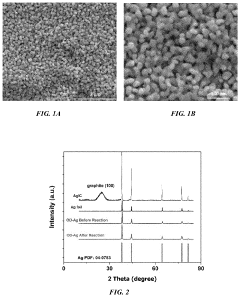
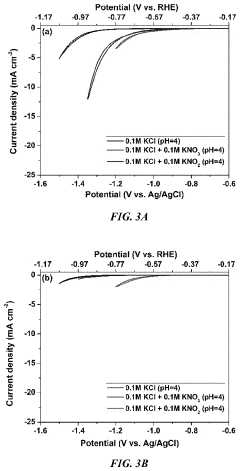
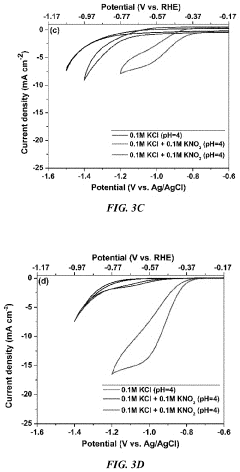
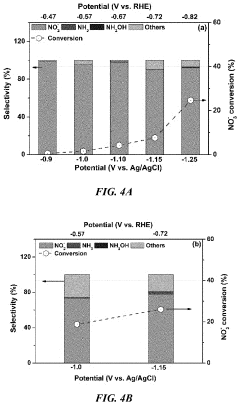
Role Of Electrolyte pH In Selectivity Of Electrocatalytic Nitrogen Reduction
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalytic NRR pH Influence Background and Objectives
Electrocatalytic nitrogen reduction reaction (NRR) has emerged as a promising alternative to the conventional Haber-Bosch process for ammonia synthesis, offering potential advantages in terms of energy efficiency, operational flexibility, and environmental impact. The evolution of this technology can be traced back to early electrochemical studies in the 20th century, but significant advancements have only materialized in the past decade with the development of novel catalysts and improved understanding of reaction mechanisms.
The pH of the electrolyte has been identified as a critical parameter that significantly influences the selectivity and efficiency of the NRR process. Historical data indicates that early research predominantly focused on catalyst materials and applied potentials, with limited attention to electrolyte properties. However, recent studies have revealed that pH conditions can dramatically alter reaction pathways, competing reactions, and ultimately the Faradaic efficiency toward ammonia production.
The technological trajectory in this field shows an increasing emphasis on understanding the complex interplay between catalyst surface properties and electrolyte characteristics. This shift represents a more holistic approach to catalyst design, moving beyond material composition alone to consider the entire electrochemical interface. Current research trends suggest that optimizing the local pH environment near the catalyst surface could be as important as the catalyst material itself.
The primary technical objective of investigating electrolyte pH effects on NRR selectivity is to overcome the persistent challenge of low Faradaic efficiency, which has been a major barrier to practical implementation. Specifically, the competing hydrogen evolution reaction (HER) often dominates under many experimental conditions, resulting in ammonia yields that are too low for commercial viability.
Secondary objectives include developing fundamental insights into how proton availability and transfer kinetics at different pH values affect the rate-determining steps in the NRR mechanism. This knowledge is essential for rational catalyst design and process optimization. Additionally, there is growing interest in understanding how pH gradients form near the electrode surface during operation and how these microenvironments differ from bulk electrolyte conditions.
From a broader perspective, this research aims to establish design principles that can guide the development of next-generation electrocatalysts with enhanced selectivity toward nitrogen reduction. The ultimate goal is to achieve Faradaic efficiencies exceeding 50% at industrially relevant current densities, which would represent a significant milestone toward practical electrochemical ammonia synthesis.
Understanding the role of electrolyte pH also intersects with sustainability objectives, as optimized pH conditions may reduce energy requirements and enable the use of more abundant catalyst materials, thereby improving the overall economic and environmental profile of electrochemical ammonia production.
The pH of the electrolyte has been identified as a critical parameter that significantly influences the selectivity and efficiency of the NRR process. Historical data indicates that early research predominantly focused on catalyst materials and applied potentials, with limited attention to electrolyte properties. However, recent studies have revealed that pH conditions can dramatically alter reaction pathways, competing reactions, and ultimately the Faradaic efficiency toward ammonia production.
