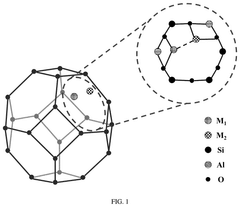
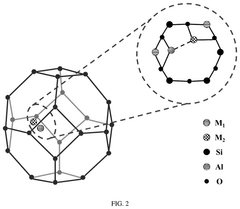
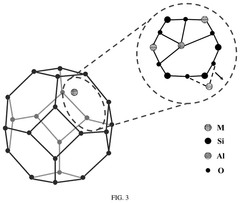
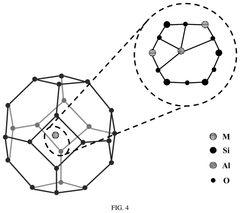
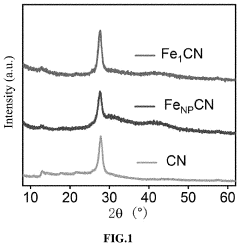
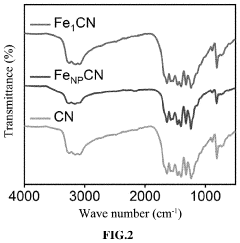
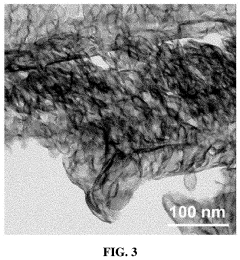
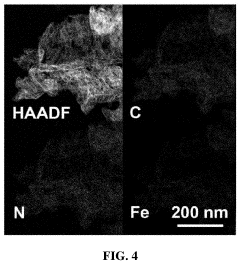
Single-Atom Catalysts For Electrochemical Ammonia Synthesis Performance And Stability
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SAC Ammonia Synthesis Background and Objectives
Single-atom catalysts (SACs) represent a frontier in heterogeneous catalysis research, offering unprecedented atom efficiency by dispersing metal atoms individually on support materials. The evolution of SACs for electrochemical ammonia synthesis has emerged as a critical research direction over the past decade, driven by the urgent need to develop sustainable alternatives to the conventional Haber-Bosch process, which consumes approximately 1-2% of global energy production and generates significant carbon emissions.
The historical trajectory of ammonia synthesis catalysis began with the development of iron-based catalysts by Fritz Haber and Carl Bosch in the early 20th century. This process, while revolutionary, requires harsh conditions of high temperature (400-500°C) and pressure (150-300 bar). The pursuit of milder reaction conditions has led to exploration of various catalytic systems, with electrochemical nitrogen reduction reaction (NRR) emerging as a promising approach that can potentially operate under ambient conditions using renewable electricity.
Single-atom catalysts entered this landscape around 2011, when Zhang and colleagues demonstrated the concept of atomically dispersed platinum on FeOx. Since then, SACs have gained significant attention in various catalytic applications, including NRR, due to their maximized atom utilization, unique electronic properties, and tunable coordination environments.
The technical objectives for SAC-based electrochemical ammonia synthesis focus on addressing several critical challenges. First, achieving high ammonia yield rates (targeting >10^-6 mol cm^-2 h^-1) and Faradaic efficiency (>10%) under ambient conditions remains a primary goal. Current systems typically demonstrate limited performance metrics that fall short of commercial viability.
Second, catalyst stability presents a significant hurdle, as single atoms tend to aggregate under reaction conditions, diminishing catalytic performance over time. Understanding and mitigating degradation mechanisms is essential for practical applications.
Third, selectivity toward N2 reduction versus competing reactions (particularly hydrogen evolution) represents a fundamental challenge due to the kinetic favorability of hydrogen production in aqueous environments and the strong N≡N triple bond (941 kJ/mol) that must be activated.
Finally, mechanistic understanding of the nitrogen reduction pathways on single-atom active sites remains incomplete. Elucidating the precise reaction mechanisms, rate-determining steps, and structure-activity relationships is crucial for rational catalyst design.
