Strategies To Improve Faradaic Efficiency At Industrial Current Densities
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Faradaic Efficiency Background and Objectives
Faradaic efficiency represents a critical parameter in electrochemical processes, measuring the percentage of electrical charge that contributes to the desired reaction versus competing side reactions. The concept originated in the 19th century with Michael Faraday's laws of electrolysis but has gained renewed significance in modern industrial applications. Understanding and optimizing Faradaic efficiency has become increasingly important as industries seek more sustainable and economically viable electrochemical processes.
The evolution of Faradaic efficiency research has progressed from fundamental electrochemical studies to sophisticated industrial applications. Initially focused on basic metal electrodeposition, the field has expanded to encompass complex reactions including CO2 reduction, water splitting for hydrogen production, and organic electrosynthesis. This progression reflects broader technological trends toward green chemistry, renewable energy storage, and carbon-neutral industrial processes.
Current industrial applications face significant challenges when operating at high current densities, which are essential for commercial viability. As current density increases, Faradaic efficiency typically decreases due to mass transport limitations, increased side reactions, and heat generation. This inverse relationship creates a technological bottleneck that limits the economic feasibility of many promising electrochemical technologies.
The primary objective of improving Faradaic efficiency at industrial current densities is to bridge the gap between laboratory demonstrations and commercial implementation. Achieving high efficiency (>90%) while maintaining industrially relevant current densities (>200 mA/cm²) would enable widespread adoption of electrochemical processes as alternatives to traditional chemical manufacturing routes. This would significantly reduce energy consumption and environmental impact across multiple industries.
Recent technological breakthroughs have demonstrated promising approaches to this challenge, including advanced catalyst design, innovative cell architectures, and precise control of reaction conditions. These developments suggest that dramatic improvements in Faradaic efficiency at high current densities are achievable through systematic research and engineering optimization.
The economic implications of such improvements are substantial. Even modest increases in Faradaic efficiency can translate to significant cost savings in large-scale operations through reduced energy consumption and increased product yield. For emerging technologies like CO2 electroreduction and green hydrogen production, enhanced Faradaic efficiency could be the determining factor in achieving market competitiveness against established processes.
This technical investigation aims to comprehensively examine strategies for improving Faradaic efficiency at industrial current densities, with particular focus on identifying approaches that can be implemented in near-term industrial settings while establishing a foundation for longer-term technological development.
The evolution of Faradaic efficiency research has progressed from fundamental electrochemical studies to sophisticated industrial applications. Initially focused on basic metal electrodeposition, the field has expanded to encompass complex reactions including CO2 reduction, water splitting for hydrogen production, and organic electrosynthesis. This progression reflects broader technological trends toward green chemistry, renewable energy storage, and carbon-neutral industrial processes.
Current industrial applications face significant challenges when operating at high current densities, which are essential for commercial viability. As current density increases, Faradaic efficiency typically decreases due to mass transport limitations, increased side reactions, and heat generation. This inverse relationship creates a technological bottleneck that limits the economic feasibility of many promising electrochemical technologies.
The primary objective of improving Faradaic efficiency at industrial current densities is to bridge the gap between laboratory demonstrations and commercial implementation. Achieving high efficiency (>90%) while maintaining industrially relevant current densities (>200 mA/cm²) would enable widespread adoption of electrochemical processes as alternatives to traditional chemical manufacturing routes. This would significantly reduce energy consumption and environmental impact across multiple industries.
Recent technological breakthroughs have demonstrated promising approaches to this challenge, including advanced catalyst design, innovative cell architectures, and precise control of reaction conditions. These developments suggest that dramatic improvements in Faradaic efficiency at high current densities are achievable through systematic research and engineering optimization.
The economic implications of such improvements are substantial. Even modest increases in Faradaic efficiency can translate to significant cost savings in large-scale operations through reduced energy consumption and increased product yield. For emerging technologies like CO2 electroreduction and green hydrogen production, enhanced Faradaic efficiency could be the determining factor in achieving market competitiveness against established processes.
This technical investigation aims to comprehensively examine strategies for improving Faradaic efficiency at industrial current densities, with particular focus on identifying approaches that can be implemented in near-term industrial settings while establishing a foundation for longer-term technological development.
