods when accounting for cooling requirements. However, water quality impacts from electrolyte disposal require careful management to prevent localized environmental degradation.
Material intensity analysis reveals that electrochemical systems generally require more specialized materials per unit of ammonia produced compared to conventional plants, particularly regarding catalyst materials containing precious metals. This creates potential resource constraints that must be addressed through recycling strategies and alternative material development.
Comparative LCA studies demonstrate that electrochemical ammonia production can achieve carbon intensity reductions of 40-95% versus conventional methods when powered by low-carbon electricity. However, these systems may show increased impacts in categories such as resource depletion and potential toxicity, highlighting the importance of holistic assessment approaches.
Sensitivity analysis of LCA results identifies electricity source, system lifetime, and catalyst loading as the most influential parameters affecting environmental outcomes. This underscores the need for policy frameworks that incentivize renewable energy integration and extended producer responsibility to maximize sustainability benefits of electrochemical ammonia technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!