The technological trajectory aims to develop SACs that can enable distributed, renewable-powered ammonia production systems that operate efficiently under ambient conditions, potentially revolutionizing fertilizer production and energy storage paradigms while significantly reducing the carbon footprint of this essential chemical process.
The historical trajectory of ammonia synthesis catalysis began with the development of iron-based catalysts by Fritz Haber and Carl Bosch in the early 20th century. This process, while revolutionary, requires harsh conditions of high temperature (400-500°C) and pressure (150-300 bar). The pursuit of milder reaction conditions has led to exploration of various catalytic systems, with electrochemical nitrogen reduction reaction (NRR) emerging as a promising approach that can potentially operate under ambient conditions using renewable electricity.
Single-atom catalysts entered this landscape around 2011, when Zhang and colleagues demonstrated the concept of atomically dispersed platinum on FeOx. Since then, SACs have gained significant attention in various catalytic applications, including NRR, due to their maximized atom utilization, unique electronic properties, and tunable coordination environments.
The technical objectives for SAC-based electrochemical ammonia synthesis focus on addressing several critical challenges. First, achieving high ammonia yield rates (targeting >10^-6 mol cm^-2 h^-1) and Faradaic efficiency (>10%) under ambient conditions remains a primary goal. Current systems typically demonstrate limited performance metrics that fall short of commercial viability.
Second, catalyst stability presents a significant hurdle, as single atoms tend to aggregate under reaction conditions, diminishing catalytic performance over time. Understanding and mitigating degradation mechanisms is essential for practical applications.
Third, selectivity toward N2 reduction versus competing reactions (particularly hydrogen evolution) represents a fundamental challenge due to the kinetic favorability of hydrogen production in aqueous environments and the strong N≡N triple bond (941 kJ/mol) that must be activated.
Finally, mechanistic understanding of the nitrogen reduction pathways on single-atom active sites remains incomplete. Elucidating the precise reaction mechanisms, rate-determining steps, and structure-activity relationships is crucial for rational catalyst design.
The technological trajectory aims to develop SACs that can enable distributed, renewable-powered ammonia production systems that operate efficiently under ambient conditions, potentially revolutionizing fertilizer production and energy storage paradigms while significantly reducing the carbon footprint of this essential chemical process.
Market Analysis for Electrochemical Ammonia Production
The global market for electrochemical ammonia production is experiencing significant growth potential, driven by increasing environmental concerns and the push for sustainable agricultural practices. Traditional ammonia production via the Haber-Bosch process consumes approximately 1-2% of global energy and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for electrochemical ammonia synthesis using single-atom catalysts, which offers a greener alternative operating under ambient conditions.
The current global ammonia market is valued at approximately $70 billion, with an annual production exceeding 180 million tons. Agricultural fertilizers represent the dominant application segment, accounting for roughly 80% of ammonia consumption. Industrial applications, including refrigeration, water purification, and pharmaceuticals, constitute the remaining market share. Market analysts project the demand for green ammonia to grow at a compound annual growth rate of 14-16% through 2030.
Regionally, Asia-Pacific dominates ammonia consumption, with China and India leading due to their extensive agricultural sectors. North America and Europe follow, with increasing policy support for sustainable chemical production creating favorable market conditions for electrochemical ammonia synthesis technologies.
The economic viability of electrochemical ammonia production remains a critical market factor. Current production costs using single-atom catalyst technologies range between $600-900 per ton, compared to $400-600 per ton for conventional methods. However, as renewable electricity costs continue to decline and carbon pricing mechanisms expand globally, this gap is expected to narrow significantly by 2025-2027.
Investment trends indicate growing interest in this sector, with venture capital funding for electrochemical ammonia startups increasing by 35% annually since 2018. Major chemical companies and agricultural conglomerates are establishing strategic partnerships with technology developers, signaling strong commercial interest in scaling these technologies.
