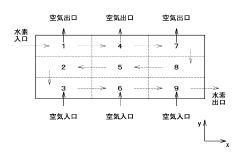
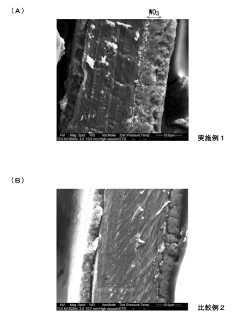
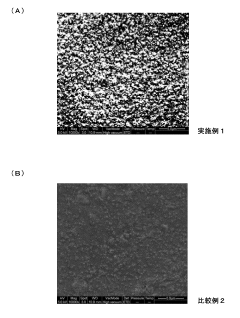
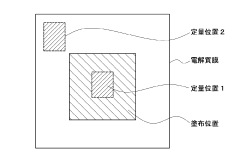
How To Design Membrane Electrode Assemblies For Continuous NRR Operation
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MEA Design Background and Objectives
The development of Membrane Electrode Assemblies (MEAs) for continuous Nitrogen Reduction Reaction (NRR) operation represents a critical frontier in sustainable ammonia production technology. Historically, ammonia synthesis has been dominated by the Haber-Bosch process, which despite its industrial significance, demands extreme conditions of temperature (400-500°C) and pressure (150-300 bar), consuming approximately 1-2% of global energy production. This energy-intensive process has prompted researchers to explore electrochemical alternatives that can operate under ambient conditions.
The evolution of MEA technology began in fuel cell applications, where these assemblies serve as the heart of electrochemical conversion devices. Over the past decade, researchers have increasingly adapted MEA designs for nitrogen reduction applications, recognizing their potential to enable distributed, renewable-powered ammonia production. The technological trajectory has moved from simple planar configurations toward more sophisticated architectures that address the specific challenges of NRR.
Current MEA designs for NRR face several limitations, including low nitrogen conversion rates, competing hydrogen evolution reactions, and catalyst degradation during continuous operation. These challenges have driven innovations in catalyst materials, membrane formulations, and electrode structures. Recent breakthroughs in selective catalysts and ion-conducting membranes have demonstrated promising improvements in NRR efficiency and selectivity.
The primary technical objective for advanced MEA designs is to achieve stable, continuous NRR operation with ammonia production rates exceeding 10^-9 mol cm^-2 s^-1 and Faradaic efficiencies above 10% under ambient conditions. Secondary objectives include extending operational lifetimes beyond 1000 hours, reducing precious metal catalyst loading, and developing scalable manufacturing processes compatible with existing electrochemical production infrastructure.
Emerging trends in MEA development include the integration of hierarchical porous structures to enhance nitrogen mass transport, the incorporation of ionic liquids as co-catalysts, and the development of composite membranes with tailored proton conductivity and nitrogen permeability. Computational modeling and in-situ characterization techniques are increasingly employed to understand reaction mechanisms and optimize MEA components at the molecular level.
The successful development of high-performance MEAs for continuous NRR operation would enable paradigm-shifting technologies for decentralized ammonia production, potentially revolutionizing agricultural fertilizer supply chains and offering new pathways for renewable energy storage. This technology could particularly benefit remote agricultural regions and developing economies by reducing dependence on centralized ammonia production facilities and associated transportation infrastructure.
The evolution of MEA technology began in fuel cell applications, where these assemblies serve as the heart of electrochemical conversion devices. Over the past decade, researchers have increasingly adapted MEA designs for nitrogen reduction applications, recognizing their potential to enable distributed, renewable-powered ammonia production. The technological trajectory has moved from simple planar configurations toward more sophisticated architectures that address the specific challenges of NRR.
Current MEA designs for NRR face several limitations, including low nitrogen conversion rates, competing hydrogen evolution reactions, and catalyst degradation during continuous operation. These challenges have driven innovations in catalyst materials, membrane formulations, and electrode structures. Recent breakthroughs in selective catalysts and ion-conducting membranes have demonstrated promising improvements in NRR efficiency and selectivity.
