How To Reduce Overpotential In NRR Through Catalyst And Electrode Engineering
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NRR Overpotential Reduction Background and Objectives
The Nitrogen Reduction Reaction (NRR) represents a promising pathway for sustainable ammonia production, offering an alternative to the energy-intensive Haber-Bosch process which currently consumes approximately 1-2% of global energy production. Since the early 2000s, electrochemical NRR has gained significant attention as researchers recognized its potential for ambient condition operation and integration with renewable energy sources.
The evolution of NRR technology has progressed through several distinct phases. Initial research focused primarily on proof-of-concept demonstrations, followed by a period of catalyst diversification. Recent years have witnessed a shift toward understanding fundamental reaction mechanisms and addressing the critical challenge of overpotential - the excess voltage required beyond the thermodynamic potential to drive the reaction at practical rates.
Overpotential represents one of the most significant barriers to NRR commercialization, directly impacting energy efficiency and economic viability. High overpotentials (often exceeding 300mV) substantially increase energy consumption, rendering the process less competitive against conventional ammonia production methods. This technical limitation has become increasingly prominent as the field matures from laboratory demonstrations toward practical applications.
The scientific community has identified several contributing factors to NRR overpotential, including sluggish electron transfer kinetics, competing hydrogen evolution reactions, and suboptimal catalyst-electrolyte interfaces. These challenges are compounded by the inherent stability of the N≡N triple bond (945 kJ/mol), which necessitates significant activation energy for cleavage.
Current technological trajectories suggest multiple promising approaches to overpotential reduction, including rational catalyst design, electrode architecture engineering, and electrolyte optimization. Computational studies increasingly guide experimental work, enabling more targeted development of materials with optimized binding energies and reaction pathways.
The primary objective of this technical investigation is to comprehensively evaluate strategies for reducing NRR overpotential through innovations in catalyst design and electrode engineering. Specifically, we aim to identify approaches that can achieve overpotential reductions of at least 100mV while maintaining ammonia production rates above 10^-10 mol cm^-2 s^-1 and Faradaic efficiencies exceeding 10% under ambient conditions.
Secondary objectives include assessing the scalability of promising approaches, evaluating their compatibility with renewable energy integration, and identifying potential synergies between catalyst and electrode engineering strategies that may yield multiplicative benefits for overall system performance.
The evolution of NRR technology has progressed through several distinct phases. Initial research focused primarily on proof-of-concept demonstrations, followed by a period of catalyst diversification. Recent years have witnessed a shift toward understanding fundamental reaction mechanisms and addressing the critical challenge of overpotential - the excess voltage required beyond the thermodynamic potential to drive the reaction at practical rates.
Overpotential represents one of the most significant barriers to NRR commercialization, directly impacting energy efficiency and economic viability. High overpotentials (often exceeding 300mV) substantially increase energy consumption, rendering the process less competitive against conventional ammonia production methods. This technical limitation has become increasingly prominent as the field matures from laboratory demonstrations toward practical applications.
The scientific community has identified several contributing factors to NRR overpotential, including sluggish electron transfer kinetics, competing hydrogen evolution reactions, and suboptimal catalyst-electrolyte interfaces. These challenges are compounded by the inherent stability of the N≡N triple bond (945 kJ/mol), which necessitates significant activation energy for cleavage.
Current technological trajectories suggest multiple promising approaches to overpotential reduction, including rational catalyst design, electrode architecture engineering, and electrolyte optimization. Computational studies increasingly guide experimental work, enabling more targeted development of materials with optimized binding energies and reaction pathways.
The primary objective of this technical investigation is to comprehensively evaluate strategies for reducing NRR overpotential through innovations in catalyst design and electrode engineering. Specifically, we aim to identify approaches that can achieve overpotential reductions of at least 100mV while maintaining ammonia production rates above 10^-10 mol cm^-2 s^-1 and Faradaic efficiencies exceeding 10% under ambient conditions.