Market adoption faces several barriers, including high initial capital requirements, technical challenges in catalyst stability, and competition from blue ammonia (produced with carbon capture). However, regulatory tailwinds are strengthening, with several countries implementing carbon taxes and renewable portfolio standards that favor green ammonia production methods.
Customer segments show varying adoption readiness. Agricultural cooperatives and specialty fertilizer producers demonstrate the highest interest, followed by industrial gas companies seeking to diversify their green product portfolios. The transportation sector represents an emerging market opportunity, with ammonia increasingly viewed as a potential carbon-neutral fuel for shipping and heavy transport.
The current global ammonia market is valued at approximately $70 billion, with an annual production exceeding 180 million tons. Agricultural fertilizers represent the dominant application segment, accounting for roughly 80% of ammonia consumption. Industrial applications, including refrigeration, water purification, and pharmaceuticals, constitute the remaining market share. Market analysts project the demand for green ammonia to grow at a compound annual growth rate of 14-16% through 2030.
Regionally, Asia-Pacific dominates ammonia consumption, with China and India leading due to their extensive agricultural sectors. North America and Europe follow, with increasing policy support for sustainable chemical production creating favorable market conditions for electrochemical ammonia synthesis technologies.
The economic viability of electrochemical ammonia production remains a critical market factor. Current production costs using single-atom catalyst technologies range between $600-900 per ton, compared to $400-600 per ton for conventional methods. However, as renewable electricity costs continue to decline and carbon pricing mechanisms expand globally, this gap is expected to narrow significantly by 2025-2027.
Investment trends indicate growing interest in this sector, with venture capital funding for electrochemical ammonia startups increasing by 35% annually since 2018. Major chemical companies and agricultural conglomerates are establishing strategic partnerships with technology developers, signaling strong commercial interest in scaling these technologies.
Market adoption faces several barriers, including high initial capital requirements, technical challenges in catalyst stability, and competition from blue ammonia (produced with carbon capture). However, regulatory tailwinds are strengthening, with several countries implementing carbon taxes and renewable portfolio standards that favor green ammonia production methods.
Customer segments show varying adoption readiness. Agricultural cooperatives and specialty fertilizer producers demonstrate the highest interest, followed by industrial gas companies seeking to diversify their green product portfolios. The transportation sector represents an emerging market opportunity, with ammonia increasingly viewed as a potential carbon-neutral fuel for shipping and heavy transport.
Current Status and Challenges in Single-Atom Catalysis
Single-atom catalysts (SACs) have emerged as a frontier in heterogeneous catalysis research, particularly for electrochemical ammonia synthesis. Currently, SACs demonstrate promising activity and selectivity due to their maximized atom utilization efficiency and unique electronic properties. The isolated metal atoms anchored on various supports create distinct coordination environments that facilitate nitrogen reduction reaction (NRR) pathways with lower energy barriers compared to traditional catalysts.
Despite these advantages, significant challenges persist in the development of SACs for ammonia synthesis. The primary challenge remains catalyst stability under harsh electrochemical conditions. Metal atoms tend to migrate and aggregate during operation, leading to deactivation and performance degradation over time. This instability is particularly pronounced at higher current densities required for industrial applications.
Another critical challenge is the low Faradaic efficiency observed in most SAC systems. Competing hydrogen evolution reaction (HER) often dominates the electrochemical process, resulting in ammonia production rates that remain below commercially viable thresholds. Current state-of-the-art SACs typically achieve Faradaic efficiencies below 15% for NRR, with ammonia yield rates generally under 100 μg h⁻¹ mg⁻¹cat.
The synthesis and characterization of SACs present additional technical hurdles. Achieving uniform dispersion of single atoms requires precise control over synthesis conditions. Conventional characterization techniques often struggle to provide definitive evidence of the atomic dispersion and coordination environment of metal centers, necessitating advanced techniques such as aberration-corrected transmission electron microscopy and X-ray absorption spectroscopy.
