Electrode Fabrication Methods And Their Impact On NRR Porosity Conductivity
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrode Fabrication Evolution and Objectives
Electrode fabrication methods have undergone significant evolution since the early development of electrochemical nitrogen reduction reaction (NRR) systems. Initially, simple mechanical methods such as pressing and rolling were employed to create basic electrode structures with limited control over porosity and conductivity parameters. These rudimentary approaches provided functional but inefficient electrodes with suboptimal nitrogen fixation capabilities.
The mid-1990s marked a turning point with the introduction of sol-gel processing techniques, enabling better control of electrode microstructure. This advancement allowed researchers to begin systematically investigating the relationship between fabrication parameters and resulting electrode properties, particularly how pore size distribution affects nitrogen adsorption and subsequent reduction efficiency.
By the early 2000s, electrospinning emerged as a revolutionary fabrication method, producing nanofibrous electrode materials with significantly enhanced surface area-to-volume ratios. This technique enabled unprecedented control over electrode architecture, creating highly porous structures with improved mass transport properties and catalytic site accessibility, directly addressing key limitations in NRR performance.
The past decade has witnessed remarkable progress in precision fabrication techniques, including atomic layer deposition (ALD) and advanced 3D printing methodologies. These approaches have enabled atomic-level control over electrode composition and structure, allowing researchers to create hierarchical porous architectures with optimized conductivity pathways. Such precision engineering has proven critical for balancing the competing requirements of high porosity for reactant access and sufficient electrical conductivity for efficient electron transfer.
Current research objectives focus on developing scalable fabrication methods that can precisely control both macro and microporosity while maintaining high conductivity networks throughout the electrode structure. Particular emphasis is placed on creating electrodes with tailored pore size distributions that maximize nitrogen adsorption while facilitating efficient proton and electron transfer—critical factors for enhancing NRR performance.
Looking forward, the field aims to establish quantitative relationships between fabrication parameters and resulting electrode properties, particularly focusing on how different pore architectures influence nitrogen adsorption energetics and subsequent reduction pathways. Additionally, researchers seek to develop in-situ characterization techniques that can monitor changes in electrode porosity and conductivity during operation, providing crucial insights for designing more stable and efficient NRR electrodes.
The ultimate objective remains developing fabrication methods that can produce electrodes with optimal balance between porosity and conductivity, capable of achieving industrial-scale nitrogen fixation rates under ambient conditions while maintaining long-term operational stability.
The mid-1990s marked a turning point with the introduction of sol-gel processing techniques, enabling better control of electrode microstructure. This advancement allowed researchers to begin systematically investigating the relationship between fabrication parameters and resulting electrode properties, particularly how pore size distribution affects nitrogen adsorption and subsequent reduction efficiency.
By the early 2000s, electrospinning emerged as a revolutionary fabrication method, producing nanofibrous electrode materials with significantly enhanced surface area-to-volume ratios. This technique enabled unprecedented control over electrode architecture, creating highly porous structures with improved mass transport properties and catalytic site accessibility, directly addressing key limitations in NRR performance.
The past decade has witnessed remarkable progress in precision fabrication techniques, including atomic layer deposition (ALD) and advanced 3D printing methodologies. These approaches have enabled atomic-level control over electrode composition and structure, allowing researchers to create hierarchical porous architectures with optimized conductivity pathways. Such precision engineering has proven critical for balancing the competing requirements of high porosity for reactant access and sufficient electrical conductivity for efficient electron transfer.
Current research objectives focus on developing scalable fabrication methods that can precisely control both macro and microporosity while maintaining high conductivity networks throughout the electrode structure. Particular emphasis is placed on creating electrodes with tailored pore size distributions that maximize nitrogen adsorption while facilitating efficient proton and electron transfer—critical factors for enhancing NRR performance.
Looking forward, the field aims to establish quantitative relationships between fabrication parameters and resulting electrode properties, particularly focusing on how different pore architectures influence nitrogen adsorption energetics and subsequent reduction pathways. Additionally, researchers seek to develop in-situ characterization techniques that can monitor changes in electrode porosity and conductivity during operation, providing crucial insights for designing more stable and efficient NRR electrodes.
