How To Develop Standardized Test Cells For Cross-Lab NRR Reproducibility
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NRR Test Cell Standardization Background & Objectives
Noise Reduction Rating (NRR) testing has evolved significantly since its inception in the 1970s, becoming a critical standard for evaluating hearing protection devices (HPDs) across various industries. Despite its widespread adoption, the field has been plagued by inconsistent test results when identical products are evaluated across different laboratories. This reproducibility challenge undermines confidence in NRR values and creates regulatory compliance issues for manufacturers operating in global markets.
The historical development of NRR testing reveals a gradual recognition of this cross-laboratory variability problem. Early standards such as ANSI S3.19-1974 and subsequent revisions provided foundational methodologies, but lacked specific controls for test environment standardization. By the 1990s, researchers began documenting significant variations in NRR results—sometimes exceeding 5-7 dB for identical products—when tested in different facilities.
These discrepancies stem from multiple factors including variations in test chamber acoustics, differences in subject positioning, inconsistent fitting procedures, and equipment calibration disparities. The absence of standardized test cells—the physical environments where NRR measurements occur—represents a fundamental gap in current testing protocols that directly impacts measurement consistency.
Recent technological advancements in acoustic measurement, materials science, and digital modeling have created new opportunities to address these longstanding challenges. Computational acoustic modeling now enables precise simulation of sound fields, while advanced materials can create more consistent acoustic environments. These developments provide the technical foundation for establishing truly standardized test cells.
The primary objective of this research initiative is to develop comprehensive specifications for standardized NRR test cells that ensure measurement reproducibility across different laboratories worldwide. This standardization aims to reduce inter-laboratory variability to less than 2 dB, a threshold identified by industry experts as necessary for meaningful cross-facility comparisons.
Secondary objectives include creating scalable implementation guidelines that accommodate laboratories with varying resource levels, establishing calibration protocols that maintain consistency over time, and developing validation methodologies to verify test cell performance. The initiative also seeks to align with evolving international standards, particularly the harmonization efforts between ANSI/ASA S12.6 and ISO 4869 testing protocols.
The successful development of standardized test cells would represent a significant advancement in hearing protection evaluation, benefiting manufacturers through reduced compliance costs, regulatory bodies through increased confidence in reported values, and ultimately end-users through more reliable hearing protection performance information. This initiative addresses a critical technical gap while supporting broader industry goals of global standards harmonization and improved occupational safety outcomes.
The historical development of NRR testing reveals a gradual recognition of this cross-laboratory variability problem. Early standards such as ANSI S3.19-1974 and subsequent revisions provided foundational methodologies, but lacked specific controls for test environment standardization. By the 1990s, researchers began documenting significant variations in NRR results—sometimes exceeding 5-7 dB for identical products—when tested in different facilities.
These discrepancies stem from multiple factors including variations in test chamber acoustics, differences in subject positioning, inconsistent fitting procedures, and equipment calibration disparities. The absence of standardized test cells—the physical environments where NRR measurements occur—represents a fundamental gap in current testing protocols that directly impacts measurement consistency.
Recent technological advancements in acoustic measurement, materials science, and digital modeling have created new opportunities to address these longstanding challenges. Computational acoustic modeling now enables precise simulation of sound fields, while advanced materials can create more consistent acoustic environments. These developments provide the technical foundation for establishing truly standardized test cells.
The primary objective of this research initiative is to develop comprehensive specifications for standardized NRR test cells that ensure measurement reproducibility across different laboratories worldwide. This standardization aims to reduce inter-laboratory variability to less than 2 dB, a threshold identified by industry experts as necessary for meaningful cross-facility comparisons.
Secondary objectives include creating scalable implementation guidelines that accommodate laboratories with varying resource levels, establishing calibration protocols that maintain consistency over time, and developing validation methodologies to verify test cell performance. The initiative also seeks to align with evolving international standards, particularly the harmonization efforts between ANSI/ASA S12.6 and ISO 4869 testing protocols.