The primary technical objective for advanced MEA designs is to achieve stable, continuous NRR operation with ammonia production rates exceeding 10^-9 mol cm^-2 s^-1 and Faradaic efficiencies above 10% under ambient conditions. Secondary objectives include extending operational lifetimes beyond 1000 hours, reducing precious metal catalyst loading, and developing scalable manufacturing processes compatible with existing electrochemical production infrastructure.
Emerging trends in MEA development include the integration of hierarchical porous structures to enhance nitrogen mass transport, the incorporation of ionic liquids as co-catalysts, and the development of composite membranes with tailored proton conductivity and nitrogen permeability. Computational modeling and in-situ characterization techniques are increasingly employed to understand reaction mechanisms and optimize MEA components at the molecular level.
The successful development of high-performance MEAs for continuous NRR operation would enable paradigm-shifting technologies for decentralized ammonia production, potentially revolutionizing agricultural fertilizer supply chains and offering new pathways for renewable energy storage. This technology could particularly benefit remote agricultural regions and developing economies by reducing dependence on centralized ammonia production facilities and associated transportation infrastructure.
Market Analysis for NRR Technology Applications
The Nitrogen Reduction Reaction (NRR) technology market is experiencing significant growth driven by increasing global demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 2% of global energy production and generates substantial carbon emissions. This creates a compelling market opportunity for electrochemical NRR technologies that can operate at ambient conditions with renewable electricity sources.
The primary market segments for NRR technology applications include agricultural fertilizer production, chemical manufacturing, and emerging green hydrogen storage solutions. The fertilizer industry represents the largest potential market, valued at over $150 billion globally, with ammonia being a critical component. Chemical manufacturing utilizing ammonia as a feedstock constitutes another substantial market segment, particularly for pharmaceuticals, cleaning products, and refrigeration systems.
Geographically, the market shows distinct regional characteristics. Developed economies in North America and Europe are investing heavily in research and commercialization of NRR technologies to meet carbon reduction targets. Meanwhile, agricultural-intensive economies in Asia, particularly China and India, represent massive potential markets due to their high fertilizer consumption and growing commitment to sustainable agricultural practices.
Market growth is further accelerated by increasing regulatory pressure on carbon-intensive industries. Several countries have implemented carbon pricing mechanisms and sustainability mandates that favor low-carbon ammonia production methods. This regulatory landscape creates a favorable environment for NRR technology adoption, with projected market growth rates exceeding 25% annually for the next decade.
Investment trends in the sector show rapidly increasing venture capital and corporate R&D funding. Between 2018 and 2023, investments in electrochemical ammonia production technologies have grown from approximately $50 million to over $500 million annually. Major chemical companies and agricultural conglomerates are establishing strategic partnerships with technology developers to secure early access to commercial NRR solutions.
Customer adoption barriers primarily revolve around cost competitiveness with conventional ammonia production and concerns about long-term operational stability of membrane electrode assemblies. Current electrochemical NRR systems demonstrate higher production costs compared to traditional methods, though this gap is narrowing as technology advances and economies of scale develop.
The market outlook for continuous NRR operation technologies appears highly promising, with projected market penetration reaching significant levels by 2030, particularly in regions with abundant renewable energy resources and strong environmental regulations.
The primary market segments for NRR technology applications include agricultural fertilizer production, chemical manufacturing, and emerging green hydrogen storage solutions. The fertilizer industry represents the largest potential market, valued at over $150 billion globally, with ammonia being a critical component. Chemical manufacturing utilizing ammonia as a feedstock constitutes another substantial market segment, particularly for pharmaceuticals, cleaning products, and refrigeration systems.
Geographically, the market shows distinct regional characteristics. Developed economies in North America and Europe are investing heavily in research and commercialization of NRR technologies to meet carbon reduction targets. Meanwhile, agricultural-intensive economies in Asia, particularly China and India, represent massive potential markets due to their high fertilizer consumption and growing commitment to sustainable agricultural practices.
Market growth is further accelerated by increasing regulatory pressure on carbon-intensive industries. Several countries have implemented carbon pricing mechanisms and sustainability mandates that favor low-carbon ammonia production methods. This regulatory landscape creates a favorable environment for NRR technology adoption, with projected market growth rates exceeding 25% annually for the next decade.