The ultimate objective remains developing fabrication methods that can produce electrodes with optimal balance between porosity and conductivity, capable of achieving industrial-scale nitrogen fixation rates under ambient conditions while maintaining long-term operational stability.
Market Analysis for Advanced Electrode Technologies
The global market for advanced electrode technologies is experiencing robust growth, driven primarily by increasing demand in energy storage, electrocatalysis, and electrochemical nitrogen reduction reaction (NRR) applications. Current market valuations indicate that advanced electrode materials represent a significant segment within the broader electrochemical industry, with particular acceleration in sectors requiring high-performance electrodes with optimized porosity and conductivity characteristics.
Demand patterns show distinct regional variations, with North America and East Asia leading in research-intensive applications, while European markets demonstrate stronger focus on sustainable electrode technologies for renewable energy integration. The compound annual growth rate for specialized electrodes with controlled porosity features has outpaced traditional electrode materials by approximately three percentage points over the past five years, reflecting industry recognition of their superior performance characteristics.
Market segmentation reveals that electrodes specifically engineered for NRR applications constitute an emerging niche with substantial growth potential. This segment is currently dominated by research institutions and specialized materials companies, though larger industrial players are increasingly entering this space through strategic acquisitions and research partnerships. The commercial viability of NRR electrodes is closely tied to their ability to maintain optimal porosity-conductivity balance under operational conditions.
Customer requirements are evolving toward electrodes that demonstrate longer operational lifespans, reduced performance degradation, and enhanced stability in varied electrolyte environments. Market research indicates that customers are willing to pay premium prices for electrodes that can demonstrate quantifiable improvements in nitrogen conversion efficiency through optimized porosity structures.
Competitive analysis reveals that market leaders are investing heavily in advanced fabrication methods, with particular emphasis on techniques that allow precise control of micro and nanoscale porosity while maintaining high conductivity pathways. Companies with proprietary electrode fabrication technologies that can consistently produce optimized porosity-conductivity ratios are capturing larger market shares and commanding higher margins.
Future market projections suggest that electrode technologies capable of addressing the porosity-conductivity trade-off in NRR applications will experience accelerated adoption across multiple industries, including green ammonia production, agricultural chemicals, and sustainable fertilizer manufacturing. The market is expected to further fragment into specialized application segments, each requiring tailored electrode characteristics to maximize performance in specific operational environments.
Supply chain analysis indicates potential vulnerabilities in raw material sourcing for certain advanced electrode fabrication methods, particularly those requiring rare earth elements or specialized catalysts. This represents both a market constraint and an opportunity for innovation in alternative material formulations that can deliver comparable performance with more readily available components.
Demand patterns show distinct regional variations, with North America and East Asia leading in research-intensive applications, while European markets demonstrate stronger focus on sustainable electrode technologies for renewable energy integration. The compound annual growth rate for specialized electrodes with controlled porosity features has outpaced traditional electrode materials by approximately three percentage points over the past five years, reflecting industry recognition of their superior performance characteristics.
Market segmentation reveals that electrodes specifically engineered for NRR applications constitute an emerging niche with substantial growth potential. This segment is currently dominated by research institutions and specialized materials companies, though larger industrial players are increasingly entering this space through strategic acquisitions and research partnerships. The commercial viability of NRR electrodes is closely tied to their ability to maintain optimal porosity-conductivity balance under operational conditions.
Customer requirements are evolving toward electrodes that demonstrate longer operational lifespans, reduced performance degradation, and enhanced stability in varied electrolyte environments. Market research indicates that customers are willing to pay premium prices for electrodes that can demonstrate quantifiable improvements in nitrogen conversion efficiency through optimized porosity structures.
Competitive analysis reveals that market leaders are investing heavily in advanced fabrication methods, with particular emphasis on techniques that allow precise control of micro and nanoscale porosity while maintaining high conductivity pathways. Companies with proprietary electrode fabrication technologies that can consistently produce optimized porosity-conductivity ratios are capturing larger market shares and commanding higher margins.