Secondary objectives include assessing the scalability of promising approaches, evaluating their compatibility with renewable energy integration, and identifying potential synergies between catalyst and electrode engineering strategies that may yield multiplicative benefits for overall system performance.
Market Analysis for Efficient Nitrogen Reduction Technologies
The global market for nitrogen reduction technologies is experiencing significant growth, driven by increasing demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial CO2 emissions. This creates a compelling market opportunity for electrochemical nitrogen reduction reaction (NRR) technologies that can operate under ambient conditions with renewable electricity sources.
The market for efficient NRR technologies spans multiple sectors, including agricultural fertilizers, chemical manufacturing, and emerging clean energy applications. The fertilizer industry represents the largest potential market, valued at over $150 billion globally, with ammonia serving as the primary nitrogen source. Chemical manufacturing constitutes another substantial segment, where ammonia serves as a precursor for numerous industrial chemicals and materials.
Market analysis indicates that early adoption of advanced NRR technologies is occurring in regions with high renewable energy penetration and stringent carbon regulations, particularly in Europe, Japan, and parts of North America. These regions offer favorable policy environments and financial incentives for green ammonia production technologies.
Demand forecasts suggest the market for electrochemical nitrogen fixation technologies could grow at a compound annual growth rate of 25-30% over the next decade, reaching significant commercial scale by 2030. This growth trajectory is supported by increasing corporate sustainability commitments and government decarbonization targets worldwide.
Investment trends show accelerating capital flows into NRR technology development, with venture capital funding for startups in this space exceeding $500 million in the past three years. Major industrial gas companies and agricultural input suppliers are also increasing R&D expenditures in this area, recognizing the strategic importance of low-overpotential NRR systems.
Customer requirements analysis reveals that commercial viability hinges on achieving Faradaic efficiency above 30%, current densities exceeding 100 mA/cm², and significant reductions in overpotential to below 0.4V. These performance metrics would enable cost-competitive ammonia production compared to conventional methods when powered by renewable electricity.
Market barriers include high capital costs for initial deployment, technical challenges in catalyst stability and selectivity, and established infrastructure favoring conventional ammonia production. However, the increasing price of carbon emissions and volatility in natural gas markets are improving the comparative economics of electrochemical approaches.
The competitive landscape features both established industrial players and innovative startups developing proprietary catalyst and electrode technologies, with intellectual property activity showing a 40% annual increase in patent filings related to low-overpotential NRR systems.
The market for efficient NRR technologies spans multiple sectors, including agricultural fertilizers, chemical manufacturing, and emerging clean energy applications. The fertilizer industry represents the largest potential market, valued at over $150 billion globally, with ammonia serving as the primary nitrogen source. Chemical manufacturing constitutes another substantial segment, where ammonia serves as a precursor for numerous industrial chemicals and materials.
Market analysis indicates that early adoption of advanced NRR technologies is occurring in regions with high renewable energy penetration and stringent carbon regulations, particularly in Europe, Japan, and parts of North America. These regions offer favorable policy environments and financial incentives for green ammonia production technologies.
Demand forecasts suggest the market for electrochemical nitrogen fixation technologies could grow at a compound annual growth rate of 25-30% over the next decade, reaching significant commercial scale by 2030. This growth trajectory is supported by increasing corporate sustainability commitments and government decarbonization targets worldwide.
Investment trends show accelerating capital flows into NRR technology development, with venture capital funding for startups in this space exceeding $500 million in the past three years. Major industrial gas companies and agricultural input suppliers are also increasing R&D expenditures in this area, recognizing the strategic importance of low-overpotential NRR systems.
Customer requirements analysis reveals that commercial viability hinges on achieving Faradaic efficiency above 30%, current densities exceeding 100 mA/cm², and significant reductions in overpotential to below 0.4V. These performance metrics would enable cost-competitive ammonia production compared to conventional methods when powered by renewable electricity.