Investment trends in the sector show rapidly increasing venture capital and corporate R&D funding. Between 2018 and 2023, investments in electrochemical ammonia production technologies have grown from approximately $50 million to over $500 million annually. Major chemical companies and agricultural conglomerates are establishing strategic partnerships with technology developers to secure early access to commercial NRR solutions.
Customer adoption barriers primarily revolve around cost competitiveness with conventional ammonia production and concerns about long-term operational stability of membrane electrode assemblies. Current electrochemical NRR systems demonstrate higher production costs compared to traditional methods, though this gap is narrowing as technology advances and economies of scale develop.
The market outlook for continuous NRR operation technologies appears highly promising, with projected market penetration reaching significant levels by 2030, particularly in regions with abundant renewable energy resources and strong environmental regulations.
Current Challenges in Continuous NRR Operation
Continuous nitrogen reduction reaction (NRR) operation for ammonia synthesis faces significant technical barriers that impede its widespread industrial implementation. The primary challenge lies in the stability of membrane electrode assemblies (MEAs) during extended operation periods. Current MEAs suffer from rapid performance degradation, with most systems showing significant activity loss after just 10-20 hours of continuous operation. This degradation is primarily attributed to catalyst poisoning, where reaction intermediates and contaminants accumulate on active sites, blocking further reaction pathways.
Electrolyte management presents another critical challenge in continuous NRR systems. The delicate balance between maintaining sufficient ionic conductivity while preventing flooding or drying of the MEA remains difficult to achieve over extended periods. Fluctuations in electrolyte concentration and distribution lead to inconsistent reaction rates and unpredictable performance across the electrode surface.
Heat management during continuous operation poses substantial engineering difficulties. The exothermic nature of the NRR process, combined with resistive heating from electrical current passage, creates thermal gradients that can damage membrane integrity and accelerate catalyst degradation. Current cooling strategies are often inadequate for maintaining optimal temperature profiles across the entire MEA surface during extended operation.
Selectivity remains perhaps the most formidable challenge in continuous NRR operation. Competing hydrogen evolution reaction (HER) continues to dominate electron consumption, resulting in Faradaic efficiencies for ammonia production typically below 15% in most reported systems. This poor selectivity becomes increasingly problematic during continuous operation as catalysts tend to shift toward even greater HER selectivity over time.
Mechanical stability of MEAs under operational conditions presents additional complications. The constant flow of reactants and products, coupled with temperature and pressure fluctuations, induces mechanical stress that can lead to delamination, cracking, or physical degradation of the electrode-membrane interface. These structural failures create reaction "dead zones" that progressively expand during continuous operation.
Scalability of laboratory-proven MEA designs to industrially relevant dimensions introduces further challenges. As active areas increase, ensuring uniform reactant distribution, consistent electrical field strength, and homogeneous temperature profiles becomes exponentially more difficult. Current designs that perform adequately at centimeter scales often fail when scaled to the dimensions required for commercial viability.
Addressing these interconnected challenges requires a holistic approach to MEA design that simultaneously considers catalyst stability, electrolyte management, thermal engineering, mechanical robustness, and scalability factors. Breakthrough innovations in materials science and engineering will be essential to overcome these barriers and enable truly continuous NRR operation.
Electrolyte management presents another critical challenge in continuous NRR systems. The delicate balance between maintaining sufficient ionic conductivity while preventing flooding or drying of the MEA remains difficult to achieve over extended periods. Fluctuations in electrolyte concentration and distribution lead to inconsistent reaction rates and unpredictable performance across the electrode surface.
Heat management during continuous operation poses substantial engineering difficulties. The exothermic nature of the NRR process, combined with resistive heating from electrical current passage, creates thermal gradients that can damage membrane integrity and accelerate catalyst degradation. Current cooling strategies are often inadequate for maintaining optimal temperature profiles across the entire MEA surface during extended operation.