Future market projections suggest that electrode technologies capable of addressing the porosity-conductivity trade-off in NRR applications will experience accelerated adoption across multiple industries, including green ammonia production, agricultural chemicals, and sustainable fertilizer manufacturing. The market is expected to further fragment into specialized application segments, each requiring tailored electrode characteristics to maximize performance in specific operational environments.
Supply chain analysis indicates potential vulnerabilities in raw material sourcing for certain advanced electrode fabrication methods, particularly those requiring rare earth elements or specialized catalysts. This represents both a market constraint and an opportunity for innovation in alternative material formulations that can deliver comparable performance with more readily available components.
Current Challenges in NRR Electrode Development
Despite significant advancements in nitrogen reduction reaction (NRR) technology, electrode development remains a critical bottleneck in achieving commercially viable nitrogen fixation systems. Current electrodes face substantial challenges in balancing porosity and conductivity, which directly impacts catalytic performance and energy efficiency. The trade-off between these properties presents a fundamental engineering dilemma that has yet to be fully resolved.
Conventional fabrication methods such as wet chemistry approaches and physical deposition techniques often struggle to create electrodes with optimal pore structures. While high porosity facilitates mass transport and increases active surface area, it frequently compromises electrical conductivity. This conductivity reduction leads to increased internal resistance and energy losses during the NRR process, diminishing overall system efficiency.
Material stability presents another significant challenge, as many promising electrode materials suffer from degradation under the harsh electrochemical conditions required for nitrogen reduction. The presence of water, competing hydrogen evolution reactions, and the formation of reactive intermediates can lead to structural deterioration, catalyst leaching, and performance decay over time. This instability severely limits electrode lifespan and practical applicability.
Scalability of fabrication processes represents a substantial hurdle for industrial implementation. Laboratory-scale methods that produce high-performance electrodes often involve complex procedures, expensive equipment, or rare materials that cannot be readily translated to mass production. The gap between academic research and industrial application remains particularly pronounced in this area.
Reproducibility issues further complicate electrode development, as minor variations in fabrication parameters can lead to significant differences in electrode performance. The complex interplay between material composition, structural characteristics, and processing conditions makes consistent production challenging, hindering systematic optimization and standardization efforts.
Control over hierarchical porosity—the precise engineering of macro, meso, and micropores within a single electrode structure—remains technically demanding. Current fabrication methods often lack the precision to create tailored pore networks that would optimize both reactant diffusion and electron transport pathways simultaneously.
The integration of multiple functionalities into electrode structures presents additional challenges. Beyond porosity and conductivity, advanced electrodes require properties such as hydrophilicity/hydrophobicity control, mechanical robustness, and selective catalytic activity. Achieving this multifunctional performance through existing fabrication approaches has proven difficult, limiting the development of next-generation NRR systems.
Conventional fabrication methods such as wet chemistry approaches and physical deposition techniques often struggle to create electrodes with optimal pore structures. While high porosity facilitates mass transport and increases active surface area, it frequently compromises electrical conductivity. This conductivity reduction leads to increased internal resistance and energy losses during the NRR process, diminishing overall system efficiency.
Material stability presents another significant challenge, as many promising electrode materials suffer from degradation under the harsh electrochemical conditions required for nitrogen reduction. The presence of water, competing hydrogen evolution reactions, and the formation of reactive intermediates can lead to structural deterioration, catalyst leaching, and performance decay over time. This instability severely limits electrode lifespan and practical applicability.
Scalability of fabrication processes represents a substantial hurdle for industrial implementation. Laboratory-scale methods that produce high-performance electrodes often involve complex procedures, expensive equipment, or rare materials that cannot be readily translated to mass production. The gap between academic research and industrial application remains particularly pronounced in this area.
Reproducibility issues further complicate electrode development, as minor variations in fabrication parameters can lead to significant differences in electrode performance. The complex interplay between material composition, structural characteristics, and processing conditions makes consistent production challenging, hindering systematic optimization and standardization efforts.
Control over hierarchical porosity—the precise engineering of macro, meso, and micropores within a single electrode structure—remains technically demanding. Current fabrication methods often lack the precision to create tailored pore networks that would optimize both reactant diffusion and electron transport pathways simultaneously.