The successful development of standardized test cells would represent a significant advancement in hearing protection evaluation, benefiting manufacturers through reduced compliance costs, regulatory bodies through increased confidence in reported values, and ultimately end-users through more reliable hearing protection performance information. This initiative addresses a critical technical gap while supporting broader industry goals of global standards harmonization and improved occupational safety outcomes.
Market Demand for Cross-Laboratory NRR Testing
The nitrogen reduction reaction (NRR) testing market is experiencing significant growth driven by the increasing focus on sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 2% of global energy and contributes substantially to greenhouse gas emissions. This environmental concern has accelerated research into electrochemical NRR as a greener alternative, creating demand for standardized testing protocols across laboratories.
Research institutions, universities, and industrial R&D departments are increasingly investing in NRR technology development, with global research funding for sustainable ammonia production technologies exceeding $500 million annually. The market for advanced NRR catalysts alone is projected to reach $2.3 billion by 2030, highlighting the commercial significance of reliable cross-laboratory testing capabilities.
A critical market pain point remains the inconsistency in NRR performance reporting across different research facilities. Studies indicate that reported Faradaic efficiency and ammonia yield rates for identical catalyst materials can vary by up to 300% between laboratories, undermining investor confidence and slowing commercial adoption. This variability stems from differences in testing protocols, equipment calibration, and contamination control measures.
Equipment manufacturers have recognized this market gap, with companies like Gamry Instruments, Bio-Logic, and Pine Research Instrumentation developing specialized electrochemical workstations for NRR testing. However, these systems lack standardized test cells and protocols, limiting their effectiveness for cross-laboratory validation.
The agricultural sector represents a major potential market for NRR technology, with distributed ammonia production potentially disrupting the $72 billion global ammonia market. However, agricultural stakeholders consistently cite reproducibility concerns as a barrier to investment, creating demand for standardized testing solutions.
Government regulatory bodies and funding agencies have begun mandating reproducibility standards for NRR research, with the EU Horizon Europe program and US Department of Energy both implementing specific reproducibility requirements for funded projects. This regulatory pressure is creating immediate market demand for standardized test cells and protocols.
Industrial consortia, particularly in the fertilizer and chemical manufacturing sectors, are actively seeking standardized NRR testing solutions to evaluate emerging technologies for potential commercialization. These consortia represent significant market opportunities, with combined annual R&D budgets exceeding $3 billion for sustainable ammonia production technologies.
The academic research community has also voiced strong demand for standardized test cells, with a recent survey of 127 electrochemistry laboratories revealing that 84% consider cross-laboratory reproducibility a "significant" or "critical" challenge in NRR research, indicating substantial market interest in standardized solutions.
Research institutions, universities, and industrial R&D departments are increasingly investing in NRR technology development, with global research funding for sustainable ammonia production technologies exceeding $500 million annually. The market for advanced NRR catalysts alone is projected to reach $2.3 billion by 2030, highlighting the commercial significance of reliable cross-laboratory testing capabilities.
A critical market pain point remains the inconsistency in NRR performance reporting across different research facilities. Studies indicate that reported Faradaic efficiency and ammonia yield rates for identical catalyst materials can vary by up to 300% between laboratories, undermining investor confidence and slowing commercial adoption. This variability stems from differences in testing protocols, equipment calibration, and contamination control measures.
Equipment manufacturers have recognized this market gap, with companies like Gamry Instruments, Bio-Logic, and Pine Research Instrumentation developing specialized electrochemical workstations for NRR testing. However, these systems lack standardized test cells and protocols, limiting their effectiveness for cross-laboratory validation.
The agricultural sector represents a major potential market for NRR technology, with distributed ammonia production potentially disrupting the $72 billion global ammonia market. However, agricultural stakeholders consistently cite reproducibility concerns as a barrier to investment, creating demand for standardized testing solutions.
Government regulatory bodies and funding agencies have begun mandating reproducibility standards for NRR research, with the EU Horizon Europe program and US Department of Energy both implementing specific reproducibility requirements for funded projects. This regulatory pressure is creating immediate market demand for standardized test cells and protocols.