The technological trajectory in this field shows an increasing emphasis on understanding the complex interplay between catalyst surface properties and electrolyte characteristics. This shift represents a more holistic approach to catalyst design, moving beyond material composition alone to consider the entire electrochemical interface. Current research trends suggest that optimizing the local pH environment near the catalyst surface could be as important as the catalyst material itself.
The primary technical objective of investigating electrolyte pH effects on NRR selectivity is to overcome the persistent challenge of low Faradaic efficiency, which has been a major barrier to practical implementation. Specifically, the competing hydrogen evolution reaction (HER) often dominates under many experimental conditions, resulting in ammonia yields that are too low for commercial viability.
Secondary objectives include developing fundamental insights into how proton availability and transfer kinetics at different pH values affect the rate-determining steps in the NRR mechanism. This knowledge is essential for rational catalyst design and process optimization. Additionally, there is growing interest in understanding how pH gradients form near the electrode surface during operation and how these microenvironments differ from bulk electrolyte conditions.
From a broader perspective, this research aims to establish design principles that can guide the development of next-generation electrocatalysts with enhanced selectivity toward nitrogen reduction. The ultimate goal is to achieve Faradaic efficiencies exceeding 50% at industrially relevant current densities, which would represent a significant milestone toward practical electrochemical ammonia synthesis.
Understanding the role of electrolyte pH also intersects with sustainability objectives, as optimized pH conditions may reduce energy requirements and enable the use of more abundant catalyst materials, thereby improving the overall economic and environmental profile of electrochemical ammonia production.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns, with electrocatalytic nitrogen reduction (NRR) emerging as a promising alternative to the traditional Haber-Bosch process. The current ammonia market, valued at approximately $72 billion in 2022, is projected to reach $110 billion by 2030, growing at a CAGR of 5.3%. This growth is primarily fueled by increasing demand for fertilizers, which account for nearly 80% of ammonia consumption worldwide.
Traditional ammonia production via the Haber-Bosch process consumes about 1-2% of global energy and generates substantial CO2 emissions—approximately 1.8 tons of CO2 per ton of ammonia produced. This environmental footprint has created a substantial market opportunity for sustainable alternatives, particularly electrochemical nitrogen reduction processes where pH control plays a crucial role in selectivity and efficiency.
The market for green ammonia production technologies is expected to grow exponentially, with investments in electrochemical ammonia synthesis projects increasing by over 200% in the past three years. Major agricultural regions including North America, Europe, and Asia-Pacific represent the largest potential markets for sustainable ammonia production technologies, with China alone consuming over 30% of global ammonia production.
Industrial stakeholders are increasingly recognizing the economic potential of pH-optimized electrocatalytic systems. Recent market analyses indicate that achieving even a 10% improvement in NRR selectivity through optimal pH control could reduce production costs by 15-20%, potentially creating a competitive advantage against conventional methods when coupled with renewable energy sources.
Government policies and environmental regulations are accelerating market adoption of sustainable ammonia production technologies. The European Union's carbon border adjustment mechanism, carbon pricing initiatives, and green hydrogen strategies in various countries are creating favorable market conditions for electrochemical ammonia synthesis technologies that demonstrate high selectivity through pH optimization.
Venture capital funding for startups focusing on electrocatalytic nitrogen reduction has reached $450 million in 2022, a threefold increase from 2019. Companies demonstrating superior control over reaction selectivity through electrolyte engineering are attracting premium valuations, highlighting the market's recognition of pH control as a critical factor in commercialization potential.
The market for specialized electrolytes and pH control systems for nitrogen reduction is emerging as a distinct segment, with specialized chemical suppliers developing tailored solutions for different catalyst systems. This ancillary market is projected to reach $300 million by 2025, representing a new value chain opportunity within the broader sustainable ammonia production landscape.
Traditional ammonia production via the Haber-Bosch process consumes about 1-2% of global energy and generates substantial CO2 emissions—approximately 1.8 tons of CO2 per ton of ammonia produced. This environmental footprint has created a substantial market opportunity for sustainable alternatives, particularly electrochemical nitrogen reduction processes where pH control plays a crucial role in selectivity and efficiency.
The market for green ammonia production technologies is expected to grow exponentially, with investments in electrochemical ammonia synthesis projects increasing by over 200% in the past three years. Major agricultural regions including North America, Europe, and Asia-Pacific represent the largest potential markets for sustainable ammonia production technologies, with China alone consuming over 30% of global ammonia production.