Industrial Market Demand for Enhanced Faradaic Efficiency
The global market for electrochemical processes that rely on high Faradaic efficiency is experiencing unprecedented growth, driven by the increasing demand for sustainable chemical production, energy storage solutions, and green hydrogen generation. Industries such as chlor-alkali, metal electrowinning, water electrolysis, and CO2 electroreduction are particularly invested in technologies that can maximize energy utilization while minimizing waste.
Current market analysis indicates that the industrial electrolysis sector alone is projected to reach significant market value by 2030, with energy efficiency improvements representing a critical competitive advantage. Companies operating large-scale electrochemical plants face mounting pressure from rising electricity costs, which typically constitute 30-70% of operational expenses in electrochemical industries.
The economic imperative for enhanced Faradaic efficiency becomes clear when considering that even a 5% improvement in efficiency can translate to millions in annual savings for large industrial operations. This is particularly relevant in regions with high electricity costs or carbon pricing mechanisms, where inefficient processes incur additional financial penalties.
Market research reveals growing customer demand for electrochemical technologies that can maintain high Faradaic efficiency at industrial-scale current densities (typically >200 mA/cm²). Traditional systems often experience dramatic efficiency drops when scaled to these commercially viable current densities, creating a significant technology gap that innovative solutions must address.
The renewable energy sector represents another major market driver, as intermittent power sources require flexible electrochemical systems that can maintain efficiency across variable operating conditions. This has created new market segments for electrolyzers and flow batteries that can operate efficiently under dynamic load conditions.
Regulatory frameworks worldwide are increasingly incentivizing energy-efficient industrial processes through carbon taxes, efficiency standards, and green certification programs. The European Union's carbon border adjustment mechanism and similar policies in other regions are creating strong market pull for technologies that can demonstrate superior Faradaic efficiency metrics.
Industry surveys indicate that manufacturing companies are willing to invest in capital equipment upgrades that promise efficiency improvements with payback periods under three years. This creates a substantial market opportunity for technologies that can deliver verifiable efficiency gains at industrial scales without requiring complete system overhauls.
The competitive landscape shows increasing collaboration between material science companies, catalyst developers, and equipment manufacturers to create integrated solutions that address Faradaic efficiency challenges. This trend toward vertical integration reflects the complex, multidisciplinary nature of efficiency improvements in industrial electrochemical processes.
Current market analysis indicates that the industrial electrolysis sector alone is projected to reach significant market value by 2030, with energy efficiency improvements representing a critical competitive advantage. Companies operating large-scale electrochemical plants face mounting pressure from rising electricity costs, which typically constitute 30-70% of operational expenses in electrochemical industries.
The economic imperative for enhanced Faradaic efficiency becomes clear when considering that even a 5% improvement in efficiency can translate to millions in annual savings for large industrial operations. This is particularly relevant in regions with high electricity costs or carbon pricing mechanisms, where inefficient processes incur additional financial penalties.
Market research reveals growing customer demand for electrochemical technologies that can maintain high Faradaic efficiency at industrial-scale current densities (typically >200 mA/cm²). Traditional systems often experience dramatic efficiency drops when scaled to these commercially viable current densities, creating a significant technology gap that innovative solutions must address.
The renewable energy sector represents another major market driver, as intermittent power sources require flexible electrochemical systems that can maintain efficiency across variable operating conditions. This has created new market segments for electrolyzers and flow batteries that can operate efficiently under dynamic load conditions.
Regulatory frameworks worldwide are increasingly incentivizing energy-efficient industrial processes through carbon taxes, efficiency standards, and green certification programs. The European Union's carbon border adjustment mechanism and similar policies in other regions are creating strong market pull for technologies that can demonstrate superior Faradaic efficiency metrics.
Industry surveys indicate that manufacturing companies are willing to invest in capital equipment upgrades that promise efficiency improvements with payback periods under three years. This creates a substantial market opportunity for technologies that can deliver verifiable efficiency gains at industrial scales without requiring complete system overhauls.
The competitive landscape shows increasing collaboration between material science companies, catalyst developers, and equipment manufacturers to create integrated solutions that address Faradaic efficiency challenges. This trend toward vertical integration reflects the complex, multidisciplinary nature of efficiency improvements in industrial electrochemical processes.
Current Challenges in High Current Density Electrocatalysis
The electrocatalytic industry faces significant challenges when operating at high current densities, particularly in maintaining optimal Faradaic efficiency. As industrial applications demand increasingly higher throughput, the fundamental limitations of electrocatalytic systems become more pronounced. The primary challenge stems from the competition between desired and parasitic reactions at the electrode surface, which intensifies at elevated current densities.