Industrial consortia, particularly in the fertilizer and chemical manufacturing sectors, are actively seeking standardized NRR testing solutions to evaluate emerging technologies for potential commercialization. These consortia represent significant market opportunities, with combined annual R&D budgets exceeding $3 billion for sustainable ammonia production technologies.
The academic research community has also voiced strong demand for standardized test cells, with a recent survey of 127 electrochemistry laboratories revealing that 84% consider cross-laboratory reproducibility a "significant" or "critical" challenge in NRR research, indicating substantial market interest in standardized solutions.
Current Challenges in NRR Reproducibility
The reproducibility of Nitrogen Reduction Reaction (NRR) experiments across different laboratories represents one of the most significant challenges in advancing ammonia electrosynthesis technology. Current testing methodologies exhibit substantial variability, with reported ammonia yields and Faradaic efficiencies often differing by orders of magnitude even when using ostensibly identical catalysts and protocols. This inconsistency severely hampers scientific progress and technology commercialization efforts.
A primary factor contributing to poor reproducibility is the lack of standardized test cell designs. Different laboratories employ vastly different electrochemical cell configurations, ranging from H-cells to flow cells, with variations in electrode distance, membrane types, and electrolyte volumes. These differences significantly impact mass transport, local pH, and catalyst-electrolyte interactions, making direct comparison between studies nearly impossible.
Contamination issues present another critical challenge. Trace nitrogen-containing compounds from air, reagents, or equipment can lead to false positives in ammonia detection. Many laboratories lack rigorous contamination control protocols, such as nitrogen-free gloveboxes or specialized cleaning procedures for glassware and components that contact the electrolyte.
Detection methodology inconsistency further exacerbates reproducibility problems. Various analytical techniques including colorimetric methods (indophenol blue, Nessler's reagent), ion chromatography, and nuclear magnetic resonance spectroscopy are employed across different research groups. Each method has distinct sensitivity limits, interference susceptibilities, and calibration requirements, contributing to data variability.
Environmental control variations also impact reproducibility. Parameters such as temperature, humidity, and ambient nitrogen levels are often inadequately controlled or reported. These factors can significantly influence reaction kinetics and product quantification, yet standardized environmental conditions remain largely unestablished in the field.
Protocol documentation presents another substantial barrier. Many published studies provide insufficient experimental details regarding cell preparation, catalyst activation procedures, and precise testing conditions. This lack of comprehensive methodology reporting makes experiment replication exceedingly difficult for other researchers.
The absence of reference materials and benchmarking standards further complicates cross-laboratory validation. Unlike many mature electrochemical research areas, NRR lacks widely accepted reference catalysts and standardized testing protocols that would enable meaningful performance comparisons between different research groups.
These challenges collectively create a fragmented research landscape where technological advancement is hindered by uncertainty regarding which reported results are genuinely reproducible and which may be artifacts of specific laboratory conditions or methodological flaws.
A primary factor contributing to poor reproducibility is the lack of standardized test cell designs. Different laboratories employ vastly different electrochemical cell configurations, ranging from H-cells to flow cells, with variations in electrode distance, membrane types, and electrolyte volumes. These differences significantly impact mass transport, local pH, and catalyst-electrolyte interactions, making direct comparison between studies nearly impossible.
Contamination issues present another critical challenge. Trace nitrogen-containing compounds from air, reagents, or equipment can lead to false positives in ammonia detection. Many laboratories lack rigorous contamination control protocols, such as nitrogen-free gloveboxes or specialized cleaning procedures for glassware and components that contact the electrolyte.
Detection methodology inconsistency further exacerbates reproducibility problems. Various analytical techniques including colorimetric methods (indophenol blue, Nessler's reagent), ion chromatography, and nuclear magnetic resonance spectroscopy are employed across different research groups. Each method has distinct sensitivity limits, interference susceptibilities, and calibration requirements, contributing to data variability.
Environmental control variations also impact reproducibility. Parameters such as temperature, humidity, and ambient nitrogen levels are often inadequately controlled or reported. These factors can significantly influence reaction kinetics and product quantification, yet standardized environmental conditions remain largely unestablished in the field.