Market barriers include high capital costs for initial deployment, technical challenges in catalyst stability and selectivity, and established infrastructure favoring conventional ammonia production. However, the increasing price of carbon emissions and volatility in natural gas markets are improving the comparative economics of electrochemical approaches.
The competitive landscape features both established industrial players and innovative startups developing proprietary catalyst and electrode technologies, with intellectual property activity showing a 40% annual increase in patent filings related to low-overpotential NRR systems.
Current Challenges in NRR Catalyst and Electrode Engineering
The field of nitrogen reduction reaction (NRR) for ammonia synthesis faces significant challenges in catalyst and electrode engineering, particularly regarding overpotential reduction. Current NRR systems typically require high overpotentials (>0.5V) to achieve meaningful ammonia production rates, severely limiting energy efficiency and commercial viability. This fundamental challenge stems from the inherent stability of the N≡N triple bond (941 kJ/mol), requiring substantial energy input for activation.
Catalyst design presents a critical bottleneck in NRR advancement. Most existing catalysts struggle with the competing hydrogen evolution reaction (HER), which dominates in aqueous environments due to its kinetic favorability. Noble metal catalysts (Ru, Pt) demonstrate promising activity but remain economically prohibitive for large-scale implementation. Meanwhile, transition metal-based alternatives often suffer from poor selectivity and stability under reaction conditions.
Electrode architecture compounds these challenges through inadequate mass transport properties. Current electrode designs frequently exhibit limited active site accessibility, poor nitrogen gas diffusion characteristics, and suboptimal three-phase boundaries where electrolyte, catalyst, and nitrogen gas interact. These limitations create concentration polarization effects that contribute significantly to overall overpotential.
Surface engineering approaches face the dilemma of balancing nitrogen adsorption strength with intermediate reduction capabilities. Too strong adsorption leads to catalyst poisoning, while weak adsorption results in insufficient activation. The optimal binding energy window remains elusive across different material systems, with theoretical predictions often diverging from experimental results.
Stability issues further complicate catalyst development, as many promising materials degrade under the harsh electrochemical conditions required for NRR. Metal leaching, surface oxidation, and structural collapse frequently occur during extended operation, necessitating more robust design strategies that maintain catalytic performance over thousands of hours.
Electrolyte engineering presents additional challenges, as proton availability must be carefully balanced to support NRR while minimizing HER. Current approaches using aprotic solvents or controlled proton donors show promise but introduce new complications regarding conductivity, viscosity, and compatibility with electrode materials.
Mechanistic understanding remains incomplete, with reaction pathways varying significantly across catalyst types. This knowledge gap hinders rational design approaches, as the rate-determining steps and intermediate species differ between systems. Advanced in-situ characterization techniques are needed to elucidate these mechanisms under realistic operating conditions.
Catalyst design presents a critical bottleneck in NRR advancement. Most existing catalysts struggle with the competing hydrogen evolution reaction (HER), which dominates in aqueous environments due to its kinetic favorability. Noble metal catalysts (Ru, Pt) demonstrate promising activity but remain economically prohibitive for large-scale implementation. Meanwhile, transition metal-based alternatives often suffer from poor selectivity and stability under reaction conditions.
Electrode architecture compounds these challenges through inadequate mass transport properties. Current electrode designs frequently exhibit limited active site accessibility, poor nitrogen gas diffusion characteristics, and suboptimal three-phase boundaries where electrolyte, catalyst, and nitrogen gas interact. These limitations create concentration polarization effects that contribute significantly to overall overpotential.
Surface engineering approaches face the dilemma of balancing nitrogen adsorption strength with intermediate reduction capabilities. Too strong adsorption leads to catalyst poisoning, while weak adsorption results in insufficient activation. The optimal binding energy window remains elusive across different material systems, with theoretical predictions often diverging from experimental results.