Industrial stakeholders are increasingly recognizing the economic potential of pH-optimized electrocatalytic systems. Recent market analyses indicate that achieving even a 10% improvement in NRR selectivity through optimal pH control could reduce production costs by 15-20%, potentially creating a competitive advantage against conventional methods when coupled with renewable energy sources.
Government policies and environmental regulations are accelerating market adoption of sustainable ammonia production technologies. The European Union's carbon border adjustment mechanism, carbon pricing initiatives, and green hydrogen strategies in various countries are creating favorable market conditions for electrochemical ammonia synthesis technologies that demonstrate high selectivity through pH optimization.
Venture capital funding for startups focusing on electrocatalytic nitrogen reduction has reached $450 million in 2022, a threefold increase from 2019. Companies demonstrating superior control over reaction selectivity through electrolyte engineering are attracting premium valuations, highlighting the market's recognition of pH control as a critical factor in commercialization potential.
The market for specialized electrolytes and pH control systems for nitrogen reduction is emerging as a distinct segment, with specialized chemical suppliers developing tailored solutions for different catalyst systems. This ancillary market is projected to reach $300 million by 2025, representing a new value chain opportunity within the broader sustainable ammonia production landscape.
Current Challenges in Electrocatalytic Nitrogen Reduction
Electrocatalytic nitrogen reduction reaction (NRR) faces significant challenges that impede its practical implementation for sustainable ammonia production. The primary obstacle remains the low Faradaic efficiency, typically below 15% in ambient conditions, which severely limits industrial viability. This inefficiency stems from the competing hydrogen evolution reaction (HER), which is thermodynamically more favorable and kinetically faster than NRR, especially in aqueous electrolytes.
The selectivity challenge is further complicated by the strong triple bond of N₂ (941 kJ/mol), requiring substantial energy input for activation. Current catalysts struggle to efficiently adsorb and activate N₂ molecules while simultaneously suppressing proton reduction to hydrogen. Even state-of-the-art catalysts demonstrate limited ability to overcome this fundamental selectivity issue.
Electrolyte pH plays a crucial role in NRR selectivity, yet its precise influence remains inadequately understood. At low pH values, the abundance of protons accelerates HER, diminishing NRR efficiency. Conversely, high pH environments can suppress HER but may introduce mass transport limitations for N₂ and alter the reaction pathway. The optimal pH window appears highly catalyst-dependent, creating significant challenges for standardized approaches.
Another critical challenge is the accurate quantification of produced ammonia. Current detection methods, including spectrophotometric techniques like the indophenol blue method, suffer from interference from catalyst leaching and electrolyte contaminants. This has led to questionable reproducibility in reported results, complicating meaningful comparison between different catalytic systems.
The stability of electrocatalysts presents another significant hurdle. Many promising materials exhibit performance degradation during extended operation due to surface poisoning, structural changes, or dissolution. This instability is often exacerbated by pH fluctuations at the electrode-electrolyte interface during reaction, creating localized conditions that may differ substantially from bulk electrolyte properties.
Energy efficiency remains suboptimal, with current systems requiring excessive overpotentials to drive NRR at appreciable rates. This high energy input contradicts the sustainability goals of electrochemical ammonia synthesis. The relationship between applied potential, electrolyte pH, and selectivity forms a complex interdependent system that has not been systematically mapped for most catalyst materials.
Scaling up laboratory results to industrially relevant current densities introduces additional challenges related to mass transport limitations, heat management, and maintaining pH stability across larger electrode surfaces. The gap between fundamental understanding at the laboratory scale and practical implementation remains substantial, particularly regarding the role of electrolyte pH in maintaining selectivity at higher production rates.
The selectivity challenge is further complicated by the strong triple bond of N₂ (941 kJ/mol), requiring substantial energy input for activation. Current catalysts struggle to efficiently adsorb and activate N₂ molecules while simultaneously suppressing proton reduction to hydrogen. Even state-of-the-art catalysts demonstrate limited ability to overcome this fundamental selectivity issue.
Electrolyte pH plays a crucial role in NRR selectivity, yet its precise influence remains inadequately understood. At low pH values, the abundance of protons accelerates HER, diminishing NRR efficiency. Conversely, high pH environments can suppress HER but may introduce mass transport limitations for N₂ and alter the reaction pathway. The optimal pH window appears highly catalyst-dependent, creating significant challenges for standardized approaches.