Mass transport limitations represent a critical bottleneck in high-current operations. As reaction rates increase, the depletion of reactants near the electrode surface creates concentration gradients that favor unwanted side reactions. This phenomenon is particularly problematic in CO2 and N2 reduction reactions, where the limited solubility of gaseous reactants in aqueous electrolytes constrains the maximum achievable current density while maintaining acceptable efficiency.
Heat management emerges as another significant challenge. Higher current densities generate substantial thermal energy through resistive heating, potentially altering local reaction environments and catalyst stability. Temperature fluctuations can dramatically impact reaction selectivity, often favoring thermodynamically preferred but commercially undesirable pathways, such as hydrogen evolution in CO2 reduction systems.
Electrode degradation accelerates markedly at industrial current densities. The increased electrochemical stress can lead to structural changes in catalysts, including surface reconstruction, particle agglomeration, and active site poisoning. These degradation mechanisms not only reduce catalytic activity but also shift selectivity away from target products, directly impacting Faradaic efficiency over time.
Bubble formation and management present unique challenges at high current densities. Gas evolution reactions create bubbles that adhere to electrode surfaces, blocking active sites and creating non-uniform current distributions. This effect is particularly pronounced in industrial settings where electrode dimensions are significantly larger than laboratory-scale experiments.
Electrolyte stability becomes increasingly problematic as current densities rise. Local pH changes near electrode surfaces can be extreme, potentially leading to precipitation of salts, degradation of electrolyte components, or unwanted side reactions. These effects are often underestimated in laboratory studies but become dominant factors in industrial implementations.
System engineering challenges compound these scientific obstacles. Maintaining uniform current distribution across large-scale electrodes requires sophisticated cell design and flow management. The interplay between electrode geometry, electrolyte flow patterns, and bubble dynamics creates complex optimization problems that must be solved to achieve commercially viable Faradaic efficiencies at industrial scales.
Mass transport limitations represent a critical bottleneck in high-current operations. As reaction rates increase, the depletion of reactants near the electrode surface creates concentration gradients that favor unwanted side reactions. This phenomenon is particularly problematic in CO2 and N2 reduction reactions, where the limited solubility of gaseous reactants in aqueous electrolytes constrains the maximum achievable current density while maintaining acceptable efficiency.
Heat management emerges as another significant challenge. Higher current densities generate substantial thermal energy through resistive heating, potentially altering local reaction environments and catalyst stability. Temperature fluctuations can dramatically impact reaction selectivity, often favoring thermodynamically preferred but commercially undesirable pathways, such as hydrogen evolution in CO2 reduction systems.
Electrode degradation accelerates markedly at industrial current densities. The increased electrochemical stress can lead to structural changes in catalysts, including surface reconstruction, particle agglomeration, and active site poisoning. These degradation mechanisms not only reduce catalytic activity but also shift selectivity away from target products, directly impacting Faradaic efficiency over time.
Bubble formation and management present unique challenges at high current densities. Gas evolution reactions create bubbles that adhere to electrode surfaces, blocking active sites and creating non-uniform current distributions. This effect is particularly pronounced in industrial settings where electrode dimensions are significantly larger than laboratory-scale experiments.
Electrolyte stability becomes increasingly problematic as current densities rise. Local pH changes near electrode surfaces can be extreme, potentially leading to precipitation of salts, degradation of electrolyte components, or unwanted side reactions. These effects are often underestimated in laboratory studies but become dominant factors in industrial implementations.
System engineering challenges compound these scientific obstacles. Maintaining uniform current distribution across large-scale electrodes requires sophisticated cell design and flow management. The interplay between electrode geometry, electrolyte flow patterns, and bubble dynamics creates complex optimization problems that must be solved to achieve commercially viable Faradaic efficiencies at industrial scales.