Stability issues further complicate catalyst development, as many promising materials degrade under the harsh electrochemical conditions required for NRR. Metal leaching, surface oxidation, and structural collapse frequently occur during extended operation, necessitating more robust design strategies that maintain catalytic performance over thousands of hours.
Electrolyte engineering presents additional challenges, as proton availability must be carefully balanced to support NRR while minimizing HER. Current approaches using aprotic solvents or controlled proton donors show promise but introduce new complications regarding conductivity, viscosity, and compatibility with electrode materials.
Mechanistic understanding remains incomplete, with reaction pathways varying significantly across catalyst types. This knowledge gap hinders rational design approaches, as the rate-determining steps and intermediate species differ between systems. Advanced in-situ characterization techniques are needed to elucidate these mechanisms under realistic operating conditions.
State-of-the-Art Catalyst and Electrode Design Approaches
01 Metal-based catalysts for NRR
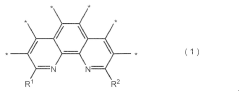
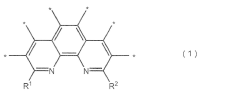
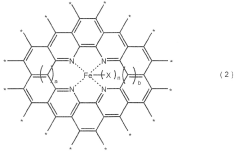
Metal-based catalysts play a crucial role in nitrogen reduction reactions by providing active sites for N2 adsorption and activation. Various metals such as iron, ruthenium, molybdenum, and nickel have been engineered to reduce overpotential in NRR. These catalysts can be designed with specific morphologies, crystal structures, and surface properties to enhance nitrogen binding while suppressing competing hydrogen evolution reactions. The incorporation of transition metals with d-orbital configurations favorable for N≡N bond activation has shown significant improvements in catalytic efficiency and selectivity.- Metal-based catalysts for NRR: Metal-based catalysts play a crucial role in nitrogen reduction reaction (NRR) by lowering the overpotential required for the reaction. Various metals such as iron, nickel, cobalt, and noble metals have been engineered to provide active sites for nitrogen adsorption and subsequent reduction. These catalysts can be designed with specific morphologies, crystal structures, and surface properties to enhance their catalytic activity and selectivity for NRR, thereby reducing the energy barrier for the reaction.
- Single-atom catalysts and atomically dispersed active sites: Single-atom catalysts (SACs) represent an advanced approach to catalyst design for NRR, where individual metal atoms are anchored on supporting materials. This maximizes atom utilization efficiency and provides well-defined active sites for nitrogen activation. The isolated metal centers can be tuned to optimize binding energies with reaction intermediates, significantly reducing overpotential. These catalysts often demonstrate superior performance due to their unique electronic properties and coordination environments.
- Carbon-based electrode materials and supports: Carbon-based materials serve as excellent supports for NRR catalysts and electrode structures due to their high conductivity, large surface area, and tunable porosity. Materials such as graphene, carbon nanotubes, and porous carbon frameworks can be functionalized to create nitrogen adsorption sites or to anchor metal catalysts. The carbon support not only provides structural stability but also facilitates electron transfer during the electrochemical process, helping to reduce overpotential and improve reaction kinetics.
- Electrolyte engineering and interface optimization: The composition and properties of the electrolyte significantly impact NRR performance by affecting the local environment at the electrode-electrolyte interface. Engineering the electrolyte pH, ionic strength, and additives can modify the electric double layer structure, influence proton availability, and alter the stability of reaction intermediates. Optimizing these parameters helps to control reaction pathways, suppress competing reactions like hydrogen evolution, and reduce the overpotential required for efficient nitrogen reduction.
- Defect engineering and heteroatom doping: Introducing defects and heteroatom dopants (such as N, S, P, or B) into catalyst structures creates active sites with altered electronic properties that can facilitate nitrogen adsorption and activation. These structural modifications can optimize the binding energy of reaction intermediates, lower the energy barriers for electron transfer, and enhance the selectivity toward ammonia formation. Strategic defect engineering and doping have proven effective in reducing overpotential and improving the overall efficiency of the nitrogen reduction reaction.