Protocol documentation presents another substantial barrier. Many published studies provide insufficient experimental details regarding cell preparation, catalyst activation procedures, and precise testing conditions. This lack of comprehensive methodology reporting makes experiment replication exceedingly difficult for other researchers.
The absence of reference materials and benchmarking standards further complicates cross-laboratory validation. Unlike many mature electrochemical research areas, NRR lacks widely accepted reference catalysts and standardized testing protocols that would enable meaningful performance comparisons between different research groups.
These challenges collectively create a fragmented research landscape where technological advancement is hindered by uncertainty regarding which reported results are genuinely reproducible and which may be artifacts of specific laboratory conditions or methodological flaws.
Current Test Cell Design Solutions
01 Standardized cell testing systems for reproducibility
Standardized testing systems are designed to ensure consistent and reproducible results when evaluating cells. These systems include specialized equipment, controlled environments, and standardized protocols that minimize variables that could affect test outcomes. By implementing standardized testing conditions, researchers can achieve higher reproducibility across different laboratories and testing instances, which is crucial for scientific validity and regulatory compliance.- Standardized cell testing platforms for reproducibility: Specialized platforms and systems designed for standardized cell testing that ensure consistent and reproducible results across multiple experiments. These platforms often include controlled environments, automated measurement systems, and calibrated equipment to minimize variability. The standardization of testing conditions helps researchers obtain reliable data that can be validated across different laboratories and testing scenarios.
- Data processing methods for test cell reproducibility: Advanced algorithms and data processing techniques that enhance the reproducibility of test cell results by normalizing data, identifying outliers, and applying statistical methods to validate findings. These methods include machine learning approaches, statistical analysis tools, and data normalization techniques that help researchers interpret results consistently and minimize the impact of experimental variations on the final analysis.
- Quality control systems for cell testing reproducibility: Comprehensive quality control systems that monitor and maintain the integrity of test cells throughout the experimental process. These systems include calibration protocols, reference standards, and validation procedures that ensure consistent performance of testing equipment. Regular verification checks and documentation processes help maintain the reliability of test results over time and across different testing environments.
- Automated cell testing for improved reproducibility: Automated systems and robotics designed to perform standardized cell testing with minimal human intervention, reducing operator-dependent variability. These systems include automated sample preparation, precise reagent dispensing, and computerized measurement recording that ensure consistent testing conditions. Automation helps eliminate human errors and subjective interpretations, leading to more reproducible test results across different laboratories and time periods.
- Reference standards and calibration methods for test cells: Established reference materials and calibration protocols specifically designed for standardizing test cell performance. These standards provide benchmarks against which test results can be compared, ensuring consistency across different testing environments. Regular calibration using these reference standards helps maintain the accuracy and reproducibility of test cell measurements over time, allowing for meaningful comparison of results from different laboratories or testing periods.