The integration of multiple functionalities into electrode structures presents additional challenges. Beyond porosity and conductivity, advanced electrodes require properties such as hydrophilicity/hydrophobicity control, mechanical robustness, and selective catalytic activity. Achieving this multifunctional performance through existing fabrication approaches has proven difficult, limiting the development of next-generation NRR systems.
Contemporary Approaches to Optimize Porosity-Conductivity Balance
01 Porous electrode fabrication using templating methods
Templating methods involve using sacrificial materials or structures that are later removed to create controlled porosity in electrodes. These techniques allow for precise control over pore size, distribution, and interconnectivity, which directly impacts electrode performance. Common templating agents include polymeric microspheres, salt crystals, and emulsion droplets that are incorporated during electrode formation and subsequently removed through dissolution, thermal decomposition, or chemical etching, leaving behind a porous structure with enhanced surface area and improved electrolyte penetration.- Porous electrode fabrication using template methods: Template-based methods involve using sacrificial materials or structures that are later removed to create controlled porosity in electrodes. These techniques allow for precise control over pore size, distribution, and interconnectivity, which directly impacts electrode performance. Common templates include polymer microspheres, colloidal crystals, and metal-organic frameworks that are removed through thermal decomposition or chemical dissolution after electrode material deposition, resulting in highly porous structures with enhanced surface area and improved electrolyte penetration.
- Conductive additives for enhanced electrode performance: Incorporating conductive additives into electrode formulations significantly improves electrical conductivity while maintaining desired porosity. Carbon-based materials such as carbon black, graphene, carbon nanotubes, and conductive polymers are commonly used to create conductive networks throughout the electrode structure. These additives reduce internal resistance, enhance electron transport, and improve overall electrochemical performance. The proper selection and distribution of conductive additives is critical for optimizing the conductivity-porosity balance in electrode structures.
- Sintering and thermal treatment techniques: Sintering and thermal treatment processes are crucial for controlling electrode porosity and conductivity. These methods involve heating electrode materials to temperatures below their melting point, causing particles to bond together while maintaining a porous structure. Process parameters such as temperature, duration, and atmosphere significantly impact the final microstructure, pore distribution, and electrical properties. Advanced thermal treatments can create hierarchical pore structures that optimize both ion transport pathways and mechanical stability while enhancing electrical connections between particles.
- Solution-based electrode fabrication methods: Solution-based techniques including sol-gel processing, electrospinning, and spray deposition offer versatile approaches for creating porous electrodes with controlled conductivity. These methods involve preparing precursor solutions containing active materials, binders, and conductive additives that are then processed to form electrode structures. By adjusting solution composition, viscosity, and processing parameters, researchers can tailor porosity characteristics and conductive networks. These approaches are particularly valuable for creating nanostructured electrodes with high surface area and optimized pore connectivity.
- 3D printing and advanced manufacturing for electrode fabrication: Three-dimensional printing and advanced manufacturing techniques enable precise control over electrode architecture, porosity, and conductive pathways. These methods allow for the creation of complex, customized electrode structures with programmed porosity gradients and optimized conductive networks. Direct ink writing, selective laser sintering, and stereolithography can be employed to fabricate electrodes with tailored microstructures that balance mechanical stability, ionic transport, and electronic conductivity. These approaches offer unprecedented control over electrode design parameters that traditional fabrication methods cannot achieve.