02 Single-atom catalysts and atomically dispersed active sites
Single-atom catalysts represent an advanced approach to NRR catalyst design, where isolated metal atoms are anchored on supporting materials to maximize atomic efficiency and provide well-defined active sites. These catalysts offer precise control over the electronic structure and coordination environment of the active metal centers, leading to optimized binding energies for nitrogen intermediates and reduced overpotential. The atomically dispersed nature of these catalysts allows for enhanced exposure of active sites and improved mass transport, resulting in higher catalytic activity per metal atom compared to traditional nanoparticle catalysts.Expand Specific Solutions03 Carbon-based supports and heteroatom doping
Carbon-based materials serve as excellent supports for NRR catalysts due to their high conductivity, large surface area, and tunable surface chemistry. Incorporating heteroatoms such as nitrogen, sulfur, phosphorus, or boron into carbon frameworks creates defects and active sites that can directly participate in nitrogen reduction or modulate the electronic properties of supported metal catalysts. These doped carbon materials can effectively reduce the overpotential by creating favorable adsorption sites for N2 molecules and stabilizing reaction intermediates. The synergistic effects between heteroatoms and metal active sites further enhance catalytic performance.Expand Specific Solutions04 Electrode architecture and interface engineering
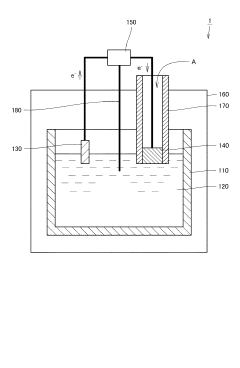
The design of electrode architectures significantly impacts NRR performance by affecting mass transport, charge transfer, and catalyst utilization. Hierarchical porous structures, 3D electrodes, and advanced substrate engineering can enhance the accessibility of active sites while maintaining electrical conductivity. Interface engineering between the catalyst and electrolyte is crucial for controlling local reaction environments, including pH gradients and electric field distributions. Optimizing the hydrophilicity/hydrophobicity balance at the electrode surface can facilitate N2 access to active sites while managing proton availability, thereby reducing overpotential and improving selectivity over competing reactions.Expand Specific Solutions05 Electrolyte composition and reaction environment control
The composition and properties of the electrolyte significantly influence NRR performance by affecting nitrogen solubility, proton availability, and the stability of reaction intermediates. Tailored electrolytes with specific pH values, ionic strengths, and additives can help reduce overpotential by creating favorable local environments for nitrogen activation. Strategies such as using ionic liquids, organic electrolytes, or introducing specific cations/anions can modify the electric double layer structure at the electrode-electrolyte interface. Controlling the reaction environment through temperature, pressure, and gas flow management further optimizes the energetics of the nitrogen reduction process.Expand Specific Solutions
Leading Research Groups and Companies in NRR Technology
The nitrogen reduction reaction (NRR) overpotential reduction landscape is currently in an early growth phase, with the market expanding as sustainable ammonia synthesis gains importance. The technology remains in development, with varying maturity levels across catalyst and electrode engineering approaches. Leading players include established materials companies like Johnson Matthey, BASF, and Umicore, who leverage their expertise in catalyst development. Academic institutions such as Zhejiang University, Monash University, and The University of Sydney are driving fundamental research breakthroughs. Industrial players including Toyota, Volkswagen, and Mitsubishi Electric are exploring applications in energy storage and transportation sectors. Research organizations like Helmholtz-Zentrum Berlin and KIST are advancing electrode engineering techniques to overcome kinetic barriers and improve selectivity in NRR catalysis.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed advanced catalyst systems for nitrogen reduction reaction (NRR) focusing on transition metal-based catalysts with engineered electronic structures. Their approach involves creating single-atom catalysts (SACs) dispersed on nitrogen-doped carbon supports, which significantly reduces overpotential by optimizing the binding energy of reaction intermediates. The company has pioneered the development of bimetallic catalysts where secondary metals modify the electronic properties of primary active sites, creating synergistic effects that lower the energy barrier for rate-determining steps in NRR. Their electrode engineering strategy incorporates hierarchical porous structures that enhance mass transport while maintaining high electrical conductivity, achieved through controlled pyrolysis techniques that create optimal micropore/mesopore distributions[1]. Johnson Matthey has also developed proprietary surface modification techniques that introduce oxygen functional groups at specific concentrations to balance hydrophilicity and nitrogen adsorption capabilities.