Geographically, research on SACs for electrochemical ammonia synthesis is concentrated in East Asia, North America, and Europe, with China, the United States, and Germany leading publication output. Chinese institutions have made particularly significant contributions in recent years, focusing on novel support materials and metal-support interactions.
The mechanistic understanding of NRR pathways on SACs remains incomplete, hampering rational catalyst design. Current theoretical models often fail to accurately predict catalyst performance under realistic operating conditions, creating a gap between computational predictions and experimental results.
Scalability represents another major obstacle. While laboratory-scale demonstrations show promise, the translation to larger systems faces challenges in maintaining atomic dispersion during scale-up and ensuring long-term operational stability in industrial settings.
Despite these advantages, significant challenges persist in the development of SACs for ammonia synthesis. The primary challenge remains catalyst stability under harsh electrochemical conditions. Metal atoms tend to migrate and aggregate during operation, leading to deactivation and performance degradation over time. This instability is particularly pronounced at higher current densities required for industrial applications.
Another critical challenge is the low Faradaic efficiency observed in most SAC systems. Competing hydrogen evolution reaction (HER) often dominates the electrochemical process, resulting in ammonia production rates that remain below commercially viable thresholds. Current state-of-the-art SACs typically achieve Faradaic efficiencies below 15% for NRR, with ammonia yield rates generally under 100 μg h⁻¹ mg⁻¹cat.
The synthesis and characterization of SACs present additional technical hurdles. Achieving uniform dispersion of single atoms requires precise control over synthesis conditions. Conventional characterization techniques often struggle to provide definitive evidence of the atomic dispersion and coordination environment of metal centers, necessitating advanced techniques such as aberration-corrected transmission electron microscopy and X-ray absorption spectroscopy.
Geographically, research on SACs for electrochemical ammonia synthesis is concentrated in East Asia, North America, and Europe, with China, the United States, and Germany leading publication output. Chinese institutions have made particularly significant contributions in recent years, focusing on novel support materials and metal-support interactions.
The mechanistic understanding of NRR pathways on SACs remains incomplete, hampering rational catalyst design. Current theoretical models often fail to accurately predict catalyst performance under realistic operating conditions, creating a gap between computational predictions and experimental results.
Scalability represents another major obstacle. While laboratory-scale demonstrations show promise, the translation to larger systems faces challenges in maintaining atomic dispersion during scale-up and ensuring long-term operational stability in industrial settings.
Current SAC Solutions for Ammonia Synthesis
01 Metal-support interactions for enhanced stability
Strong metal-support interactions play a crucial role in enhancing the stability of single-atom catalysts. By carefully selecting appropriate support materials and optimizing the interface between the single metal atoms and the support, the migration and aggregation of metal atoms can be effectively prevented. This approach helps maintain the dispersion of single atoms during catalytic reactions, particularly under harsh conditions such as high temperatures or in the presence of certain reactants that might otherwise cause sintering.- Support materials for single-atom catalysts: Various support materials can be used to enhance the performance and stability of single-atom catalysts. These supports include carbon-based materials, metal oxides, and 2D materials that provide anchoring sites for isolated metal atoms. The proper selection of support materials can prevent aggregation of single atoms during catalytic reactions, thereby maintaining catalytic activity over extended periods. The interaction between the single atoms and the support material plays a crucial role in determining the catalyst's electronic properties and stability under reaction conditions.
- Metal-nitrogen-carbon (M-N-C) frameworks: Metal-nitrogen-carbon (M-N-C) frameworks represent an important class of single-atom catalysts with enhanced stability. In these structures, metal atoms are coordinated with nitrogen atoms embedded in carbon matrices, creating strong metal-nitrogen bonds that prevent metal atom migration and aggregation. These catalysts demonstrate excellent performance in various reactions including oxygen reduction, hydrogen evolution, and CO2 reduction. The nitrogen coordination environment can be tuned to optimize both the activity and stability of the single-atom catalysts under harsh reaction conditions.