Current Strategies for Industrial-Scale Faradaic Efficiency
01 Catalyst design for improved Faradaic efficiency
The design and selection of catalysts play a crucial role in enhancing the Faradaic efficiency of electrochemical systems. Specific catalyst materials, structures, and compositions can significantly influence the selectivity and efficiency of electrochemical reactions. Advanced catalyst designs can minimize unwanted side reactions and energy losses, thereby increasing the conversion efficiency of electrical energy to desired chemical products. Optimization of catalyst surface properties and active sites can lead to substantial improvements in overall system performance.- Catalyst design for improved Faradaic efficiency: The design and selection of catalysts play a crucial role in enhancing the Faradaic efficiency of electrochemical systems. Various catalyst materials, structures, and compositions can significantly impact the selectivity and efficiency of electrochemical reactions. Optimized catalysts can reduce unwanted side reactions and energy losses, thereby increasing the conversion efficiency of electrical energy to desired chemical products. Advanced catalyst designs incorporate specific elements or nanostructures that promote targeted reaction pathways while suppressing competing reactions.
- Electrode materials and structures for enhanced efficiency: The composition, structure, and surface properties of electrode materials significantly influence the Faradaic efficiency of electrochemical systems. Engineered electrode architectures with optimized porosity, surface area, and conductivity can improve reaction kinetics and mass transport. Novel electrode materials and configurations help minimize overpotential requirements while maximizing product selectivity. Modifications to electrode surfaces through functionalization or patterning can create preferential reaction sites that enhance the efficiency of specific electrochemical conversions.
- Electrolyte composition and system parameters: The composition of electrolytes and operating parameters significantly affect the Faradaic efficiency of electrochemical systems. Factors such as pH, ionic strength, temperature, and pressure can be optimized to enhance reaction selectivity and reduce energy losses. Specialized electrolyte additives can suppress unwanted side reactions or promote desired reaction pathways. Careful control of system parameters including current density, potential, and flow rates allows for maximizing efficiency across different electrochemical applications, from energy storage to chemical synthesis.
- Monitoring and measurement techniques: Advanced techniques for monitoring and measuring Faradaic efficiency are essential for optimizing electrochemical systems. These methods include in-situ spectroscopy, chromatography, and electrochemical characterization that provide real-time data on reaction products and efficiency. Precise measurement systems help identify efficiency losses and reaction intermediates, enabling targeted improvements to system design. Computational models combined with experimental data allow for predictive analysis of efficiency under various operating conditions, accelerating the development of high-efficiency electrochemical technologies.
- System design for CO2 conversion and energy storage applications: Specialized electrochemical system designs for applications such as CO2 conversion and energy storage focus on maximizing Faradaic efficiency for specific reactions. These systems incorporate optimized cell configurations, membrane technologies, and flow patterns to enhance mass transport and reaction selectivity. Integrated approaches combining thermal management, pressure control, and product separation improve overall system efficiency. Advanced designs address challenges such as bubble formation, concentration polarization, and catalyst deactivation that can reduce Faradaic efficiency in practical applications.
02 Electrode materials and configurations for enhanced efficiency
The selection and configuration of electrode materials significantly impact the Faradaic efficiency of electrochemical systems. Various electrode designs, including structured electrodes, porous materials, and composite electrodes, can be employed to increase active surface area and improve reaction kinetics. Electrode modifications such as surface treatments and incorporation of functional groups can enhance selectivity toward desired reactions. Optimized electrode configurations can reduce overpotential requirements and improve charge transfer processes, leading to higher Faradaic efficiencies.Expand Specific Solutions03 Electrolyte composition and reaction environment control
The composition of electrolytes and control of the reaction environment are critical factors affecting Faradaic efficiency. Parameters such as pH, temperature, pressure, and electrolyte concentration can be optimized to favor desired electrochemical pathways. Addition of specific ions or compounds to the electrolyte can suppress competing reactions and enhance selectivity. Buffering systems and controlled mass transport within the electrolyte can maintain optimal conditions at the electrode-electrolyte interface, resulting in improved efficiency and product yield in electrochemical conversion processes.Expand Specific Solutions04 System design and operational parameters optimization
The overall design of electrochemical systems and optimization of operational parameters significantly influence Faradaic efficiency. Factors such as cell architecture, membrane selection, flow field design, and current density distribution affect reaction selectivity and efficiency. Advanced control strategies for applied potential, current density, and reaction time can be implemented to maximize desired product formation. Integration of monitoring and feedback systems allows for real-time adjustment of operational parameters to maintain optimal Faradaic efficiency under varying conditions.Expand Specific Solutions05 Novel measurement and characterization techniques
Advanced measurement and characterization techniques are essential for accurately determining and improving Faradaic efficiency in electrochemical systems. In-situ and operando analytical methods enable real-time monitoring of electrochemical processes and product formation. Techniques such as gas chromatography, mass spectrometry, and spectroelectrochemical methods provide detailed information about reaction mechanisms and efficiency losses. Computational modeling and simulation approaches help predict and optimize system performance, guiding experimental design for enhanced Faradaic efficiency.Expand Specific Solutions
Leading Companies and Research Institutions in Electrocatalysis
The field of improving Faradaic efficiency at industrial current densities is currently in a growth phase, with the market expected to reach significant scale as green hydrogen and carbon capture technologies gain momentum. The technology maturity varies across applications, with companies demonstrating different levels of advancement. Dioxide Materials and Ebb Carbon are pioneering electrochemical CO2 conversion with enhanced efficiency, while established players like Hitachi, Toshiba, and IBM contribute substantial R&D resources. Academic institutions including Dalian University of Technology and University of British Columbia provide fundamental research support. The competitive landscape features both specialized startups focused on novel electrode materials and catalysts, and industrial conglomerates integrating these advances into large-scale systems, creating a dynamic ecosystem driving innovation toward commercial viability.