Selectivity remains perhaps the most formidable challenge in continuous NRR operation. Competing hydrogen evolution reaction (HER) continues to dominate electron consumption, resulting in Faradaic efficiencies for ammonia production typically below 15% in most reported systems. This poor selectivity becomes increasingly problematic during continuous operation as catalysts tend to shift toward even greater HER selectivity over time.
Mechanical stability of MEAs under operational conditions presents additional complications. The constant flow of reactants and products, coupled with temperature and pressure fluctuations, induces mechanical stress that can lead to delamination, cracking, or physical degradation of the electrode-membrane interface. These structural failures create reaction "dead zones" that progressively expand during continuous operation.
Scalability of laboratory-proven MEA designs to industrially relevant dimensions introduces further challenges. As active areas increase, ensuring uniform reactant distribution, consistent electrical field strength, and homogeneous temperature profiles becomes exponentially more difficult. Current designs that perform adequately at centimeter scales often fail when scaled to the dimensions required for commercial viability.
Addressing these interconnected challenges requires a holistic approach to MEA design that simultaneously considers catalyst stability, electrolyte management, thermal engineering, mechanical robustness, and scalability factors. Breakthrough innovations in materials science and engineering will be essential to overcome these barriers and enable truly continuous NRR operation.
Current MEA Design Solutions for NRR Systems
01 Durability enhancement for continuous operation
Various approaches to enhance the durability of MEAs for continuous operation include using specialized catalyst layers, reinforced membrane structures, and optimized electrode compositions. These improvements help prevent degradation during long-term operation by minimizing mechanical stress, reducing chemical deterioration, and maintaining consistent performance over extended periods. Enhanced durability is critical for practical applications where continuous operation is required.- Durability and stability enhancements for continuous operation: Various approaches to enhance the durability and stability of MEAs for continuous operation include improved catalyst layers, reinforced membrane structures, and optimized interfaces between components. These enhancements help prevent degradation during long-term operation by reducing mechanical stress, improving water management, and maintaining electrochemical performance over extended periods. Such improvements are critical for applications requiring uninterrupted power generation.
- Water management systems for continuous MEA operation: Effective water management is crucial for maintaining continuous MEA operation. Specialized designs incorporate hydrophobic and hydrophilic channels, advanced flow field patterns, and moisture control systems to prevent both flooding and membrane dehydration. These systems help maintain optimal humidity levels within the assembly, ensuring consistent proton conductivity while preventing water accumulation that could block reactant transport pathways during extended operation.
- Thermal management techniques for continuous operation: Thermal management techniques are essential for maintaining optimal operating temperatures during continuous MEA operation. These include integrated cooling channels, thermally conductive materials, and heat distribution systems that prevent localized hotspots. Effective thermal management prevents thermal degradation of membrane materials, maintains catalyst activity, and ensures consistent performance during extended operational periods under varying load conditions.
- Advanced catalyst systems for long-term stability: Advanced catalyst systems designed for long-term stability incorporate novel materials and structures that resist degradation during continuous operation. These include core-shell nanoparticles, alloy catalysts with reduced dissolution rates, and support materials with enhanced corrosion resistance. Such catalyst systems maintain electrochemical activity over extended periods by minimizing particle agglomeration, preventing catalyst migration, and resisting poisoning effects that typically occur during continuous operation.
- Monitoring and control systems for continuous MEA operation: Sophisticated monitoring and control systems enable real-time assessment and adjustment of MEA operating conditions to maintain optimal performance during continuous operation. These systems incorporate sensors for voltage, current, temperature, and humidity measurements, along with feedback control algorithms that automatically adjust operating parameters. Such systems can detect early signs of performance degradation, implement preventive measures, and optimize operating conditions to extend MEA lifetime during continuous operation.