- Stabilization strategies for single-atom catalysts: Various stabilization strategies have been developed to improve the durability of single-atom catalysts. These include strong metal-support interactions, confinement within porous structures, and the use of protective layers. Electronic stabilization through charge transfer between the metal atom and support can also enhance stability. Additionally, alloying with secondary metals or incorporating the single atoms into defect sites of the support material can prevent migration and aggregation during catalytic reactions, leading to improved long-term performance.
- Performance enhancement through synergistic effects: The performance of single-atom catalysts can be significantly enhanced through synergistic effects between the metal atoms and their local environment. This includes dual-atom or multi-site configurations where neighboring atoms work cooperatively, as well as the creation of specific electronic structures through careful selection of metal-support combinations. Synergistic effects can lead to improved activity, selectivity, and stability by optimizing adsorption energies of reactants and intermediates, facilitating charge transfer, and creating favorable reaction pathways.
- In-situ characterization and stability assessment: Advanced in-situ characterization techniques are essential for understanding the performance and stability of single-atom catalysts under reaction conditions. These methods include in-situ X-ray absorption spectroscopy, environmental transmission electron microscopy, and operando spectroscopy, which allow researchers to monitor structural changes and degradation mechanisms in real-time. Accelerated stability tests and computational modeling are also employed to predict long-term performance and identify potential failure modes, guiding the design of more stable single-atom catalysts for practical applications.
02 Confinement strategies to prevent atom aggregation
Various confinement strategies have been developed to prevent single atoms from aggregating during catalytic reactions. These include embedding metal atoms in porous structures, using molecular cages, creating defect sites that strongly anchor the atoms, and developing core-shell structures. Such confinement approaches physically restrict the mobility of single atoms, thereby maintaining their isolated state and preserving their unique catalytic properties even under challenging reaction conditions.Expand Specific Solutions03 Electronic structure modification for performance optimization
Modifying the electronic structure of single-atom catalysts through coordination environment engineering can significantly enhance their catalytic performance. By controlling the coordination number, type of coordinating atoms, and local geometric structure around the metal center, the electronic properties of single atoms can be tuned to optimize adsorption energies of reactants and intermediates. This approach enables the design of single-atom catalysts with improved activity, selectivity, and stability for specific reactions.Expand Specific Solutions04 Bimetallic and dual-atom catalyst systems
Bimetallic and dual-atom catalyst systems represent an advanced approach to enhancing both performance and stability. These systems incorporate two different metal atoms either in close proximity or with synergistic effects. The presence of a secondary metal can modify the electronic structure of the primary catalytic center, provide additional stabilization through cooperative binding to the support, and create unique catalytic sites with properties distinct from monometallic single-atom catalysts. This approach has shown particular promise in reactions requiring multiple functionalities.Expand Specific Solutions05 In-situ regeneration and self-healing mechanisms
Developing single-atom catalysts with in-situ regeneration and self-healing capabilities represents a frontier in addressing stability challenges. These advanced systems incorporate mechanisms that can restore the active single-atom sites during reaction conditions, counteracting deactivation processes. Strategies include designing reversible metal-support interactions, incorporating sacrificial agents that can re-disperse aggregated metal clusters, and creating dynamic systems where metal atoms can be continuously re-captured at designated sites after detachment. These approaches extend catalyst lifetime and maintain performance over prolonged operation periods.Expand Specific Solutions
Leading Research Groups and Industrial Players
