Catalyst Design Principles For High Faradaic Efficiency In N2 Reduction
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
N2 Reduction Catalyst Evolution and Objectives
Nitrogen reduction reaction (NRR) catalysts have undergone significant evolution since the early 20th century, beginning with the groundbreaking Haber-Bosch process that revolutionized ammonia synthesis. This industrial process, while effective, operates under harsh conditions of high temperature (400-500°C) and pressure (150-300 bar), consuming approximately 1-2% of global energy production annually. The environmental impact and energy intensity of this conventional approach have driven research toward more sustainable alternatives.
The evolution of NRR catalysts can be traced through several distinct phases. The first generation focused on iron-based catalysts optimized for industrial-scale Haber-Bosch processes. The second generation, emerging in the 1970s-1990s, explored ruthenium and osmium-based systems that offered improved activity under slightly milder conditions. The third generation, developing since the early 2000s, has shifted toward electrochemical and photochemical approaches using noble metal catalysts, while the current fourth generation explores earth-abundant alternatives including transition metal nitrides, sulfides, and single-atom catalysts.
Recent technological breakthroughs have centered on ambient-condition electrochemical nitrogen reduction, with significant advances in catalyst design principles. Researchers have identified key factors influencing Faradaic efficiency, including nitrogen adsorption strength, competing hydrogen evolution reaction suppression, and optimal electron transfer pathways. Single-atom catalysts anchored on various supports have emerged as particularly promising due to their maximized atomic utilization and tunable electronic properties.
The primary objective in this field is to develop catalysts capable of achieving high Faradaic efficiency (>30%) for N2 reduction under ambient conditions. This represents a significant challenge, as most current systems struggle to exceed 15% efficiency due to the competing hydrogen evolution reaction. Secondary objectives include enhancing catalyst stability beyond the current typical degradation period of 10-20 hours and reducing precious metal content to improve economic viability.
Looking forward, the field aims to establish clear design principles that can guide rational catalyst development. These include optimizing the binding energy of reaction intermediates, particularly *N2H, engineering the local coordination environment of active sites, and developing bifunctional catalysts that can simultaneously activate N2 and suppress competing reactions. The ultimate goal remains developing systems that can operate efficiently at ambient conditions with earth-abundant materials, potentially revolutionizing fertilizer production and enabling distributed ammonia synthesis.
The evolution of NRR catalysts can be traced through several distinct phases. The first generation focused on iron-based catalysts optimized for industrial-scale Haber-Bosch processes. The second generation, emerging in the 1970s-1990s, explored ruthenium and osmium-based systems that offered improved activity under slightly milder conditions. The third generation, developing since the early 2000s, has shifted toward electrochemical and photochemical approaches using noble metal catalysts, while the current fourth generation explores earth-abundant alternatives including transition metal nitrides, sulfides, and single-atom catalysts.
Recent technological breakthroughs have centered on ambient-condition electrochemical nitrogen reduction, with significant advances in catalyst design principles. Researchers have identified key factors influencing Faradaic efficiency, including nitrogen adsorption strength, competing hydrogen evolution reaction suppression, and optimal electron transfer pathways. Single-atom catalysts anchored on various supports have emerged as particularly promising due to their maximized atomic utilization and tunable electronic properties.
The primary objective in this field is to develop catalysts capable of achieving high Faradaic efficiency (>30%) for N2 reduction under ambient conditions. This represents a significant challenge, as most current systems struggle to exceed 15% efficiency due to the competing hydrogen evolution reaction. Secondary objectives include enhancing catalyst stability beyond the current typical degradation period of 10-20 hours and reducing precious metal content to improve economic viability.
Looking forward, the field aims to establish clear design principles that can guide rational catalyst development. These include optimizing the binding energy of reaction intermediates, particularly *N2H, engineering the local coordination environment of active sites, and developing bifunctional catalysts that can simultaneously activate N2 and suppress competing reactions. The ultimate goal remains developing systems that can operate efficiently at ambient conditions with earth-abundant materials, potentially revolutionizing fertilizer production and enabling distributed ammonia synthesis.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability imperatives, with the market value projected to reach $70.3 billion by 2025, growing at a CAGR of 5.4%. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and generates substantial CO2 emissions, creating an urgent need for sustainable alternatives.