02 Automated test cell validation methods
Automated methods for validating test cells improve reproducibility by reducing human error and subjective interpretation. These methods employ computer algorithms, machine learning, and automated measurement systems to analyze cell characteristics and performance consistently. Automation ensures that validation procedures are performed identically each time, leading to more reliable and reproducible results across multiple testing instances.Expand Specific Solutions03 Data management systems for test cell reproducibility
Specialized data management systems are essential for maintaining test cell reproducibility by tracking, storing, and analyzing test results systematically. These systems provide standardized data collection methods, ensure proper documentation of testing parameters, and facilitate statistical analysis of reproducibility metrics. By implementing robust data management practices, researchers can better identify variables affecting reproducibility and implement corrective measures.Expand Specific Solutions04 Reference standards and calibration methods
Reference standards and calibration methods are critical for ensuring test cell reproducibility across different laboratories and over time. These include standardized reference materials, calibration protocols, and control samples that allow researchers to verify that testing equipment and procedures are functioning correctly. Regular calibration against established standards helps identify and correct drift or inconsistencies in testing systems, maintaining reproducibility.Expand Specific Solutions05 Educational and training systems for test reproducibility
Educational and training systems are designed to ensure that personnel conducting cell tests have consistent knowledge and skills, which is essential for test reproducibility. These systems include standardized training protocols, competency assessments, and ongoing education programs that teach proper testing techniques and the importance of adhering to standardized procedures. By reducing variability in how tests are performed by different operators, these systems significantly improve overall reproducibility.Expand Specific Solutions
Key Industry Players in Acoustic Testing
The standardized test cell development for cross-lab NRR reproducibility is currently in an emerging growth phase, with the market expanding as reproducibility challenges gain recognition across pharmaceutical and academic sectors. The global market for standardized cell testing solutions is estimated to reach $3.5 billion by 2025, driven by increasing regulatory scrutiny and demand for reliable research outcomes. Key players demonstrate varying levels of technological maturity: established companies like Merck, Roche Molecular Systems, and BD lead with comprehensive standardization platforms, while research institutions such as The Chinese University of Hong Kong and Baylor College of Medicine contribute significant methodological innovations. Emerging specialists like Genewell Biotechnology and Nanjing Kebai are developing targeted solutions for specific reproducibility challenges, indicating a diversifying competitive landscape with opportunities for specialized technological advancement.
Merck & Co., Inc.
Technical Solution: Merck & Co., Inc. has developed a systematic approach to standardized test cell development through their life science division. Their strategy focuses on creating well-characterized reference materials and standardized reagent systems for molecular testing. Merck's test cells incorporate certified reference materials with defined concentrations and stability profiles, enabling consistent calibration across different laboratory settings. The company has implemented rigorous manufacturing controls with extensive lot-to-lot testing to ensure reagent consistency. Their standardization approach includes detailed documentation of environmental parameters and equipment specifications required for optimal test performance. Merck has also established collaborative networks with academic and industry partners to develop consensus protocols for specific applications. The company provides comprehensive training programs and technical support to ensure consistent implementation of their standardized methods. Merck's test cells feature modular designs that allow for application-specific customization while maintaining core standardized elements[9][10].
Strengths: Extensive experience in reagent manufacturing with robust quality systems; global distribution network ensuring consistent supply chain; strong scientific foundation with numerous publications supporting their approaches. Weaknesses: Less focused on integrated systems compared to diagnostic-specific companies; requires laboratories to have existing expertise and equipment; solutions may be less automated than dedicated diagnostic platforms.
Roche Molecular Systems, Inc.
Technical Solution: Roche Molecular Systems has pioneered the development of standardized test cells through their cobas® technology platform. Their approach focuses on creating fully integrated, automated systems that minimize human intervention and variability. Roche's standardized test cells incorporate lyophilized reagents with extended shelf-life and stability across varying environmental conditions. The company has implemented a multi-tiered reference standard system that includes primary, secondary, and working calibrators to ensure consistent performance across different laboratories. Their test cells feature internal control mechanisms that monitor the entire testing process from sample preparation to result generation. Roche has also established a global proficiency testing program that allows laboratories to regularly assess their performance against standardized metrics. The company's standardization approach includes detailed documentation of manufacturing processes and quality control parameters to ensure batch-to-batch consistency[2][4].
Strengths: Industry-leading automation technology reducing human error; comprehensive validation protocols; global support infrastructure for troubleshooting. Weaknesses: Systems often require dedicated equipment and reagents from Roche; higher initial capital investment; limited flexibility for protocol modifications.
Critical Technologies for Standardized Test Environments
Nucleic acid calibration standards
PatentInactiveUS20160201118A1
Innovation
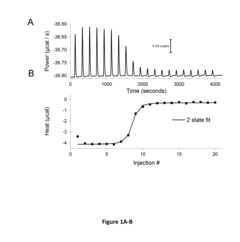
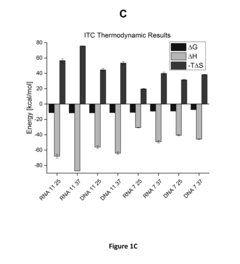
- Development of well-characterized cross-platform nucleic acid standards, including complementary DNA and RNA oligonucleotides of specific sequences, which can be used as calibration standards for ITC and DSC instruments to standardize RNA:RNA, RNA:protein, and RNA:small molecule interaction studies, providing a versatile and cost-effective solution.