Dioxide Materials, Inc.
Technical Solution: Dioxide Materials has developed advanced catalyst systems specifically designed to improve Faradaic efficiency at industrial current densities for CO2 electroreduction. Their proprietary Sustainion® anion exchange membranes and ionomers enable stable operation at high current densities (>200 mA/cm²) while maintaining Faradaic efficiencies above 90% for extended periods. The company's approach combines specially designed electrode structures with optimized mass transport characteristics to mitigate concentration polarization effects that typically limit efficiency at industrial scales. Their catalyst design incorporates nanostructured copper-based materials with controlled morphology and surface chemistry to selectively produce valuable C2+ products like ethylene and ethanol. Additionally, they've implemented innovative cell designs with enhanced reactant delivery systems that maintain high local CO2 concentrations at the electrode surface even at high reaction rates, addressing a key limitation in industrial electrolyzers.
Strengths: Proprietary membrane technology enables stable high-current operation; integrated system approach addresses multiple efficiency bottlenecks simultaneously; demonstrated long-term stability in industrial conditions. Weaknesses: Higher system complexity increases capital costs; requires precise control of operating parameters; may face challenges in scaling to very large industrial installations.
Ebb Carbon, Inc.
Technical Solution: Ebb Carbon has pioneered an electrochemical approach to improving Faradaic efficiency at industrial current densities through their innovative pH-gradient enabled system. Their technology focuses on decoupling the electrochemical reaction environment from the overall cell conditions, creating localized optimal conditions for high efficiency even at elevated current densities (>500 mA/cm²). The company employs specially engineered electrode assemblies with gradient structures that maintain reactant concentration and pH at ideal levels throughout the reaction zone. Their system incorporates advanced flow field designs that ensure uniform current distribution across large electrode surfaces, a critical factor for maintaining efficiency at industrial scales. Ebb Carbon's approach also features dynamic operational protocols that continuously adjust electrolyte composition and flow rates in response to changing current densities, preventing efficiency losses that typically occur during industrial operation. Their technology has demonstrated sustained Faradaic efficiencies exceeding 85% for carbon dioxide reduction to formate at current densities relevant for commercial applications.
Strengths: Dynamic system adaptation maintains efficiency across varying operating conditions; innovative pH control strategy addresses fundamental limitations; demonstrated scalability to industrial-relevant parameters. Weaknesses: System complexity requires sophisticated control systems; higher operational costs compared to conventional approaches; potential challenges with long-term stability of specialized components.
Key Innovations in Electrode Materials and Catalyst Design
Nano-engineered catalyst for improving the faradaic efficiency of energy conversion and electrolysis systems
PatentPendingUS20240384425A1
Innovation
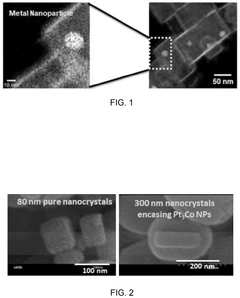
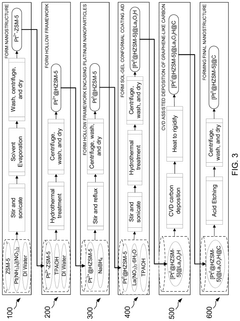
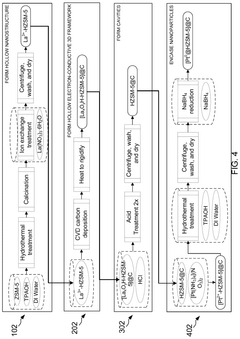
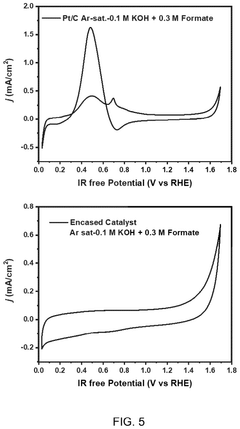
- A nanoengineered catalyst is developed, comprising a metal or metal alloy nanoparticle encased in an electron-conductive nano-sized hierarchically porous three-dimensional inorganic framework, specifically a hollow zeolitic framework, which selectively blocks undesired reactions and promotes desired electrochemical processes.