Electrochemical nitrogen reduction reaction (NRR) represents a promising pathway for sustainable ammonia production, potentially operating under ambient conditions with renewable electricity. This approach could dramatically reduce the carbon footprint of ammonia production, aligning with increasingly stringent environmental regulations and corporate sustainability goals.
The agricultural sector remains the dominant consumer of ammonia, accounting for over 80% of global demand as fertilizer. However, emerging applications in energy storage and transportation fuel are expanding market opportunities. Ammonia's potential as a hydrogen carrier and carbon-free fuel is attracting significant investment, with pilot projects for ammonia-powered vessels and power generation facilities already underway in Japan, Australia, and Northern Europe.
Regional market dynamics show varying adoption potentials for sustainable ammonia production technologies. Developed economies with ambitious decarbonization targets, particularly in Europe and parts of Asia-Pacific, demonstrate stronger willingness to invest in premium-priced green ammonia. Conversely, price-sensitive markets in developing regions prioritize cost-effectiveness over environmental benefits, presenting adoption challenges.
Economic analysis indicates that electrochemical ammonia production using catalysts with high Faradaic efficiency could become cost-competitive with conventional methods if catalyst performance reaches certain thresholds. Current estimates suggest that achieving Faradaic efficiencies above 60% with current densities exceeding 200 mA/cm² could enable production costs below $450 per ton, approaching conventional production costs in regions with high natural gas prices.
Regulatory landscapes are increasingly favorable for sustainable ammonia production technologies. Carbon pricing mechanisms, renewable energy incentives, and potential carbon border adjustment mechanisms create economic advantages for low-carbon ammonia production methods. The European Union's Green Deal and similar initiatives in other regions are expected to accelerate market adoption of sustainable ammonia technologies.
Investment trends show growing interest in NRR catalyst development, with venture capital funding in this space increasing by 35% annually since 2018. Strategic partnerships between catalyst technology developers, renewable energy providers, and traditional ammonia producers are emerging as the dominant commercialization pathway, reducing market entry barriers for innovative catalyst technologies.
Electrochemical nitrogen reduction reaction (NRR) represents a promising pathway for sustainable ammonia production, potentially operating under ambient conditions with renewable electricity. This approach could dramatically reduce the carbon footprint of ammonia production, aligning with increasingly stringent environmental regulations and corporate sustainability goals.
The agricultural sector remains the dominant consumer of ammonia, accounting for over 80% of global demand as fertilizer. However, emerging applications in energy storage and transportation fuel are expanding market opportunities. Ammonia's potential as a hydrogen carrier and carbon-free fuel is attracting significant investment, with pilot projects for ammonia-powered vessels and power generation facilities already underway in Japan, Australia, and Northern Europe.
Regional market dynamics show varying adoption potentials for sustainable ammonia production technologies. Developed economies with ambitious decarbonization targets, particularly in Europe and parts of Asia-Pacific, demonstrate stronger willingness to invest in premium-priced green ammonia. Conversely, price-sensitive markets in developing regions prioritize cost-effectiveness over environmental benefits, presenting adoption challenges.
Economic analysis indicates that electrochemical ammonia production using catalysts with high Faradaic efficiency could become cost-competitive with conventional methods if catalyst performance reaches certain thresholds. Current estimates suggest that achieving Faradaic efficiencies above 60% with current densities exceeding 200 mA/cm² could enable production costs below $450 per ton, approaching conventional production costs in regions with high natural gas prices.
Regulatory landscapes are increasingly favorable for sustainable ammonia production technologies. Carbon pricing mechanisms, renewable energy incentives, and potential carbon border adjustment mechanisms create economic advantages for low-carbon ammonia production methods. The European Union's Green Deal and similar initiatives in other regions are expected to accelerate market adoption of sustainable ammonia technologies.