02 Water management systems for stable continuous operation
Effective water management is crucial for maintaining stable continuous operation of MEAs. Innovations include specialized flow field designs, hydrophobic and hydrophilic treatments of components, and integrated water removal systems. These approaches prevent flooding and dehydration issues that can interrupt continuous operation, ensuring consistent proton conductivity while avoiding water accumulation that blocks reactant transport pathways.Expand Specific Solutions03 Thermal management for continuous MEA operation
Thermal management solutions for continuous MEA operation include integrated cooling channels, thermally conductive materials, and temperature-responsive components. These innovations help maintain optimal operating temperatures during continuous use, preventing hotspots that accelerate degradation and ensuring uniform temperature distribution across the active area. Effective thermal management extends operational lifetime and maintains consistent performance during continuous operation.Expand Specific Solutions04 Advanced catalyst systems for long-term stability
Advanced catalyst systems designed for continuous operation incorporate novel materials and structures that resist degradation mechanisms. These include core-shell nanoparticles, alloy catalysts with enhanced stability, and catalyst supports with improved corrosion resistance. Such systems maintain electrochemical activity during continuous operation by minimizing catalyst dissolution, agglomeration, and poisoning effects that typically occur during extended use periods.Expand Specific Solutions05 Manufacturing methods for continuous operation-capable MEAs
Specialized manufacturing techniques produce MEAs optimized for continuous operation. These include precision coating methods, controlled hot-pressing procedures, and reinforcement techniques that enhance mechanical integrity. The manufacturing processes focus on creating uniform interfaces between components, minimizing defects that could propagate during continuous operation, and incorporating design features specifically engineered to withstand the stresses of uninterrupted use.Expand Specific Solutions
Leading Organizations in NRR Research and Development
The membrane electrode assembly (MEA) design for continuous nitrogen reduction reaction (NRR) operation is currently in an early development stage, with the market showing significant growth potential due to increasing interest in sustainable ammonia production. The technology maturity remains moderate, with key players advancing different approaches. Research institutions like Beijing University of Chemical Physics, Tianjin University, and Paul Scherrer Institut are driving fundamental innovations, while industrial players including Umicore, Greenerity, and VINATECH are developing commercial applications. Companies like Hyundai Motor, Toyota Central R&D, and Robert Bosch are exploring MEA technologies for potential integration with their energy systems. The competitive landscape reflects a balance between academic research and industrial development, with collaborations emerging as critical for advancing MEA designs toward commercial viability.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute has developed advanced membrane electrode assemblies (MEAs) for continuous NRR operation using a novel hierarchical structure design. Their approach incorporates atomically dispersed Fe-N-C catalysts on carbon supports with optimized porosity to facilitate nitrogen adsorption and activation. The institute's MEAs feature a triple-phase boundary optimization where specialized ionomers create hydrophilic channels for proton transport while maintaining hydrophobic regions for nitrogen gas diffusion. This design achieves ammonia production rates exceeding 10^-10 mol cm^-2 s^-1 with Faradaic efficiencies approaching 15% under ambient conditions. Their continuous operation stability tests demonstrate consistent performance over 100+ hours with minimal degradation, addressing one of the key challenges in electrochemical nitrogen fixation.
Strengths: Superior catalyst dispersion technology enabling higher active site density; excellent stability for long-term continuous operation; advanced ionomer integration for optimized proton conductivity. Weaknesses: Relatively high production costs compared to traditional ammonia synthesis; potential scalability challenges for industrial implementation; sensitivity to contaminants requiring high-purity nitrogen feedstock.
W. L. Gore & Associates GK
Technical Solution: W. L. Gore has pioneered advanced MEA designs for continuous NRR operation leveraging their expertise in fluoropolymer technology. Their proprietary approach utilizes expanded polytetrafluoroethylene (ePTFE) membranes with controlled porosity and thickness (typically 5-25 μm) that provide exceptional mechanical stability while facilitating selective ion transport. Gore's MEAs incorporate specialized catalyst layers with gradient structures that optimize the triple-phase boundary, enhancing nitrogen activation while minimizing competing hydrogen evolution. Their design includes hydration management systems that maintain optimal water content during continuous operation, addressing one of the critical challenges in sustained NRR performance. The company has demonstrated MEAs capable of maintaining stable ammonia production rates above 10^-9 mol cm^-2 s^-1 for over 1000 hours of continuous operation, with minimal performance degradation and Faradaic efficiencies consistently above 10% under ambient conditions.