Another critical challenge is the accurate quantification of produced ammonia. Current detection methods, including spectrophotometric techniques like the indophenol blue method, suffer from interference from catalyst leaching and electrolyte contaminants. This has led to questionable reproducibility in reported results, complicating meaningful comparison between different catalytic systems.
The stability of electrocatalysts presents another significant hurdle. Many promising materials exhibit performance degradation during extended operation due to surface poisoning, structural changes, or dissolution. This instability is often exacerbated by pH fluctuations at the electrode-electrolyte interface during reaction, creating localized conditions that may differ substantially from bulk electrolyte properties.
Energy efficiency remains suboptimal, with current systems requiring excessive overpotentials to drive NRR at appreciable rates. This high energy input contradicts the sustainability goals of electrochemical ammonia synthesis. The relationship between applied potential, electrolyte pH, and selectivity forms a complex interdependent system that has not been systematically mapped for most catalyst materials.
Scaling up laboratory results to industrially relevant current densities introduces additional challenges related to mass transport limitations, heat management, and maintaining pH stability across larger electrode surfaces. The gap between fundamental understanding at the laboratory scale and practical implementation remains substantial, particularly regarding the role of electrolyte pH in maintaining selectivity at higher production rates.
Current pH Modulation Strategies for NRR Selectivity
01 pH-selective ion-sensitive electrodes
Ion-sensitive electrodes designed to selectively measure pH in various electrolyte solutions. These electrodes incorporate specialized membranes or sensing elements that respond specifically to hydrogen ion concentration while minimizing interference from other ions present in the electrolyte. The selectivity is achieved through careful material selection and electrode design, allowing for accurate pH measurements even in complex electrolyte environments.- pH-selective electrochemical sensors: Electrochemical sensors designed with specific pH selectivity capabilities allow for accurate measurement of pH in various environments. These sensors utilize specialized electrode materials and coatings that respond selectively to hydrogen ion concentration while minimizing interference from other ions. The technology enables precise pH monitoring in complex electrolyte solutions and can be integrated into portable devices for field applications.
- Electrolyte composition for pH-selective applications: Specialized electrolyte compositions can be formulated to enhance pH selectivity in electrochemical systems. These compositions typically include buffering agents, ionic carriers, and selective membranes that facilitate specific ion transport while maintaining stability across a wide pH range. The formulations can be tailored for different applications, from analytical chemistry to industrial process monitoring, providing improved sensitivity and reduced interference from competing ions.
- pH-selective membrane technology: Advanced membrane technologies enable highly selective pH measurements in complex electrolyte environments. These membranes incorporate ionophores, polymeric matrices, and functional groups that selectively interact with hydrogen ions while blocking other interfering species. The design of these membranes can be optimized for specific pH ranges and operating conditions, improving the performance of electrochemical sensors and analytical devices.
- Microfluidic pH-selective systems: Microfluidic platforms incorporating pH-selective elements enable precise control and measurement of electrolyte pH in miniaturized systems. These systems integrate electrodes, reference elements, and selective membranes within microchannels to create compact analytical devices. The technology allows for real-time pH monitoring with minimal sample volumes, making it suitable for biomedical applications, environmental monitoring, and lab-on-a-chip devices.
- Calibration and standardization methods for pH-selective measurements: Specialized calibration techniques and standardization protocols ensure accurate pH measurements across different electrolyte compositions. These methods account for variations in ionic strength, temperature, and interfering species that can affect pH-selective measurements. Advanced algorithms and reference systems are employed to maintain measurement accuracy and reliability in diverse applications, from laboratory analysis to industrial process control.