Investment trends show growing interest in NRR catalyst development, with venture capital funding in this space increasing by 35% annually since 2018. Strategic partnerships between catalyst technology developers, renewable energy providers, and traditional ammonia producers are emerging as the dominant commercialization pathway, reducing market entry barriers for innovative catalyst technologies.
Current Challenges in N2 Reduction Catalysts
Despite significant advancements in N2 reduction reaction (NRR) catalyst development, researchers continue to face substantial challenges in achieving high Faradaic efficiency. The primary obstacle remains the competitive hydrogen evolution reaction (HER), which dominates electron consumption in aqueous environments. This parasitic reaction significantly reduces NRR efficiency, with most catalysts showing Faradaic efficiencies below 15% under ambient conditions.
Material stability presents another critical challenge, as many promising catalysts suffer from degradation during operation. Metal-based catalysts often experience leaching or surface reconstruction, while carbon-based materials may undergo oxidation or structural changes. These degradation mechanisms not only reduce catalyst longevity but also compromise selectivity over extended operation periods.
Nitrogen activation remains fundamentally difficult due to the exceptional stability of the N≡N triple bond (945 kJ/mol), requiring significant energy input for cleavage. Current catalysts struggle to efficiently adsorb and activate N2 molecules while simultaneously facilitating proton and electron transfer. The optimization of binding energies represents a delicate balance - too weak and N2 activation becomes inefficient, too strong and product release becomes rate-limiting.
Reaction pathway control presents significant complexity, as NRR can proceed through either associative or dissociative mechanisms with multiple possible intermediates. Current catalysts lack precise control over these pathways, leading to incomplete reduction and formation of unwanted byproducts like N2H2 or NH2OH, further reducing Faradaic efficiency.
Mass transport limitations also hinder performance, particularly N2 solubility in aqueous electrolytes (approximately 0.6 mM at room temperature), creating diffusion constraints that limit reaction rates. This challenge is compounded by the need to optimize electrolyte composition, as pH, ionic strength, and buffer capacity significantly impact both N2 reduction and competing reactions.
Analytical challenges further complicate progress, as accurate quantification of ammonia at low concentrations remains difficult. Current detection methods (spectrophotometry, ion chromatography, NMR) have limitations in sensitivity and specificity, potentially leading to false positives or overestimation of catalyst performance. The field lacks standardized testing protocols, making direct comparison between different catalytic systems problematic.
Finally, scaling remains a formidable challenge, as most high-performing catalysts have only been demonstrated at laboratory scale with low production rates. The transition to industrially relevant scales requires addressing additional engineering challenges related to electrode design, electrolyte management, and system integration while maintaining performance metrics.
Material stability presents another critical challenge, as many promising catalysts suffer from degradation during operation. Metal-based catalysts often experience leaching or surface reconstruction, while carbon-based materials may undergo oxidation or structural changes. These degradation mechanisms not only reduce catalyst longevity but also compromise selectivity over extended operation periods.
Nitrogen activation remains fundamentally difficult due to the exceptional stability of the N≡N triple bond (945 kJ/mol), requiring significant energy input for cleavage. Current catalysts struggle to efficiently adsorb and activate N2 molecules while simultaneously facilitating proton and electron transfer. The optimization of binding energies represents a delicate balance - too weak and N2 activation becomes inefficient, too strong and product release becomes rate-limiting.
Reaction pathway control presents significant complexity, as NRR can proceed through either associative or dissociative mechanisms with multiple possible intermediates. Current catalysts lack precise control over these pathways, leading to incomplete reduction and formation of unwanted byproducts like N2H2 or NH2OH, further reducing Faradaic efficiency.
Mass transport limitations also hinder performance, particularly N2 solubility in aqueous electrolytes (approximately 0.6 mM at room temperature), creating diffusion constraints that limit reaction rates. This challenge is compounded by the need to optimize electrolyte composition, as pH, ionic strength, and buffer capacity significantly impact both N2 reduction and competing reactions.