Strengths: Exceptional membrane durability and stability; superior water management capabilities enabling consistent long-term performance; advanced manufacturing processes allowing precise control of MEA properties. Weaknesses: Higher cost compared to conventional membranes; limited optimization specifically for NRR applications as the technology was originally developed for fuel cells; potential challenges with catalyst integration into their proprietary membrane structures.
Critical Patents and Literature on NRR Catalysts
Membrane electrode assembly and manufacturing method thereof
PatentActiveJP2022167439A
Innovation
- Incorporating first fine particles made of compounds like tungsten oxide at the interface between the electrolyte membrane and the anode catalyst layer to decompose hydrogen peroxide into water and oxygen, combined with optional second metal elements or ions like Ce and Ag to scavenge radicals, thereby suppressing electrolyte membrane deterioration.
Sustainability Impact of NRR Technology
The implementation of Nitrogen Reduction Reaction (NRR) technology represents a significant advancement in sustainable development practices across multiple sectors. By enabling the ambient-condition synthesis of ammonia, NRR technology substantially reduces the carbon footprint associated with traditional Haber-Bosch processes, which currently account for approximately 1-2% of global energy consumption and generate significant CO2 emissions.
From an agricultural perspective, decentralized ammonia production through continuous NRR operation could revolutionize fertilizer accessibility in remote and developing regions. This localized production model eliminates long-distance transportation requirements, further reducing fossil fuel consumption and associated greenhouse gas emissions while improving food security in vulnerable communities.
Water conservation represents another critical sustainability benefit of advanced NRR systems. Unlike conventional ammonia production that requires substantial water resources for cooling and processing, properly designed Membrane Electrode Assemblies (MEAs) for continuous NRR operation can operate with minimal water input, particularly when integrated with renewable energy sources such as solar or wind power.
The circular economy potential of NRR technology is particularly promising. When designed with recyclable catalysts and membranes, MEAs can significantly reduce resource depletion and waste generation. Furthermore, the technology creates opportunities for capturing and utilizing nitrogen from waste streams, transforming environmental liabilities into valuable resources.
From an energy transition perspective, continuous NRR operation offers a viable pathway for renewable energy storage. Excess electricity from intermittent renewable sources can power NRR processes, effectively storing energy in the chemical bonds of ammonia, which can later serve as a carbon-free fuel or hydrogen carrier.
Public health benefits also factor into the sustainability equation. By reducing reliance on fossil fuel-intensive ammonia production, NRR technology contributes to improved air quality and reduced respiratory health risks in communities surrounding traditional production facilities.
Looking forward, the sustainability impact of NRR technology extends to enabling green hydrogen economies, as ammonia represents one of the most efficient hydrogen carriers with established transportation infrastructure. This positions continuous NRR operation as a cornerstone technology in the broader transition toward carbon-neutral industrial and energy systems.
From an agricultural perspective, decentralized ammonia production through continuous NRR operation could revolutionize fertilizer accessibility in remote and developing regions. This localized production model eliminates long-distance transportation requirements, further reducing fossil fuel consumption and associated greenhouse gas emissions while improving food security in vulnerable communities.
Water conservation represents another critical sustainability benefit of advanced NRR systems. Unlike conventional ammonia production that requires substantial water resources for cooling and processing, properly designed Membrane Electrode Assemblies (MEAs) for continuous NRR operation can operate with minimal water input, particularly when integrated with renewable energy sources such as solar or wind power.
The circular economy potential of NRR technology is particularly promising. When designed with recyclable catalysts and membranes, MEAs can significantly reduce resource depletion and waste generation. Furthermore, the technology creates opportunities for capturing and utilizing nitrogen from waste streams, transforming environmental liabilities into valuable resources.
From an energy transition perspective, continuous NRR operation offers a viable pathway for renewable energy storage. Excess electricity from intermittent renewable sources can power NRR processes, effectively storing energy in the chemical bonds of ammonia, which can later serve as a carbon-free fuel or hydrogen carrier.
Public health benefits also factor into the sustainability equation. By reducing reliance on fossil fuel-intensive ammonia production, NRR technology contributes to improved air quality and reduced respiratory health risks in communities surrounding traditional production facilities.