Hydrogen rich gas generator
PatentInactiveEP2373576A1
Innovation
- The method involves electrically isolating each electrolyte solution within electrolysis cells from the supply solution and using electrodes coated with nanomaterials, such as nickel and iron nanoparticles, to enhance voltage efficiency at current densities exceeding 100 mA/cm2, while applying constant current DC power for optimized control.
Energy Efficiency and Sustainability Considerations
Improving Faradaic efficiency at industrial current densities is intrinsically linked to broader energy efficiency and sustainability considerations. The electrochemical processes involved in industrial applications consume significant amounts of electricity, making energy efficiency a critical factor in both economic viability and environmental impact. When Faradaic efficiency is suboptimal, energy is wasted in side reactions, generating unwanted byproducts and heat rather than the desired products. This inefficiency translates directly into higher energy consumption per unit of product, increasing both operational costs and carbon footprint.
From a sustainability perspective, optimizing Faradaic efficiency contributes significantly to reducing the environmental impact of electrochemical processes. Higher efficiency means less electricity consumption for the same production output, which, depending on the energy source, can substantially reduce greenhouse gas emissions. For instance, in chlor-alkali production, improving Faradaic efficiency by just 5% could reduce energy consumption by approximately 150-200 kWh per ton of product, representing significant carbon emission reductions when scaled to industrial production volumes.
Life cycle assessment (LCA) studies indicate that the operational phase of electrochemical processes typically accounts for 60-80% of their total environmental impact, primarily due to energy consumption. Therefore, strategies that enhance Faradaic efficiency directly contribute to improving the overall sustainability profile of these processes. This becomes increasingly important as industries face stricter environmental regulations and carbon pricing mechanisms worldwide.
The economic implications of improved Faradaic efficiency are equally compelling. Energy costs typically represent 30-50% of operational expenses in electrochemical industries. At current industrial scales, even marginal improvements in efficiency can yield substantial cost savings. For example, in large-scale hydrogen production via water electrolysis, increasing Faradaic efficiency from 85% to 95% could reduce production costs by approximately 10-15%, potentially saving millions of dollars annually for large facilities.
Furthermore, enhanced energy efficiency through improved Faradaic efficiency aligns with global sustainability goals and circular economy principles. By maximizing resource utilization and minimizing waste, these improvements help decouple economic growth from environmental degradation. This alignment becomes particularly relevant as industries increasingly adopt Environmental, Social, and Governance (ESG) criteria and seek to demonstrate progress toward sustainability targets to stakeholders and investors.
From a sustainability perspective, optimizing Faradaic efficiency contributes significantly to reducing the environmental impact of electrochemical processes. Higher efficiency means less electricity consumption for the same production output, which, depending on the energy source, can substantially reduce greenhouse gas emissions. For instance, in chlor-alkali production, improving Faradaic efficiency by just 5% could reduce energy consumption by approximately 150-200 kWh per ton of product, representing significant carbon emission reductions when scaled to industrial production volumes.
Life cycle assessment (LCA) studies indicate that the operational phase of electrochemical processes typically accounts for 60-80% of their total environmental impact, primarily due to energy consumption. Therefore, strategies that enhance Faradaic efficiency directly contribute to improving the overall sustainability profile of these processes. This becomes increasingly important as industries face stricter environmental regulations and carbon pricing mechanisms worldwide.
The economic implications of improved Faradaic efficiency are equally compelling. Energy costs typically represent 30-50% of operational expenses in electrochemical industries. At current industrial scales, even marginal improvements in efficiency can yield substantial cost savings. For example, in large-scale hydrogen production via water electrolysis, increasing Faradaic efficiency from 85% to 95% could reduce production costs by approximately 10-15%, potentially saving millions of dollars annually for large facilities.