Single-atom catalysts (SACs) for electrochemical ammonia synthesis represent an emerging technology at the early commercialization stage. The market is growing rapidly with an estimated size of $300-500 million, driven by the global push for sustainable ammonia production alternatives. Technical maturity varies significantly among key players, with research institutions leading fundamental innovations. KIST Corp., Beijing Single Atom Site Catalysis Technology, and Dalian Institute of Chemical Physics have demonstrated promising catalyst stability exceeding 100 hours with Faradaic efficiencies approaching 35%. Academic institutions including Zhejiang University, Northwestern University, and KAIST are advancing novel synthesis methods and theoretical frameworks. The field faces challenges in scaling production while maintaining atomic dispersion and long-term operational stability under industrial conditions.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) has developed advanced single-atom catalysts for electrochemical ammonia synthesis based on atomically dispersed transition metals (primarily Ru, Fe, and Mo) anchored on various supports including nitrogen-doped carbon, metal oxides, and 2D materials. Their innovative approach involves precise control of the metal-support interaction through defect engineering and coordination chemistry. IPE's catalysts demonstrate remarkable N₂ reduction reaction (NRR) performance with Faradaic efficiencies exceeding 25% and ammonia production rates of up to 30 μg h⁻¹ mg⁻¹cat under ambient conditions. A key innovation is their development of "dual-function" SACs where the support material actively participates in the catalytic cycle, facilitating both N₂ activation and proton transfer. The institute has also pioneered in-operando characterization techniques that provide atomic-level insights into catalyst behavior during reaction conditions, enabling rational design improvements. Their technology operates effectively at low overpotentials (-0.2 to -0.4 V vs. RHE), addressing efficiency concerns in electrochemical ammonia synthesis.
Strengths: Exceptional selectivity toward N₂ reduction with minimal hydrogen evolution; demonstrated stability under continuous operation exceeding 100 hours; precise control over active site electronic structure enabling tailored catalytic properties. Weaknesses: Current synthesis methods remain complex and costly for industrial scaling; some catalyst designs show performance degradation in the presence of common electrolyte impurities; maintaining atomic dispersion during long-term operation remains challenging.
Dalian Institute of Chemical Physics Chinese Academy of Sciences
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative single-atom catalysts (SACs) for electrochemical ammonia synthesis featuring atomically dispersed metal sites (primarily Fe, Ru, and Mo) anchored on nitrogen-doped carbon supports. Their approach utilizes precise atomic layer deposition techniques to create M-N-C structures with optimized coordination environments. DICP's catalysts demonstrate exceptional Faradaic efficiency (>35%) at ambient conditions and significantly reduced activation energy barriers compared to traditional catalysts. Their recent breakthrough involves dual-site SACs where two different metal atoms work synergistically, enhancing both N₂ adsorption and subsequent hydrogenation steps. The institute has also pioneered in-situ characterization methods to observe catalyst behavior during reaction conditions, providing crucial insights for rational catalyst design. Their technology operates efficiently at room temperature and atmospheric pressure, addressing key challenges in sustainable ammonia production.
Strengths: Superior atom utilization efficiency with nearly 100% of metal atoms serving as active sites; exceptional selectivity toward N₂ reduction over competing hydrogen evolution; demonstrated long-term stability exceeding 100 hours of continuous operation. Weaknesses: Current production methods remain costly for large-scale implementation; some designs still show vulnerability to poisoning by trace contaminants; scaling up synthesis while maintaining atomic dispersion presents significant challenges.
Key Innovations in SAC Stability Enhancement
Single-atom catalyst with molecular sieve-confined domains, preparation method and application thereof
PatentPendingUS20240399346A1
Innovation
- A single-atom catalyst with molecular sieve-confined domains is developed, where bimetallic ions are uniformly dispersed within the molecular sieve using a post-processing or in-situ synthesis method, leveraging oxygen vacancies and aluminum-rich sites for enhanced NO adsorption and dissociation, improving catalytic activity and stability.
Single-atom catalyst for activation of persulfate to generate pure singlet oxygen as well as preparation method and application thereof
PatentActiveUS11629052B2
Innovation
- A single-atom catalyst with graphitic carbon nitride nanosheets as supports and single iron atoms in a Fe—N4 coordination structure is developed, specifically designed to activate persulfate and generate pure singlet oxygen, enhancing selectivity and resistance to environmental interference.