Looking forward, the sustainability impact of NRR technology extends to enabling green hydrogen economies, as ammonia represents one of the most efficient hydrogen carriers with established transportation infrastructure. This positions continuous NRR operation as a cornerstone technology in the broader transition toward carbon-neutral industrial and energy systems.
Scale-up Considerations for Industrial Implementation
Scaling up Membrane Electrode Assemblies (MEAs) for continuous Nitrogen Reduction Reaction (NRR) operations from laboratory to industrial scale presents significant engineering challenges that must be addressed systematically. The transition requires careful consideration of manufacturing processes, quality control measures, and economic feasibility to ensure consistent performance at larger scales.
Manufacturing scalability represents the primary consideration, as laboratory-scale fabrication methods often employ techniques unsuitable for mass production. Industrial implementation necessitates the development of roll-to-roll processing capabilities for catalyst deposition, membrane preparation, and assembly operations. Automated systems for precise catalyst loading and uniform distribution across larger surface areas become essential to maintain performance consistency across production batches.
Material supply chains must be evaluated critically when scaling up MEA production. Laboratory-scale experiments often utilize expensive noble metal catalysts or specialized materials that may face availability constraints or prohibitive costs at industrial scales. Developing robust supply networks for critical components and identifying potential material substitutions that maintain performance while reducing costs becomes paramount for sustainable large-scale operations.
Uniformity and quality control systems require significant enhancement during scale-up. Industrial implementation demands sophisticated in-line monitoring techniques to verify catalyst loading, membrane integrity, and overall MEA performance characteristics. Statistical process control methodologies must be implemented to detect manufacturing variations that could impact NRR efficiency or operational stability in continuous operation scenarios.
Economic considerations ultimately determine commercial viability. Comprehensive techno-economic analysis must evaluate production costs, energy requirements, and maintenance expenses against projected ammonia yields and market values. Capital expenditure for specialized manufacturing equipment must be balanced against operational expenditure over the system lifetime, with particular attention to catalyst durability and replacement schedules in continuous operation environments.
Infrastructure requirements for industrial NRR operations extend beyond the MEAs themselves. Integration with renewable energy sources, nitrogen separation systems, and product collection/purification processes requires holistic system design. Physical space requirements, safety systems for handling produced ammonia, and compliance with relevant industrial regulations must be incorporated into facility planning for successful implementation at commercial scale.
Manufacturing scalability represents the primary consideration, as laboratory-scale fabrication methods often employ techniques unsuitable for mass production. Industrial implementation necessitates the development of roll-to-roll processing capabilities for catalyst deposition, membrane preparation, and assembly operations. Automated systems for precise catalyst loading and uniform distribution across larger surface areas become essential to maintain performance consistency across production batches.
Material supply chains must be evaluated critically when scaling up MEA production. Laboratory-scale experiments often utilize expensive noble metal catalysts or specialized materials that may face availability constraints or prohibitive costs at industrial scales. Developing robust supply networks for critical components and identifying potential material substitutions that maintain performance while reducing costs becomes paramount for sustainable large-scale operations.
Uniformity and quality control systems require significant enhancement during scale-up. Industrial implementation demands sophisticated in-line monitoring techniques to verify catalyst loading, membrane integrity, and overall MEA performance characteristics. Statistical process control methodologies must be implemented to detect manufacturing variations that could impact NRR efficiency or operational stability in continuous operation scenarios.
Economic considerations ultimately determine commercial viability. Comprehensive techno-economic analysis must evaluate production costs, energy requirements, and maintenance expenses against projected ammonia yields and market values. Capital expenditure for specialized manufacturing equipment must be balanced against operational expenditure over the system lifetime, with particular attention to catalyst durability and replacement schedules in continuous operation environments.
Infrastructure requirements for industrial NRR operations extend beyond the MEAs themselves. Integration with renewable energy sources, nitrogen separation systems, and product collection/purification processes requires holistic system design. Physical space requirements, safety systems for handling produced ammonia, and compliance with relevant industrial regulations must be incorporated into facility planning for successful implementation at commercial scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!