02 Electrolyte composition optimization for pH selectivity
Formulation of electrolyte compositions with specific additives to enhance pH selectivity in electrochemical systems. These formulations may include buffer components, ionic strength adjusters, and selective complexing agents that help maintain stable pH conditions while allowing for selective ion transport. The optimized electrolyte compositions improve the performance of pH-dependent processes and measurements by reducing interference from competing ions.Expand Specific Solutions03 pH-selective membranes for electrolyte applications
Development of specialized membrane materials that exhibit selective permeability based on pH conditions in electrolyte environments. These membranes incorporate functional groups that respond to changes in hydrogen ion concentration, allowing for controlled ion transport across the membrane barrier. The pH-selective membranes find applications in sensors, separation processes, and controlled release systems where electrolyte pH plays a critical role.Expand Specific Solutions04 Electrochemical sensors with pH-dependent selectivity
Advanced electrochemical sensing systems designed to exhibit different selectivity profiles depending on the pH of the electrolyte solution. These sensors utilize pH-responsive materials or electrode configurations that can selectively detect target analytes under specific pH conditions. The pH-dependent selectivity enables more accurate measurements in complex samples by minimizing interference from competing species through pH optimization.Expand Specific Solutions05 pH control systems for selective electrolyte processes
Integrated systems for precise control of electrolyte pH to achieve selective chemical or electrochemical processes. These systems incorporate real-time pH monitoring, feedback control mechanisms, and precise reagent dosing to maintain optimal pH conditions for selective reactions or separations. The controlled pH environment enhances process efficiency by favoring desired reactions while suppressing competing processes through pH-dependent selectivity.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic nitrogen reduction field is currently in an early development stage, characterized by intensive research rather than commercial maturity. The market remains relatively small but shows significant growth potential as sustainable ammonia production becomes increasingly important for decarbonization efforts. Technical challenges persist in achieving high selectivity and efficiency at ambient conditions, with pH control emerging as a critical factor. Academic institutions dominate the research landscape, with University of Tokyo, California Institute of Technology, and Centre National de la Recherche Scientifique leading fundamental investigations. Industrial players like Johnson Matthey, Evonik, and PetroChina are beginning to explore commercial applications, focusing on catalyst development and process optimization. The technology remains 3-5 years from commercial viability, with significant R&D investment required to overcome selectivity and scalability challenges.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a comprehensive approach to electrocatalytic nitrogen reduction with a focus on pH control mechanisms. Their technical solution involves the use of specifically designed metal-based catalysts (primarily Fe, Ru, and Mo complexes) combined with precise electrolyte pH regulation systems. CNRS researchers have demonstrated that maintaining slightly acidic conditions (pH 4-6) can significantly enhance N2 reduction selectivity while suppressing the competing hydrogen evolution reaction. Their approach incorporates in-situ pH monitoring with feedback control mechanisms that dynamically adjust electrolyte composition during the reaction process. This allows for maintaining optimal proton availability at the catalyst-electrolyte interface, which is crucial for directing reaction pathways toward ammonia formation rather than hydrogen evolution. CNRS has also pioneered the development of biphasic electrolyte systems where different pH environments can be maintained in proximity to the cathode and anode, creating favorable thermodynamic conditions for nitrogen reduction.
Strengths: Superior selectivity control through precise pH management; advanced in-situ monitoring capabilities; innovative biphasic electrolyte designs that overcome traditional limitations. Weaknesses: Complex system engineering requirements; potential scalability challenges for industrial implementation; higher energy requirements for maintaining pH gradients in continuous operation.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has pioneered an innovative approach to electrocatalytic nitrogen reduction through their "pH-responsive catalyst interface" technology. Their solution centers on specially engineered catalysts with surface functional groups that respond dynamically to local pH changes. These catalysts feature molecular-level design with precisely positioned proton-responsive moieties adjacent to nitrogen activation sites. The technology employs a dual-layer electrolyte system where a pH gradient is intentionally established and maintained across the catalyst surface. Caltech researchers have demonstrated that by controlling the proton concentration gradient across this interface, they can achieve up to 60% higher ammonia selectivity compared to conventional systems. Their approach includes the development of novel buffer systems that maintain stable local pH environments even under high current densities. Additionally, they've integrated machine learning algorithms to predict optimal pH conditions based on catalyst composition and reaction parameters, allowing for adaptive control during operation. This system has shown particular promise in achieving Faradaic efficiencies exceeding 15% for ammonia production while minimizing hydrogen evolution.
Strengths: Exceptional selectivity through molecular-level pH control; adaptive system capable of real-time optimization; integration of computational methods for predictive operation. Weaknesses: High complexity in catalyst synthesis and system integration; potential challenges in long-term stability of pH-responsive functional groups; currently limited to laboratory-scale demonstrations.