Furthermore, enhanced energy efficiency through improved Faradaic efficiency aligns with global sustainability goals and circular economy principles. By maximizing resource utilization and minimizing waste, these improvements help decouple economic growth from environmental degradation. This alignment becomes particularly relevant as industries increasingly adopt Environmental, Social, and Governance (ESG) criteria and seek to demonstrate progress toward sustainability targets to stakeholders and investors.
Scale-up Challenges and Economic Feasibility Analysis
Scaling up electrochemical processes from laboratory to industrial scale presents significant challenges when optimizing Faradaic efficiency at high current densities. The transition from small-scale experiments to commercial operations introduces complex engineering hurdles that directly impact system performance and economic viability. Primary scale-up challenges include heat management issues, as industrial-scale operations generate substantial thermal energy that can degrade catalysts and reduce Faradaic efficiency if not properly dissipated. Additionally, maintaining uniform electrolyte distribution becomes increasingly difficult in larger systems, leading to concentration gradients that compromise reaction selectivity.
Mass transport limitations represent another critical barrier, as the delivery of reactants to electrode surfaces becomes rate-limiting at industrial scales. This often necessitates advanced electrode designs with optimized porosity and surface area characteristics. Furthermore, bubble management emerges as a significant concern at high current densities, where gas evolution can block active catalyst sites and increase ohmic resistance, ultimately reducing system efficiency.
From an economic perspective, the capital expenditure (CAPEX) for industrial electrochemical systems remains high, particularly for specialized materials like precious metal catalysts and ion-exchange membranes. A comprehensive economic feasibility analysis reveals that Faradaic efficiency directly impacts operational expenditure (OPEX) through energy consumption costs, which typically constitute 30-50% of total production costs in electrochemical processes. Sensitivity analyses indicate that a 10% improvement in Faradaic efficiency can reduce overall production costs by 7-12%, depending on electricity prices and product value.
The economic viability threshold varies by application, but generally requires Faradaic efficiencies exceeding 80% at current densities above 200 mA/cm² for commodity chemicals production. For higher-value products, the economic requirements may be less stringent. Importantly, lifecycle assessments demonstrate that improved Faradaic efficiency delivers environmental benefits through reduced carbon footprints, potentially qualifying for carbon credits or regulatory advantages in certain markets.
Recent techno-economic analyses suggest that membrane durability and replacement frequency significantly impact long-term operational costs, with membrane degradation often accelerated at higher current densities. This creates a complex optimization problem where maximum production rates must be balanced against component lifetime considerations. Successful industrial implementation requires integrated engineering approaches that simultaneously address technical challenges while maintaining economic competitiveness against conventional production methods.
Mass transport limitations represent another critical barrier, as the delivery of reactants to electrode surfaces becomes rate-limiting at industrial scales. This often necessitates advanced electrode designs with optimized porosity and surface area characteristics. Furthermore, bubble management emerges as a significant concern at high current densities, where gas evolution can block active catalyst sites and increase ohmic resistance, ultimately reducing system efficiency.
From an economic perspective, the capital expenditure (CAPEX) for industrial electrochemical systems remains high, particularly for specialized materials like precious metal catalysts and ion-exchange membranes. A comprehensive economic feasibility analysis reveals that Faradaic efficiency directly impacts operational expenditure (OPEX) through energy consumption costs, which typically constitute 30-50% of total production costs in electrochemical processes. Sensitivity analyses indicate that a 10% improvement in Faradaic efficiency can reduce overall production costs by 7-12%, depending on electricity prices and product value.
The economic viability threshold varies by application, but generally requires Faradaic efficiencies exceeding 80% at current densities above 200 mA/cm² for commodity chemicals production. For higher-value products, the economic requirements may be less stringent. Importantly, lifecycle assessments demonstrate that improved Faradaic efficiency delivers environmental benefits through reduced carbon footprints, potentially qualifying for carbon credits or regulatory advantages in certain markets.
Recent techno-economic analyses suggest that membrane durability and replacement frequency significantly impact long-term operational costs, with membrane degradation often accelerated at higher current densities. This creates a complex optimization problem where maximum production rates must be balanced against component lifetime considerations. Successful industrial implementation requires integrated engineering approaches that simultaneously address technical challenges while maintaining economic competitiveness against conventional production methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!