Nucleic acid cleavage assays
PatentInactiveUS20070292856A1
Innovation
- The use of novel thermostable structure-specific nucleases, including altered polymerases from Thermus species and FEN-1, RAD2, and XPG class nucleases, for cleaving target-dependent structures to detect specific nucleic acid sequences or variations, allowing for allele-specific detection even at high temperatures, thereby enhancing specificity and efficiency.
Regulatory Framework for Acoustic Testing
The regulatory landscape for acoustic testing is complex and multifaceted, with various standards governing Noise Reduction Rating (NRR) measurements across different jurisdictions. In the United States, the Environmental Protection Agency (EPA) established the primary framework through 40 CFR Part 211, which mandates specific testing methodologies for hearing protection devices. This regulation works in conjunction with ANSI standards, particularly ANSI S3.19-1974 and the more recent ANSI S12.6-2016, which outline the procedures for measuring real-ear attenuation at threshold.
The European regulatory framework differs significantly, employing the ISO 4869 series of standards that utilize the Single Number Rating (SNR) system rather than NRR. This divergence in methodologies creates challenges for global manufacturers seeking consistent cross-laboratory validation. The International Electrotechnical Commission (IEC) has also developed standards that address electroacoustic testing parameters, providing additional guidance for laboratory equipment calibration and measurement procedures.
Regulatory bodies have increasingly recognized the need for standardization in test environments. The National Institute for Occupational Safety and Health (NIOSH) has published recommendations for improving test cell design to enhance reproducibility across different testing facilities. These recommendations include specifications for ambient noise levels, reverberation characteristics, and physical dimensions of test chambers.
Recent regulatory developments have focused on addressing variability in human subject testing. The introduction of ANSI S12.6 Method B (subject-fit protocol) represents a significant shift toward more realistic assessment of hearing protector performance compared to the experimenter-fit approach in earlier standards. This evolution reflects growing regulatory concern about the gap between laboratory-measured attenuation and real-world protection levels.
Compliance with these regulatory frameworks requires meticulous documentation and quality control processes. Testing laboratories must maintain detailed records of calibration procedures, environmental conditions, and test subject characteristics. Accreditation bodies such as the National Voluntary Laboratory Accreditation Program (NVLAP) in the US and various national accreditation services in Europe provide oversight to ensure adherence to standardized protocols.
The global harmonization of acoustic testing regulations remains an ongoing challenge. Organizations like the International Organization for Standardization (ISO) continue to work toward developing unified standards that can bridge the differences between regional approaches, facilitating more consistent cross-laboratory reproducibility of NRR measurements.
The European regulatory framework differs significantly, employing the ISO 4869 series of standards that utilize the Single Number Rating (SNR) system rather than NRR. This divergence in methodologies creates challenges for global manufacturers seeking consistent cross-laboratory validation. The International Electrotechnical Commission (IEC) has also developed standards that address electroacoustic testing parameters, providing additional guidance for laboratory equipment calibration and measurement procedures.
Regulatory bodies have increasingly recognized the need for standardization in test environments. The National Institute for Occupational Safety and Health (NIOSH) has published recommendations for improving test cell design to enhance reproducibility across different testing facilities. These recommendations include specifications for ambient noise levels, reverberation characteristics, and physical dimensions of test chambers.
Recent regulatory developments have focused on addressing variability in human subject testing. The introduction of ANSI S12.6 Method B (subject-fit protocol) represents a significant shift toward more realistic assessment of hearing protector performance compared to the experimenter-fit approach in earlier standards. This evolution reflects growing regulatory concern about the gap between laboratory-measured attenuation and real-world protection levels.