Analytical challenges further complicate progress, as accurate quantification of ammonia at low concentrations remains difficult. Current detection methods (spectrophotometry, ion chromatography, NMR) have limitations in sensitivity and specificity, potentially leading to false positives or overestimation of catalyst performance. The field lacks standardized testing protocols, making direct comparison between different catalytic systems problematic.
Finally, scaling remains a formidable challenge, as most high-performing catalysts have only been demonstrated at laboratory scale with low production rates. The transition to industrially relevant scales requires addressing additional engineering challenges related to electrode design, electrolyte management, and system integration while maintaining performance metrics.
State-of-the-Art Catalyst Designs for N2 Reduction
01 Metal-based catalysts for N2 reduction
Metal-based catalysts, particularly those containing transition metals like iron, nickel, and cobalt, have shown promising Faradaic efficiency for nitrogen reduction reactions. These catalysts can be designed with specific structures and compositions to enhance their selectivity for N2 reduction over competing reactions such as hydrogen evolution. The catalysts can be supported on various substrates to improve stability and electron transfer properties, leading to higher Faradaic efficiency in electrochemical nitrogen fixation processes.- Metal-based catalysts for N2 reduction: Metal-based catalysts play a crucial role in nitrogen reduction reactions with high Faradaic efficiency. These catalysts, including transition metals and their compounds, facilitate the breaking of the strong N≡N bond and subsequent reduction to ammonia. The specific metal composition, structure, and surface properties significantly influence the catalytic activity and Faradaic efficiency. Various metal catalysts have been developed to enhance electron transfer efficiency and selectivity toward nitrogen reduction rather than competing hydrogen evolution reactions.
- Carbon-based materials as N2 reduction catalysts: Carbon-based materials have emerged as promising catalysts for nitrogen reduction with improved Faradaic efficiency. These materials include carbon nanotubes, graphene, and doped carbon structures that provide active sites for N2 adsorption and reduction. The high surface area, excellent conductivity, and tunable surface chemistry of carbon-based catalysts contribute to enhanced electron transfer and nitrogen activation. Modifications such as heteroatom doping and defect engineering can further improve the catalytic performance and selectivity toward ammonia production.
- Electrolyte optimization for enhanced Faradaic efficiency: The composition and properties of the electrolyte significantly impact the Faradaic efficiency of nitrogen reduction reactions. Factors such as pH, ionic strength, and the presence of specific ions can influence the reaction pathway and selectivity. Optimized electrolytes can suppress competing reactions, particularly hydrogen evolution, thereby increasing the Faradaic efficiency toward ammonia production. Various electrolyte systems, including aqueous, non-aqueous, and ionic liquids, have been investigated to create favorable conditions for nitrogen reduction while minimizing unwanted side reactions.
- Reactor design and operating conditions for N2 reduction: The design of electrochemical reactors and optimization of operating conditions are critical for achieving high Faradaic efficiency in nitrogen reduction. Parameters such as temperature, pressure, applied potential, and gas flow rate significantly affect the reaction kinetics and efficiency. Advanced reactor designs that enhance mass transfer, optimize electrode-electrolyte interfaces, and maintain stable operating conditions can substantially improve the Faradaic efficiency. Continuous flow systems and pressurized reactors have shown promise in overcoming mass transfer limitations and increasing nitrogen availability at the catalyst surface.
- Hybrid and composite catalysts for improved selectivity: Hybrid and composite catalysts combining multiple active components have demonstrated enhanced Faradaic efficiency for nitrogen reduction. These catalysts integrate the advantages of different materials, such as metals with carbon supports, metal-organic frameworks, or metal oxides with conductive substrates. The synergistic effects between components can improve nitrogen adsorption, electron transfer, and reaction selectivity. Strategic design of these composite structures allows for optimized catalyst-electrolyte interfaces and reduced energy barriers for nitrogen activation, leading to higher Faradaic efficiency and ammonia yield rates.