Strengths: Extensive experience in precious metal catalysis provides superior control over atomic-level catalyst design. Their established manufacturing infrastructure enables scalable production of complex catalyst systems. Weaknesses: Higher production costs associated with precious metal components may limit commercial viability, and their catalysts may be sensitive to poisoning in real-world conditions with multiple contaminants.
Zhejiang University
Technical Solution: Zhejiang University has developed innovative approaches to reducing NRR overpotential through rational catalyst design and advanced electrode engineering. Their research focuses on single-atom catalysts (SACs) anchored on defect-rich carbon supports, where individual metal atoms (primarily Fe, Co, and Mo) are coordinated with nitrogen to create M-Nx active sites with optimized electronic structures. These sites feature precisely tuned d-band centers that facilitate N2 adsorption while weakening the N≡N bond, significantly lowering the activation energy barrier. Their catalyst design incorporates dual-site mechanisms where adjacent active centers work cooperatively to stabilize reaction intermediates[5]. For electrode engineering, Zhejiang University has pioneered hierarchical porous structures with controlled wettability gradients that enhance mass transport while maintaining high catalyst utilization. Their electrodes feature a three-dimensional architecture with interconnected macro/meso/micropores that facilitate gas diffusion, electrolyte penetration, and electron transport simultaneously. Recent work has demonstrated overpotential reductions of approximately 270mV compared to state-of-the-art materials through the integration of these catalyst and electrode design principles.
Strengths: Strong fundamental research capabilities in theoretical modeling and advanced characterization techniques enable atomic-level catalyst optimization. Their interdisciplinary approach integrates computational predictions with experimental validation. Weaknesses: Some of their most promising materials involve complex synthesis procedures with multiple steps that may challenge industrial scaling, and long-term stability under practical operating conditions requires further improvement.
Key Breakthroughs in Low-Overpotential NRR Systems
Catalyst for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction, method for producing catalyst for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction, and electrode for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction
PatentInactiveJP2021115501A
Innovation
- Incorporating Fe-N4 structures with a high active point density of 3.0 × 10^-5 to 1.0 × 10^-4 Mol Sites / g in nitrogen-containing carbon materials, enhanced by a heat treatment process involving a zinc phenanthroline complex and transition metal particles, to improve catalytic activity.
Nitrogen reduction method
PatentActiveJP2012219285A
Innovation
- The method involves generating Li2+ in a molten salt of alkali halides, using a voltage-controlled potential to indirectly reduce nitrogen at the cathode without a catalyst, leveraging the electrochemical properties of lithium ions to enhance the reaction.
Scalability and Industrial Implementation Considerations
The transition from laboratory-scale NRR catalyst systems to industrial implementation presents significant challenges that must be addressed for commercial viability. Current laboratory demonstrations typically involve small electrode areas and low current densities, which are inadequate for industrial nitrogen fixation demands. Scaling up requires not only maintaining the catalytic performance observed in laboratory settings but also ensuring economic feasibility and operational stability under industrial conditions.
Material availability represents a critical consideration for large-scale implementation. Many high-performance NRR catalysts incorporate precious metals or rare earth elements that face supply constraints and price volatility. Industrial adoption necessitates either developing catalysts based on earth-abundant elements or dramatically reducing the loading of scarce materials while maintaining performance. Recent advances in single-atom catalysts and nanostructured designs offer promising pathways to minimize precious metal content while preserving catalytic activity.