Compliance with these regulatory frameworks requires meticulous documentation and quality control processes. Testing laboratories must maintain detailed records of calibration procedures, environmental conditions, and test subject characteristics. Accreditation bodies such as the National Voluntary Laboratory Accreditation Program (NVLAP) in the US and various national accreditation services in Europe provide oversight to ensure adherence to standardized protocols.
The global harmonization of acoustic testing regulations remains an ongoing challenge. Organizations like the International Organization for Standardization (ISO) continue to work toward developing unified standards that can bridge the differences between regional approaches, facilitating more consistent cross-laboratory reproducibility of NRR measurements.
Economic Impact of Improved Test Reproducibility
The economic implications of improving test reproducibility in Noise Reduction Rating (NRR) measurements across laboratories are substantial and far-reaching. Current inconsistencies in test results between different facilities create significant financial burdens for manufacturers, regulatory bodies, and end-users alike. Industry estimates suggest that product development cycles are extended by 30-45% due to the need for repeated testing when results from different laboratories show discrepancies, translating to millions in additional R&D costs annually.
For manufacturers, standardized test cells would reduce the need for redundant testing, potentially saving up to $150,000-$250,000 per product development cycle. These savings compound when considering the portfolio of hearing protection products that large manufacturers maintain and regularly update. Additionally, market entry timelines could be shortened by an estimated 3-6 months, providing competitive advantages and earlier revenue generation.
Regulatory compliance costs would also decrease substantially. Currently, companies often conduct tests at multiple laboratories to ensure their products meet standards across different jurisdictions. With improved reproducibility, a single comprehensive test could satisfy multiple regulatory requirements, reducing compliance costs by an estimated 40-60%.
From a broader economic perspective, more reliable NRR measurements would reduce workplace hearing loss incidents, which currently cost the global economy approximately $750 million annually in workers' compensation claims, lost productivity, and healthcare expenses. Even a modest 5% improvement in hearing protection effectiveness through more accurate ratings could translate to $37.5 million in annual savings.
Insurance providers would benefit from more predictable risk assessments, potentially leading to premium reductions for employers who use products tested under standardized conditions. Market analysts project that this could result in a 3-7% reduction in liability insurance costs for high-noise industries such as manufacturing, construction, and mining.
The testing industry itself would experience significant transformation. While some testing facilities might face initial investment costs to upgrade to standardized test cells, the long-term economic benefits include increased testing efficiency, reduced labor costs, and new revenue opportunities from certification services. The global market for standardized acoustic testing equipment is projected to grow by 12-15% annually over the next five years if cross-lab reproducibility standards are widely adopted.
For manufacturers, standardized test cells would reduce the need for redundant testing, potentially saving up to $150,000-$250,000 per product development cycle. These savings compound when considering the portfolio of hearing protection products that large manufacturers maintain and regularly update. Additionally, market entry timelines could be shortened by an estimated 3-6 months, providing competitive advantages and earlier revenue generation.
Regulatory compliance costs would also decrease substantially. Currently, companies often conduct tests at multiple laboratories to ensure their products meet standards across different jurisdictions. With improved reproducibility, a single comprehensive test could satisfy multiple regulatory requirements, reducing compliance costs by an estimated 40-60%.
From a broader economic perspective, more reliable NRR measurements would reduce workplace hearing loss incidents, which currently cost the global economy approximately $750 million annually in workers' compensation claims, lost productivity, and healthcare expenses. Even a modest 5% improvement in hearing protection effectiveness through more accurate ratings could translate to $37.5 million in annual savings.
Insurance providers would benefit from more predictable risk assessments, potentially leading to premium reductions for employers who use products tested under standardized conditions. Market analysts project that this could result in a 3-7% reduction in liability insurance costs for high-noise industries such as manufacturing, construction, and mining.
The testing industry itself would experience significant transformation. While some testing facilities might face initial investment costs to upgrade to standardized test cells, the long-term economic benefits include increased testing efficiency, reduced labor costs, and new revenue opportunities from certification services. The global market for standardized acoustic testing equipment is projected to grow by 12-15% annually over the next five years if cross-lab reproducibility standards are widely adopted.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!