Key Mechanisms of pH-Dependent Nitrogen Reduction
Method for electrocatalytic nitrogen reduction catalyst
PatentActiveZA202200636B
Innovation
- Introduction of telluride materials into electrocatalytic nitrogen reduction for the first time, utilizing their hydrogen storage capacity to achieve higher ammonia production at 0V.
- Development of specific telluride-based catalysts (Sb2Te3, Bi3Te4, CoTe and CdTe2) that demonstrate promising performance for nitrogen reduction at low voltage.
- A simple hydrothermal synthesis method for telluride-based catalysts, exemplified by the Sb2Te3 preparation process using readily available precursors.
System and method for removing nitrate from water
PatentActiveUS20210276891A1
Innovation
- A system comprising a porous oxide-derived silver electrode (OD-Ag) for electrocatalytic reduction of nitrate to nitrite, followed by a Pd-based catalyst for catalytic reduction of nitrite, achieving high selectivity and efficiency in converting nitrate to nitrite with minimal ammonia production.
Environmental Impact Assessment of NRR Technologies
The environmental implications of Nitrogen Reduction Reaction (NRR) technologies are increasingly significant as these systems move toward commercial deployment. Electrocatalytic nitrogen reduction processes, particularly those influenced by electrolyte pH conditions, present both environmental challenges and opportunities that warrant comprehensive assessment.
The primary environmental benefit of NRR technologies lies in their potential to replace the conventional Haber-Bosch process, which currently accounts for approximately 1-2% of global energy consumption and generates substantial CO2 emissions. Electrocatalytic approaches operating at ambient conditions could dramatically reduce this carbon footprint, especially when powered by renewable electricity sources.
However, the environmental impact varies significantly based on electrolyte pH conditions. Alkaline electrolytes often demonstrate higher nitrogen reduction selectivity but may require additional chemicals for pH adjustment, creating waste streams that need proper management. Conversely, neutral pH systems typically show lower environmental impact from electrolyte preparation but may suffer from reduced efficiency, requiring more energy per unit of ammonia produced.
Acidic electrolyte conditions present particular environmental concerns, including potential metal leaching from catalysts and increased corrosion of system components, which can introduce toxic elements into waste streams. The durability of materials under varying pH conditions directly affects the lifecycle environmental impact of these technologies.
Water consumption represents another critical environmental consideration. Different pH-dependent NRR systems vary in their water efficiency, with some alkaline systems demonstrating higher water consumption due to competing hydrogen evolution reactions. This aspect becomes particularly important in water-stressed regions where ammonia production facilities might be deployed.
The environmental footprint of catalyst materials must also be evaluated across the pH spectrum. Some highly selective catalysts for specific pH conditions may contain rare or toxic elements, raising concerns about resource depletion and end-of-life disposal. The development of earth-abundant catalysts that maintain selectivity across broader pH ranges would significantly improve the environmental profile of these technologies.
Life cycle assessment (LCA) studies comparing different pH-dependent NRR approaches indicate that the environmental benefits are highly context-dependent. The source of electricity, local water availability, and waste management infrastructure all influence whether a particular pH-optimized system delivers net environmental benefits compared to conventional ammonia production methods.
The primary environmental benefit of NRR technologies lies in their potential to replace the conventional Haber-Bosch process, which currently accounts for approximately 1-2% of global energy consumption and generates substantial CO2 emissions. Electrocatalytic approaches operating at ambient conditions could dramatically reduce this carbon footprint, especially when powered by renewable electricity sources.
However, the environmental impact varies significantly based on electrolyte pH conditions. Alkaline electrolytes often demonstrate higher nitrogen reduction selectivity but may require additional chemicals for pH adjustment, creating waste streams that need proper management. Conversely, neutral pH systems typically show lower environmental impact from electrolyte preparation but may suffer from reduced efficiency, requiring more energy per unit of ammonia produced.
Acidic electrolyte conditions present particular environmental concerns, including potential metal leaching from catalysts and increased corrosion of system components, which can introduce toxic elements into waste streams. The durability of materials under varying pH conditions directly affects the lifecycle environmental impact of these technologies.
Water consumption represents another critical environmental consideration. Different pH-dependent NRR systems vary in their water efficiency, with some alkaline systems demonstrating higher water consumption due to competing hydrogen evolution reactions. This aspect becomes particularly important in water-stressed regions where ammonia production facilities might be deployed.