Manufacturing processes must evolve to accommodate industrial-scale production of optimized electrodes. Techniques such as electrodeposition, spray coating, and roll-to-roll processing show potential for large-area electrode fabrication with controlled morphology and composition. However, maintaining nanoscale features and precise catalyst distribution across large electrode surfaces remains challenging. Standardization of manufacturing protocols is essential to ensure reproducibility and quality control in scaled production.
Reactor design considerations become increasingly important at industrial scales. Efficient mass transport of nitrogen to catalyst sites, effective product separation, and thermal management all present engineering challenges. Membrane electrode assemblies and flow cell configurations offer advantages for industrial implementation by enabling higher current densities and improved reactant delivery. Additionally, integration with renewable energy sources requires addressing intermittency issues through system design and potentially incorporating energy storage components.
Economic viability ultimately determines industrial adoption potential. Current NRR systems typically require high overpotentials that translate to excessive energy consumption, significantly impacting operational costs. Techno-economic analyses suggest that achieving industrial viability requires catalysts that can operate below 300 mV overpotential with Faradaic efficiencies exceeding 60% at industrially relevant current densities (>200 mA/cm²). These targets represent substantial improvements over current state-of-the-art systems but provide clear benchmarks for research and development efforts.
Regulatory frameworks and sustainability metrics will increasingly influence implementation pathways. Life cycle assessments comparing electrochemical nitrogen fixation with conventional Haber-Bosch processes must consider not only energy efficiency but also environmental impacts, water usage, and carbon footprint. Developing standardized testing protocols and performance metrics will facilitate meaningful comparisons between different technological approaches and accelerate commercialization of the most promising systems.
Material availability represents a critical consideration for large-scale implementation. Many high-performance NRR catalysts incorporate precious metals or rare earth elements that face supply constraints and price volatility. Industrial adoption necessitates either developing catalysts based on earth-abundant elements or dramatically reducing the loading of scarce materials while maintaining performance. Recent advances in single-atom catalysts and nanostructured designs offer promising pathways to minimize precious metal content while preserving catalytic activity.
Manufacturing processes must evolve to accommodate industrial-scale production of optimized electrodes. Techniques such as electrodeposition, spray coating, and roll-to-roll processing show potential for large-area electrode fabrication with controlled morphology and composition. However, maintaining nanoscale features and precise catalyst distribution across large electrode surfaces remains challenging. Standardization of manufacturing protocols is essential to ensure reproducibility and quality control in scaled production.
Reactor design considerations become increasingly important at industrial scales. Efficient mass transport of nitrogen to catalyst sites, effective product separation, and thermal management all present engineering challenges. Membrane electrode assemblies and flow cell configurations offer advantages for industrial implementation by enabling higher current densities and improved reactant delivery. Additionally, integration with renewable energy sources requires addressing intermittency issues through system design and potentially incorporating energy storage components.
Economic viability ultimately determines industrial adoption potential. Current NRR systems typically require high overpotentials that translate to excessive energy consumption, significantly impacting operational costs. Techno-economic analyses suggest that achieving industrial viability requires catalysts that can operate below 300 mV overpotential with Faradaic efficiencies exceeding 60% at industrially relevant current densities (>200 mA/cm²). These targets represent substantial improvements over current state-of-the-art systems but provide clear benchmarks for research and development efforts.
Regulatory frameworks and sustainability metrics will increasingly influence implementation pathways. Life cycle assessments comparing electrochemical nitrogen fixation with conventional Haber-Bosch processes must consider not only energy efficiency but also environmental impacts, water usage, and carbon footprint. Developing standardized testing protocols and performance metrics will facilitate meaningful comparisons between different technological approaches and accelerate commercialization of the most promising systems.