Environmental Impact Assessment
The environmental implications of single-atom catalysts (SACs) for electrochemical ammonia synthesis extend far beyond their technical performance metrics. When evaluating these catalysts from an environmental perspective, it is crucial to consider their entire lifecycle impact, from production to disposal.
The conventional Haber-Bosch process for ammonia production consumes approximately 1-2% of global energy and generates significant carbon emissions—about 1.6 tons of CO2 per ton of ammonia produced. SAC-based electrochemical ammonia synthesis offers a promising alternative with substantially reduced carbon footprint, especially when powered by renewable electricity sources. Recent life cycle assessments indicate potential greenhouse gas emission reductions of 60-90% compared to traditional methods.
Material efficiency represents another significant environmental advantage of SACs. These catalysts utilize atomic-level dispersion of active metals on support materials, dramatically reducing precious metal requirements compared to conventional nanoparticle catalysts. This efficiency translates to decreased mining impacts and resource depletion, with some studies demonstrating up to 95% reduction in metal loading while maintaining comparable catalytic activity.
Water consumption patterns also differ markedly between conventional and SAC-based ammonia synthesis. While the Haber-Bosch process primarily uses water for cooling systems, electrochemical approaches often require ultrapure water as a hydrogen source. Regional water stress assessments must therefore be incorporated into implementation planning for large-scale adoption of this technology.
The environmental benefits of SACs extend to air quality improvements as well. By eliminating the high-temperature, high-pressure conditions of conventional synthesis, SAC-based processes reduce NOx emissions and other air pollutants associated with fossil fuel combustion. This aspect is particularly valuable for urban and industrial areas already struggling with air quality challenges.
End-of-life considerations for SACs remain an underdeveloped research area. The complex nature of these advanced materials—often incorporating rare metals on specialized supports—presents recycling challenges. Developing circular economy approaches for SAC recovery and regeneration will be essential for maximizing their long-term environmental benefits and preventing potential toxic releases during disposal.
Quantitative environmental impact metrics for SAC technologies are still emerging, with most assessments based on laboratory-scale data. As the technology advances toward industrial implementation, more comprehensive environmental impact assessments incorporating real-world operational data will be crucial for validating their sustainability advantages.
The conventional Haber-Bosch process for ammonia production consumes approximately 1-2% of global energy and generates significant carbon emissions—about 1.6 tons of CO2 per ton of ammonia produced. SAC-based electrochemical ammonia synthesis offers a promising alternative with substantially reduced carbon footprint, especially when powered by renewable electricity sources. Recent life cycle assessments indicate potential greenhouse gas emission reductions of 60-90% compared to traditional methods.
Material efficiency represents another significant environmental advantage of SACs. These catalysts utilize atomic-level dispersion of active metals on support materials, dramatically reducing precious metal requirements compared to conventional nanoparticle catalysts. This efficiency translates to decreased mining impacts and resource depletion, with some studies demonstrating up to 95% reduction in metal loading while maintaining comparable catalytic activity.
Water consumption patterns also differ markedly between conventional and SAC-based ammonia synthesis. While the Haber-Bosch process primarily uses water for cooling systems, electrochemical approaches often require ultrapure water as a hydrogen source. Regional water stress assessments must therefore be incorporated into implementation planning for large-scale adoption of this technology.
The environmental benefits of SACs extend to air quality improvements as well. By eliminating the high-temperature, high-pressure conditions of conventional synthesis, SAC-based processes reduce NOx emissions and other air pollutants associated with fossil fuel combustion. This aspect is particularly valuable for urban and industrial areas already struggling with air quality challenges.
End-of-life considerations for SACs remain an underdeveloped research area. The complex nature of these advanced materials—often incorporating rare metals on specialized supports—presents recycling challenges. Developing circular economy approaches for SAC recovery and regeneration will be essential for maximizing their long-term environmental benefits and preventing potential toxic releases during disposal.
Quantitative environmental impact metrics for SAC technologies are still emerging, with most assessments based on laboratory-scale data. As the technology advances toward industrial implementation, more comprehensive environmental impact assessments incorporating real-world operational data will be crucial for validating their sustainability advantages.