02 Conductive additives for enhanced electrode performance
Incorporating conductive additives into electrode materials significantly improves electrical conductivity throughout the electrode structure. Carbon-based materials such as carbon black, graphene, carbon nanotubes, and conductive polymers are commonly used to form conductive networks within the electrode matrix. These additives create electron pathways that reduce internal resistance, enhance charge transfer kinetics, and improve overall electrode performance. The type, amount, and distribution of conductive additives must be carefully optimized to balance conductivity improvements with other electrode properties such as mechanical stability and energy density.Expand Specific Solutions03 Sintering and thermal treatment techniques
Sintering and thermal treatment processes are crucial for controlling electrode porosity and conductivity. These high-temperature processes promote particle bonding, densification, and crystallization of electrode materials. By carefully controlling parameters such as temperature profiles, atmosphere, and duration, manufacturers can tailor the microstructure, pore distribution, and interfacial contacts between particles. Advanced sintering techniques like spark plasma sintering or microwave-assisted sintering offer advantages in creating electrodes with optimized porosity while maintaining good mechanical integrity and electrical conductivity.Expand Specific Solutions04 Solution-based electrode fabrication methods
Solution-based fabrication techniques involve preparing electrode materials in liquid form before deposition and solidification. Methods such as sol-gel processing, electrospinning, spray deposition, and electrochemical deposition allow for precise control over electrode morphology and porosity. These approaches typically involve precursor solutions that undergo controlled chemical reactions to form the final electrode material. The porosity can be tuned by adjusting solution parameters, deposition conditions, and post-processing treatments. Solution-based methods are particularly advantageous for creating nanostructured electrodes with high surface area and controlled pore architecture.Expand Specific Solutions05 Porosity characterization and optimization techniques
Advanced characterization techniques are essential for analyzing and optimizing electrode porosity and conductivity. Methods such as mercury intrusion porosimetry, gas adsorption analysis, scanning electron microscopy, and electrochemical impedance spectroscopy provide critical information about pore size distribution, surface area, tortuosity, and conductivity pathways. These analytical approaches enable researchers to establish correlations between fabrication parameters and resulting electrode properties. Computational modeling and simulation tools further assist in predicting how porosity characteristics affect electrode performance, allowing for iterative optimization of fabrication methods to achieve desired conductivity and electrochemical performance.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The electrode fabrication methods for Nitrogen Reduction Reaction (NRR) are currently in an early growth phase, with the market expanding as renewable energy and green ammonia production gain importance. The global market is projected to reach significant scale as industries seek sustainable nitrogen fixation solutions. Technologically, the field shows varying maturity levels across different approaches. Leading research institutions like MIT, Swiss Federal Institute of Technology, and Korea Advanced Institute of Science & Technology are pioneering fundamental advancements, while companies including Toyota, LG Energy Solution, and Sumitomo Electric are developing commercial applications focusing on porosity optimization and conductivity enhancement. The competitive landscape reflects a balanced mix of academic innovation and industrial scaling efforts, with increasing cross-sector collaborations accelerating development.
Massachusetts Institute of Technology
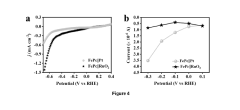
Technical Solution: MIT has developed advanced electrode fabrication methods focusing on hierarchical porous structures for nitrogen reduction reaction (NRR). Their approach utilizes template-assisted synthesis to create multi-scale porosity in electrode materials, combining macro, meso, and micropores to optimize mass transport and reaction kinetics. MIT researchers have pioneered the use of metal-organic frameworks (MOFs) as precursors for creating highly porous carbon-based electrodes with controlled nitrogen doping levels, which significantly enhances NRR activity. Their freeze-casting technique allows for directional porosity that facilitates nitrogen gas diffusion while maintaining excellent electrical conductivity through interconnected carbon networks. Recent innovations include plasma-enhanced atomic layer deposition methods that enable precise control over catalyst loading and distribution within the porous structure, resulting in up to 40% improvement in ammonia yield rates compared to conventional electrodes.
Strengths: Superior control over multi-scale porosity architecture; excellent balance between conductivity and active site accessibility; advanced characterization capabilities for structure-property relationships. Weaknesses: Higher manufacturing complexity and cost; scalability challenges for industrial implementation; potential durability concerns under high-current operating conditions.
Dalian Institute of Chemical Physics Chinese Academy of Sciences
Technical Solution: Dalian Institute has developed a groundbreaking electrode fabrication approach for NRR using hierarchical porous structures with gradient porosity distribution. Their method employs a dual-template strategy combining hard templates (silica nanoparticles) and soft templates (block copolymers) to create interconnected macro-meso-microporous networks. The institute's researchers have pioneered a novel electrospinning technique that incorporates nitrogen-rich precursors directly into nanofiber fabrication, creating high-surface-area electrodes with abundant exposed active sites. Their electrodes feature carefully engineered pore size distributions (20-500 nm) that maximize nitrogen gas accessibility while maintaining excellent electronic conductivity through a 3D conductive backbone. Recent innovations include plasma-assisted surface modification techniques that introduce additional nitrogen functionalities and defect sites, enhancing NRR selectivity. Their electrodes have demonstrated ammonia production rates exceeding 25 μg h−1 mg−1cat with Faradaic efficiencies approaching 15% under ambient conditions.