Environmental Impact and Sustainability Assessment
The reduction of overpotential in nitrogen reduction reaction (NRR) through catalyst and electrode engineering carries significant environmental implications that extend beyond technical performance metrics. The environmental footprint of ammonia production via conventional Haber-Bosch process is substantial, consuming approximately 1-2% of global energy and generating considerable greenhouse gas emissions. Advanced NRR technologies with reduced overpotential offer a pathway to dramatically decrease this environmental burden.
When evaluating the sustainability of catalyst materials for NRR, life cycle assessment (LCA) reveals important considerations. Noble metal catalysts, while often demonstrating superior performance in reducing overpotential, present sustainability challenges due to their scarcity, energy-intensive mining processes, and significant environmental impacts during extraction. Earth-abundant alternatives such as transition metal nitrides, carbides, and single-atom catalysts represent more sustainable options, though their performance optimization remains an ongoing challenge.
Electrode engineering approaches that minimize material waste through precise deposition techniques contribute positively to sustainability profiles. Techniques such as atomic layer deposition and controlled electrodeposition enable significant reductions in precious metal loading while maintaining catalytic performance, thereby improving resource efficiency. Additionally, the development of self-supporting electrodes eliminates the need for separate substrates, further reducing material requirements.
Energy efficiency improvements resulting from reduced overpotential translate directly into lower carbon emissions when powered by conventional energy sources. More importantly, lower overpotential NRR systems become increasingly compatible with renewable energy integration, potentially enabling distributed, carbon-neutral ammonia production powered by intermittent renewable sources like solar and wind.
Water consumption represents another critical environmental consideration, particularly as many high-performance NRR systems utilize aqueous electrolytes. Catalyst and electrode designs that maintain efficiency while minimizing water requirements or enabling the use of non-potable water sources enhance overall sustainability. Similarly, systems designed to prevent or capture nitrogen-containing byproducts help mitigate potential contributions to water eutrophication.
End-of-life considerations for catalysts and electrodes are increasingly important in sustainability assessments. Designs that facilitate material recovery and recycling, particularly for precious metal components, significantly improve life-cycle environmental performance. Recent advances in recoverable catalyst architectures and electrode designs with separable components represent promising developments in this area.
When evaluating the sustainability of catalyst materials for NRR, life cycle assessment (LCA) reveals important considerations. Noble metal catalysts, while often demonstrating superior performance in reducing overpotential, present sustainability challenges due to their scarcity, energy-intensive mining processes, and significant environmental impacts during extraction. Earth-abundant alternatives such as transition metal nitrides, carbides, and single-atom catalysts represent more sustainable options, though their performance optimization remains an ongoing challenge.
Electrode engineering approaches that minimize material waste through precise deposition techniques contribute positively to sustainability profiles. Techniques such as atomic layer deposition and controlled electrodeposition enable significant reductions in precious metal loading while maintaining catalytic performance, thereby improving resource efficiency. Additionally, the development of self-supporting electrodes eliminates the need for separate substrates, further reducing material requirements.
Energy efficiency improvements resulting from reduced overpotential translate directly into lower carbon emissions when powered by conventional energy sources. More importantly, lower overpotential NRR systems become increasingly compatible with renewable energy integration, potentially enabling distributed, carbon-neutral ammonia production powered by intermittent renewable sources like solar and wind.
Water consumption represents another critical environmental consideration, particularly as many high-performance NRR systems utilize aqueous electrolytes. Catalyst and electrode designs that maintain efficiency while minimizing water requirements or enabling the use of non-potable water sources enhance overall sustainability. Similarly, systems designed to prevent or capture nitrogen-containing byproducts help mitigate potential contributions to water eutrophication.
End-of-life considerations for catalysts and electrodes are increasingly important in sustainability assessments. Designs that facilitate material recovery and recycling, particularly for precious metal components, significantly improve life-cycle environmental performance. Recent advances in recoverable catalyst architectures and electrode designs with separable components represent promising developments in this area.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!