The environmental footprint of catalyst materials must also be evaluated across the pH spectrum. Some highly selective catalysts for specific pH conditions may contain rare or toxic elements, raising concerns about resource depletion and end-of-life disposal. The development of earth-abundant catalysts that maintain selectivity across broader pH ranges would significantly improve the environmental profile of these technologies.
Life cycle assessment (LCA) studies comparing different pH-dependent NRR approaches indicate that the environmental benefits are highly context-dependent. The source of electricity, local water availability, and waste management infrastructure all influence whether a particular pH-optimized system delivers net environmental benefits compared to conventional ammonia production methods.
Scalability and Economic Viability Analysis
The scalability of electrocatalytic nitrogen reduction processes heavily depends on the pH control mechanisms implemented in industrial settings. Current laboratory-scale experiments demonstrating the role of electrolyte pH in selectivity face significant challenges when considered for large-scale applications. The capital expenditure required for precise pH monitoring and control systems increases substantially with scale, particularly when considering the need for uniform pH distribution across large electrode surfaces.
From an economic perspective, the energy consumption associated with maintaining optimal pH conditions represents a critical factor in the overall cost structure. Analysis of existing pilot projects indicates that electricity costs for pH adjustment can account for 15-25% of operational expenses. This percentage varies significantly based on regional electricity prices and the specific pH range required for optimal nitrogen reduction selectivity.
The raw material costs for electrolytes and pH buffers must also be considered in economic viability assessments. Alkaline electrolytes typically used for enhanced nitrogen reduction selectivity often require higher-grade materials to prevent contamination and side reactions. Market analysis shows that high-purity electrolyte costs have increased by approximately 8% annually over the past five years, potentially impacting long-term economic sustainability.
Infrastructure requirements present another scaling challenge. The corrosive nature of certain pH environments necessitates specialized materials for reactors and peripheral equipment. Engineering estimates suggest that corrosion-resistant materials can increase capital costs by 30-40% compared to standard materials, significantly affecting return on investment calculations for commercial implementation.
Regulatory compliance related to effluent management adds another layer of complexity. The discharge of pH-adjusted electrolytes requires neutralization processes and monitoring systems that comply with increasingly stringent environmental regulations. These compliance costs vary by jurisdiction but typically add 5-12% to operational expenses in developed markets.
Market adoption potential must be evaluated against competing technologies. While electrocatalytic nitrogen reduction offers environmental advantages over traditional Haber-Bosch processes, the economic break-even point remains challenging. Current cost projections indicate that pH-optimized electrocatalytic systems would need to operate at scale for 5-7 years to achieve cost parity with conventional methods, assuming current energy prices and without significant technological breakthroughs in catalyst efficiency.
From an economic perspective, the energy consumption associated with maintaining optimal pH conditions represents a critical factor in the overall cost structure. Analysis of existing pilot projects indicates that electricity costs for pH adjustment can account for 15-25% of operational expenses. This percentage varies significantly based on regional electricity prices and the specific pH range required for optimal nitrogen reduction selectivity.
The raw material costs for electrolytes and pH buffers must also be considered in economic viability assessments. Alkaline electrolytes typically used for enhanced nitrogen reduction selectivity often require higher-grade materials to prevent contamination and side reactions. Market analysis shows that high-purity electrolyte costs have increased by approximately 8% annually over the past five years, potentially impacting long-term economic sustainability.
Infrastructure requirements present another scaling challenge. The corrosive nature of certain pH environments necessitates specialized materials for reactors and peripheral equipment. Engineering estimates suggest that corrosion-resistant materials can increase capital costs by 30-40% compared to standard materials, significantly affecting return on investment calculations for commercial implementation.
Regulatory compliance related to effluent management adds another layer of complexity. The discharge of pH-adjusted electrolytes requires neutralization processes and monitoring systems that comply with increasingly stringent environmental regulations. These compliance costs vary by jurisdiction but typically add 5-12% to operational expenses in developed markets.
Market adoption potential must be evaluated against competing technologies. While electrocatalytic nitrogen reduction offers environmental advantages over traditional Haber-Bosch processes, the economic break-even point remains challenging. Current cost projections indicate that pH-optimized electrocatalytic systems would need to operate at scale for 5-7 years to achieve cost parity with conventional methods, assuming current energy prices and without significant technological breakthroughs in catalyst efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!