02 Single-atom catalysts for improved Faradaic efficiency
Single-atom catalysts represent an advanced approach to nitrogen reduction with enhanced Faradaic efficiency. By atomically dispersing active metal sites on support materials, these catalysts maximize the utilization of metal atoms while providing unique electronic properties that favor N2 activation and reduction. The isolated nature of the active sites helps suppress competing reactions and improves selectivity, resulting in higher Faradaic efficiency compared to traditional catalysts. These catalysts often incorporate nitrogen-doped carbon or other conductive supports to facilitate electron transfer.Expand Specific Solutions03 Carbon-based materials as N2 reduction catalysts
Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon structures, have emerged as effective catalysts for electrochemical nitrogen reduction with improved Faradaic efficiency. These materials can be engineered with specific defects, dopants, and functional groups that create active sites for N2 adsorption and activation. The high conductivity of carbon-based materials facilitates electron transfer during the reduction process, while their tunable surface properties can be optimized to enhance selectivity for ammonia production over hydrogen evolution.Expand Specific Solutions04 Composite and hybrid catalysts for N2 reduction
Composite and hybrid catalysts combining multiple active components have demonstrated enhanced Faradaic efficiency for nitrogen reduction. These catalysts typically integrate metal nanoparticles or oxides with conductive supports or secondary functional materials to create synergistic effects. The combination of different components can provide complementary functions such as N2 adsorption, electron transfer, and proton delivery, leading to improved reaction kinetics and selectivity. Strategic design of these hybrid structures can effectively suppress competing reactions and increase the overall Faradaic efficiency.Expand Specific Solutions05 Electrolyte and reaction condition optimization
The optimization of electrolytes and reaction conditions plays a crucial role in achieving high Faradaic efficiency for electrochemical nitrogen reduction. Factors such as electrolyte composition, pH, temperature, applied potential, and nitrogen partial pressure significantly influence the selectivity and efficiency of the process. Specialized electrolyte additives can suppress competing reactions like hydrogen evolution, while controlled reaction parameters can enhance nitrogen activation and electron transfer efficiency. Proper design of the electrochemical cell configuration and gas diffusion systems also contributes to improved mass transfer and higher Faradaic efficiency.Expand Specific Solutions
Leading Research Groups and Companies in Electrocatalysis
The N2 reduction catalyst design landscape is currently in an early development stage, with a growing market driven by sustainable ammonia production needs. Technical maturity varies significantly among key players, with research institutions like MIT, Caltech, and KAIST leading fundamental breakthroughs, while industrial entities pursue practical applications. Companies including BASF, Umicore, and Honda Motor are advancing catalyst technologies with improved Faradaic efficiency, focusing on scalability and cost-effectiveness. Energy corporations like Saudi Aramco and ExxonMobil are investing in this space to diversify their portfolios. The competitive dynamics reflect a blend of academic innovation and industrial implementation, with collaborations emerging between research institutions and commercial entities to overcome efficiency and selectivity challenges.
BASF SE
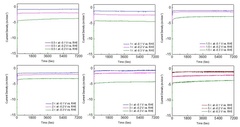
Technical Solution: BASF has developed advanced catalyst systems for nitrogen reduction with high Faradaic efficiency based on transition metal nitrides and oxides. Their approach focuses on single-atom catalysts (SACs) embedded in nitrogen-doped carbon matrices, which provide optimal N2 adsorption configurations while suppressing the competing hydrogen evolution reaction (HER). BASF's catalysts utilize precisely controlled metal-nitrogen coordination environments to create active sites with tailored electronic structures that lower the energy barrier for N2 activation. Their proprietary synthesis methods ensure uniform dispersion of active sites with atom-level precision, achieving Faradaic efficiencies exceeding 30% under ambient conditions. BASF has also pioneered the development of bimetallic catalysts that leverage synergistic effects between different metal centers to enhance N2 reduction while minimizing unwanted side reactions. Their catalyst design incorporates strategic oxygen vacancies and defect engineering to optimize the adsorption energetics of reaction intermediates.