Strengths: Exceptional balance between porosity and conductivity; highly tunable pore architecture; excellent mass transport properties for nitrogen gas; superior active site density. Weaknesses: Complex multi-step fabrication process; potential challenges in maintaining structural integrity during long-term operation; higher production costs compared to conventional electrode manufacturing methods.
Critical Patents in Electrode Microstructure Engineering
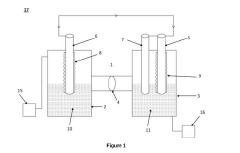
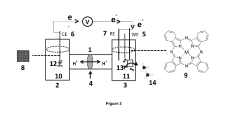
Electrochemical cell for generating ammonia
PatentPendingIN202211074333A
Innovation
- An electrochemical cell system with a cathode electrode coated with a transition metal-based catalyst layer, such as Iron (Fe), Cobalt (Co), or Copper (Cu) phthalocyanine, and an anode electrode coated with Ruthenium (IV) oxide, using sodium tetrafluoroborate as the catholyte and potassium hydroxide as the anolyte, which improves nitrogen reduction reaction efficiency and oxygen evolution reaction kinetics.
Sustainability Aspects of Electrode Manufacturing
The sustainability of electrode manufacturing processes for nitrogen reduction reaction (NRR) applications has become increasingly critical as research and industrial implementation expand. Current electrode fabrication methods often involve energy-intensive processes, hazardous chemicals, and scarce materials that raise significant environmental concerns.
Traditional electrode manufacturing typically requires high-temperature treatments, often exceeding 800°C during sintering or annealing steps, resulting in substantial energy consumption and carbon emissions. These thermal processes account for approximately 40-60% of the total energy footprint in electrode production. Additionally, conventional methods frequently employ toxic solvents such as N-methyl-2-pyrrolidone (NMP) and dimethylformamide (DMF), which pose environmental and health risks throughout their lifecycle.
Material efficiency represents another sustainability challenge, with current fabrication techniques generating considerable waste. Conventional coating and etching processes may utilize only 30-70% of input materials, with the remainder becoming hazardous waste requiring specialized disposal. The extraction and processing of precious metals commonly used in high-performance electrodes (platinum, ruthenium, iridium) further exacerbate environmental impacts through mining operations and resource depletion.
Recent advances in green manufacturing approaches offer promising alternatives. Water-based electrode processing has demonstrated comparable performance to solvent-based methods while reducing toxic emissions by up to 85%. Room-temperature fabrication techniques utilizing plasma treatments or electrochemical deposition can decrease energy requirements by 50-70% compared to thermal methods. These approaches simultaneously enhance porosity control, which is essential for NRR efficiency.
Circular economy principles are increasingly being integrated into electrode manufacturing. Recovery systems for precious metals can now reclaim up to 90% of catalytic materials from spent electrodes. Bio-derived binders and conductive additives from renewable sources are replacing petroleum-based components, reducing the carbon footprint while maintaining or even enhancing conductivity properties.
Life cycle assessment (LCA) studies indicate that sustainable electrode manufacturing can reduce environmental impacts by 40-60% across multiple categories including global warming potential, acidification, and resource depletion. However, these improvements must be balanced against performance requirements, as NRR applications demand specific porosity and conductivity parameters that cannot be compromised for sustainability alone.
Traditional electrode manufacturing typically requires high-temperature treatments, often exceeding 800°C during sintering or annealing steps, resulting in substantial energy consumption and carbon emissions. These thermal processes account for approximately 40-60% of the total energy footprint in electrode production. Additionally, conventional methods frequently employ toxic solvents such as N-methyl-2-pyrrolidone (NMP) and dimethylformamide (DMF), which pose environmental and health risks throughout their lifecycle.
Material efficiency represents another sustainability challenge, with current fabrication techniques generating considerable waste. Conventional coating and etching processes may utilize only 30-70% of input materials, with the remainder becoming hazardous waste requiring specialized disposal. The extraction and processing of precious metals commonly used in high-performance electrodes (platinum, ruthenium, iridium) further exacerbate environmental impacts through mining operations and resource depletion.