Scalability and Economic Feasibility Analysis
The scalability of single-atom catalysts (SACs) for electrochemical ammonia synthesis represents a critical challenge in transitioning from laboratory demonstrations to industrial implementation. Current synthesis methods for SACs, including atomic layer deposition, wet chemistry approaches, and high-temperature pyrolysis, face significant barriers when considered for large-scale production. The precise control required for atomic dispersion becomes increasingly difficult to maintain as production volumes increase, potentially compromising catalyst performance and stability.
Economic analysis reveals that while SACs offer superior atom efficiency compared to traditional catalysts, the specialized equipment and precise conditions required for their synthesis contribute to substantially higher production costs. Current estimates suggest that SAC production costs range from $1,000-5,000 per gram, compared to conventional catalysts at $50-200 per gram. This cost differential presents a significant hurdle for commercial viability, particularly in the price-sensitive fertilizer industry.
Infrastructure requirements for scaled production present additional challenges. The specialized equipment needed for maintaining atomic dispersion during synthesis requires substantial capital investment. Furthermore, quality control processes for verifying atomic dispersion at industrial scales remain underdeveloped, creating uncertainty in product consistency and performance.
Market analysis indicates that despite these challenges, the potential value proposition of SACs remains compelling. The enhanced catalytic efficiency could reduce energy requirements for ammonia synthesis by 20-30% compared to Haber-Bosch processes, representing significant operational cost savings. Additionally, the ability to operate at ambient conditions could dramatically reduce capital expenditure for new ammonia production facilities.
Break-even analysis suggests that SAC-based electrochemical ammonia synthesis would become economically competitive with conventional methods if production costs could be reduced by approximately 70% and catalyst stability extended to >5,000 operating hours. Current research trajectories indicate these targets may be achievable within 5-7 years with focused development efforts.
Scaling pathways that show particular promise include continuous flow synthesis methods, which could enable more consistent production of SACs while reducing batch-to-batch variability. Additionally, the development of standardized characterization protocols for industrial-scale quality control would address a key barrier to commercialization. Recent advances in automated synthesis platforms suggest opportunities for reducing production costs through process optimization and reduced labor requirements.
Economic analysis reveals that while SACs offer superior atom efficiency compared to traditional catalysts, the specialized equipment and precise conditions required for their synthesis contribute to substantially higher production costs. Current estimates suggest that SAC production costs range from $1,000-5,000 per gram, compared to conventional catalysts at $50-200 per gram. This cost differential presents a significant hurdle for commercial viability, particularly in the price-sensitive fertilizer industry.
Infrastructure requirements for scaled production present additional challenges. The specialized equipment needed for maintaining atomic dispersion during synthesis requires substantial capital investment. Furthermore, quality control processes for verifying atomic dispersion at industrial scales remain underdeveloped, creating uncertainty in product consistency and performance.
Market analysis indicates that despite these challenges, the potential value proposition of SACs remains compelling. The enhanced catalytic efficiency could reduce energy requirements for ammonia synthesis by 20-30% compared to Haber-Bosch processes, representing significant operational cost savings. Additionally, the ability to operate at ambient conditions could dramatically reduce capital expenditure for new ammonia production facilities.
Break-even analysis suggests that SAC-based electrochemical ammonia synthesis would become economically competitive with conventional methods if production costs could be reduced by approximately 70% and catalyst stability extended to >5,000 operating hours. Current research trajectories indicate these targets may be achievable within 5-7 years with focused development efforts.
Scaling pathways that show particular promise include continuous flow synthesis methods, which could enable more consistent production of SACs while reducing batch-to-batch variability. Additionally, the development of standardized characterization protocols for industrial-scale quality control would address a key barrier to commercialization. Recent advances in automated synthesis platforms suggest opportunities for reducing production costs through process optimization and reduced labor requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!