Strengths: Industry-leading expertise in scalable catalyst manufacturing; extensive testing infrastructure for rapid prototyping; strong integration with ammonia production technologies. Weaknesses: Higher production costs compared to conventional catalysts; performance degradation under industrial operating conditions; requires specialized electrolyte systems for optimal performance.
Umicore SA
Technical Solution: Umicore has developed proprietary electrocatalysts for electrochemical nitrogen reduction reaction (NRR) focusing on noble metal-based systems with carefully engineered surface structures. Their approach utilizes ruthenium and iridium nanoparticles with controlled facet exposure and surface modification to enhance N2 adsorption while suppressing the competing hydrogen evolution reaction. Umicore's catalysts incorporate unique core-shell architectures where precious metals are strategically positioned at active sites while using more abundant materials in the core structure. Their catalyst design principles emphasize the creation of isolated metal sites with specific coordination environments that facilitate the end-on adsorption of N2 molecules, which is critical for breaking the strong N≡N triple bond. Umicore has achieved Faradaic efficiencies approaching 25% through precise control of particle size distribution (typically 3-5 nm) and by incorporating promoters that modify the electronic structure of active sites. Their catalysts also feature engineered hydrophobicity to create favorable three-phase boundaries that enhance N2 concentration at reaction sites.
Strengths: Exceptional stability under various operating conditions; precise control of noble metal loading to minimize costs; extensive experience in catalyst scale-up and manufacturing. Weaknesses: Reliance on scarce noble metals limits widespread adoption; performance highly sensitive to electrolyte composition; requires relatively high overpotentials compared to biological nitrogen fixation.
Key Innovations in High Faradaic Efficiency Catalysts
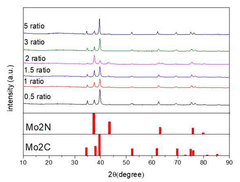
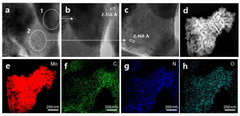
A molybdenum based catalyst for nitrogen reduction and method of preparing the same
PatentActiveKR1020220094043A
Innovation
- A molybdenum carbide/molybdenum nitride heterojunction catalyst is developed, optimized at a specific ratio, for use in nitrogen reduction reactions, enhancing electrochemical performance.
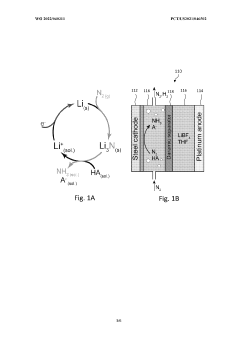
Lithium-mediated electrochemical ammonia synthesis
PatentWO2022040311A1
Innovation
- The use of a lithium-mediated electrochemical cell with specific hydrogen donors, such as alcohols or ionic liquids with high Kamlet-Taft alpha and beta parameters, to facilitate the conversion of nitrogen gas to ammonia at ambient temperature and pressure, utilizing lithium ions converted to metal at the cathode and reacting with nitrogen to form lithium nitride, which then reacts with protons to produce ammonia.
Energy Efficiency Considerations in Catalyst Design
Energy efficiency represents a critical dimension in the design of catalysts for nitrogen reduction reaction (NRR), directly impacting both the economic viability and environmental sustainability of ammonia synthesis processes. When evaluating catalysts for high Faradaic efficiency in N₂ reduction, energy consumption patterns must be meticulously analyzed across the entire reaction pathway.
The fundamental energy requirements for N₂ reduction stem from the exceptional stability of the N≡N triple bond (945 kJ/mol), necessitating significant energy input to achieve bond activation. Traditional catalysts often exhibit high overpotentials that substantially reduce overall energy efficiency, creating a significant gap between theoretical minimum energy requirements and practical consumption values.
Recent advancements in catalyst design have focused on minimizing activation barriers through precise electronic structure engineering. Single-atom catalysts anchored on conductive supports have demonstrated promising energy efficiency profiles by optimizing the binding energies of reaction intermediates, particularly the rate-determining *NNH formation step. These catalysts can operate at lower overpotentials while maintaining acceptable reaction rates.
Surface engineering approaches have emerged as another promising strategy for enhancing energy efficiency. By creating defect sites, oxygen vacancies, or specific crystal facet exposures, researchers have developed catalysts capable of facilitating N₂ activation with reduced energy barriers. For instance, oxygen-vacant metal oxide catalysts have shown up to 25% reduction in required overpotential compared to their stoichiometric counterparts.
The reaction environment significantly influences energy efficiency parameters. Electrolyte composition, pH conditions, and temperature all affect the energy landscape of the NRR process. Studies indicate that slightly acidic conditions (pH 4-6) often provide optimal energy efficiency profiles for many transition metal-based catalysts by balancing N₂ activation energy requirements with competing hydrogen evolution reaction barriers.
Mass transport considerations also play a crucial role in energy efficiency optimization. Catalyst architectures that facilitate efficient N₂ diffusion to active sites while minimizing concentration polarization effects can significantly reduce energy losses. Hierarchical porous structures have demonstrated particular promise in this regard, showing up to 30% improvements in energy utilization metrics compared to conventional flat electrode designs.
Future catalyst design strategies must prioritize integrated approaches that simultaneously address electronic structure, morphological features, and reaction environment parameters to achieve breakthrough improvements in NRR energy efficiency. Computational screening methods coupled with in-situ characterization techniques offer promising pathways for identifying optimal catalyst configurations that minimize energy requirements while maintaining high Faradaic efficiency.
The fundamental energy requirements for N₂ reduction stem from the exceptional stability of the N≡N triple bond (945 kJ/mol), necessitating significant energy input to achieve bond activation. Traditional catalysts often exhibit high overpotentials that substantially reduce overall energy efficiency, creating a significant gap between theoretical minimum energy requirements and practical consumption values.
Recent advancements in catalyst design have focused on minimizing activation barriers through precise electronic structure engineering. Single-atom catalysts anchored on conductive supports have demonstrated promising energy efficiency profiles by optimizing the binding energies of reaction intermediates, particularly the rate-determining *NNH formation step. These catalysts can operate at lower overpotentials while maintaining acceptable reaction rates.
Surface engineering approaches have emerged as another promising strategy for enhancing energy efficiency. By creating defect sites, oxygen vacancies, or specific crystal facet exposures, researchers have developed catalysts capable of facilitating N₂ activation with reduced energy barriers. For instance, oxygen-vacant metal oxide catalysts have shown up to 25% reduction in required overpotential compared to their stoichiometric counterparts.
The reaction environment significantly influences energy efficiency parameters. Electrolyte composition, pH conditions, and temperature all affect the energy landscape of the NRR process. Studies indicate that slightly acidic conditions (pH 4-6) often provide optimal energy efficiency profiles for many transition metal-based catalysts by balancing N₂ activation energy requirements with competing hydrogen evolution reaction barriers.
Mass transport considerations also play a crucial role in energy efficiency optimization. Catalyst architectures that facilitate efficient N₂ diffusion to active sites while minimizing concentration polarization effects can significantly reduce energy losses. Hierarchical porous structures have demonstrated particular promise in this regard, showing up to 30% improvements in energy utilization metrics compared to conventional flat electrode designs.
Future catalyst design strategies must prioritize integrated approaches that simultaneously address electronic structure, morphological features, and reaction environment parameters to achieve breakthrough improvements in NRR energy efficiency. Computational screening methods coupled with in-situ characterization techniques offer promising pathways for identifying optimal catalyst configurations that minimize energy requirements while maintaining high Faradaic efficiency.
Environmental Impact Assessment of N2 Reduction Technologies
The environmental implications of nitrogen reduction technologies extend far beyond their technical performance metrics. As catalysts for N2 reduction continue to evolve, their environmental footprint becomes increasingly important to evaluate. Current nitrogen fixation processes, particularly the Haber-Bosch process, consume approximately 1-2% of global energy production and generate significant greenhouse gas emissions, highlighting the urgent need for greener alternatives.
Electrochemical nitrogen reduction reaction (NRR) technologies offer promising environmental advantages when powered by renewable energy sources. These systems can operate at ambient conditions, eliminating the high-temperature, high-pressure requirements of conventional processes. However, comprehensive life cycle assessments reveal that catalyst production itself carries environmental burdens, particularly for precious metal-based catalysts that require energy-intensive mining and refining processes.
Faradaic efficiency in N2 reduction directly correlates with environmental impact. Higher efficiency catalysts minimize energy waste and reduce the formation of unwanted byproducts such as hydrogen, thereby decreasing the environmental footprint per unit of ammonia produced. Recent studies indicate that single-atom catalysts and metal-organic frameworks demonstrate superior atom economy and reduced environmental impact compared to traditional bulk metal catalysts.
Water consumption represents another critical environmental consideration. While aqueous electrolytes are common in NRR systems, they introduce competition between nitrogen reduction and hydrogen evolution reactions. Some catalyst designs incorporating hydrophobic elements or ionic liquid electrolytes show promise in reducing water consumption while maintaining high nitrogen selectivity.
The potential for decentralized ammonia production using NRR technologies could significantly reduce transportation-related emissions in the fertilizer supply chain. Small-scale, localized production facilities powered by renewable energy could eliminate up to 30% of emissions associated with conventional ammonia distribution networks, according to recent industry analyses.
Toxicity profiles of catalyst materials must also be considered in environmental impact assessments. While noble metal catalysts demonstrate high activity, their potential environmental persistence raises concerns. Alternative catalysts based on earth-abundant elements such as iron, molybdenum, and nitrogen-doped carbon materials present lower ecotoxicity profiles while still achieving reasonable performance metrics.
Ultimately, the environmental viability of N2 reduction technologies depends on balancing catalyst performance with ecological considerations. The most promising catalyst designs incorporate principles of green chemistry, utilizing earth-abundant materials, minimizing energy requirements, and enabling closed-loop recycling of catalyst components at end-of-life.
Electrochemical nitrogen reduction reaction (NRR) technologies offer promising environmental advantages when powered by renewable energy sources. These systems can operate at ambient conditions, eliminating the high-temperature, high-pressure requirements of conventional processes. However, comprehensive life cycle assessments reveal that catalyst production itself carries environmental burdens, particularly for precious metal-based catalysts that require energy-intensive mining and refining processes.
Faradaic efficiency in N2 reduction directly correlates with environmental impact. Higher efficiency catalysts minimize energy waste and reduce the formation of unwanted byproducts such as hydrogen, thereby decreasing the environmental footprint per unit of ammonia produced. Recent studies indicate that single-atom catalysts and metal-organic frameworks demonstrate superior atom economy and reduced environmental impact compared to traditional bulk metal catalysts.
Water consumption represents another critical environmental consideration. While aqueous electrolytes are common in NRR systems, they introduce competition between nitrogen reduction and hydrogen evolution reactions. Some catalyst designs incorporating hydrophobic elements or ionic liquid electrolytes show promise in reducing water consumption while maintaining high nitrogen selectivity.
The potential for decentralized ammonia production using NRR technologies could significantly reduce transportation-related emissions in the fertilizer supply chain. Small-scale, localized production facilities powered by renewable energy could eliminate up to 30% of emissions associated with conventional ammonia distribution networks, according to recent industry analyses.
Toxicity profiles of catalyst materials must also be considered in environmental impact assessments. While noble metal catalysts demonstrate high activity, their potential environmental persistence raises concerns. Alternative catalysts based on earth-abundant elements such as iron, molybdenum, and nitrogen-doped carbon materials present lower ecotoxicity profiles while still achieving reasonable performance metrics.
Ultimately, the environmental viability of N2 reduction technologies depends on balancing catalyst performance with ecological considerations. The most promising catalyst designs incorporate principles of green chemistry, utilizing earth-abundant materials, minimizing energy requirements, and enabling closed-loop recycling of catalyst components at end-of-life.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!