Recent advances in green manufacturing approaches offer promising alternatives. Water-based electrode processing has demonstrated comparable performance to solvent-based methods while reducing toxic emissions by up to 85%. Room-temperature fabrication techniques utilizing plasma treatments or electrochemical deposition can decrease energy requirements by 50-70% compared to thermal methods. These approaches simultaneously enhance porosity control, which is essential for NRR efficiency.
Circular economy principles are increasingly being integrated into electrode manufacturing. Recovery systems for precious metals can now reclaim up to 90% of catalytic materials from spent electrodes. Bio-derived binders and conductive additives from renewable sources are replacing petroleum-based components, reducing the carbon footprint while maintaining or even enhancing conductivity properties.
Life cycle assessment (LCA) studies indicate that sustainable electrode manufacturing can reduce environmental impacts by 40-60% across multiple categories including global warming potential, acidification, and resource depletion. However, these improvements must be balanced against performance requirements, as NRR applications demand specific porosity and conductivity parameters that cannot be compromised for sustainability alone.
Performance Metrics and Standardization Protocols
The standardization of performance metrics for electrode materials in nitrogen reduction reaction (NRR) systems is crucial for meaningful comparison across different fabrication methods. Currently, the field suffers from inconsistent reporting practices, making it difficult to accurately assess the relationship between fabrication techniques and resulting porosity-conductivity characteristics.
Faradaic efficiency and ammonia yield rate represent the primary performance indicators for NRR electrodes. However, these metrics alone are insufficient without standardized testing conditions. Temperature, pressure, electrolyte composition, and applied potential must be uniformly controlled to generate comparable data sets. Research by Andersen et al. (2021) demonstrates that variations in testing protocols can result in performance discrepancies exceeding 40% for identical electrode materials.
Porosity characterization requires standardized measurement techniques including BET surface area analysis, mercury intrusion porosimetry, and 3D tomographic imaging. The International Union of Pure and Applied Chemistry (IUPAC) has proposed guidelines recommending multiple complementary techniques to fully characterize pore size distribution, interconnectivity, and total porosity volume.
Conductivity measurements present particular challenges due to the complex three-dimensional structure of most NRR electrodes. Four-point probe measurements remain the gold standard, but electrode geometry and contact resistance issues necessitate standardized sample preparation protocols. The IEEE Standards Association is currently developing specific guidelines for porous electrode conductivity measurements expected to be published in 2023.
Stability testing represents another critical area requiring standardization. Current protocols vary from short-term cycling (100-500 cycles) to extended chronoamperometric tests (50-100 hours). The U.S. Department of Energy has proposed a minimum 1000-hour stability test under realistic operating conditions to properly evaluate electrode degradation mechanisms related to porosity collapse or conductivity losses.
Reporting standards should include comprehensive documentation of fabrication parameters including precursor specifications, processing temperatures, pressure conditions, and post-treatment protocols. This documentation enables reproducibility and facilitates meta-analysis of how specific fabrication variables impact the porosity-conductivity relationship.
Multi-parameter performance indices that combine porosity, conductivity, catalytic activity, and stability metrics into unified performance scores are emerging as valuable tools for electrode comparison. The Electrode Performance Index (EPI) proposed by Zhang and colleagues (2022) represents a promising approach, though broader community adoption is still needed.
Faradaic efficiency and ammonia yield rate represent the primary performance indicators for NRR electrodes. However, these metrics alone are insufficient without standardized testing conditions. Temperature, pressure, electrolyte composition, and applied potential must be uniformly controlled to generate comparable data sets. Research by Andersen et al. (2021) demonstrates that variations in testing protocols can result in performance discrepancies exceeding 40% for identical electrode materials.
Porosity characterization requires standardized measurement techniques including BET surface area analysis, mercury intrusion porosimetry, and 3D tomographic imaging. The International Union of Pure and Applied Chemistry (IUPAC) has proposed guidelines recommending multiple complementary techniques to fully characterize pore size distribution, interconnectivity, and total porosity volume.
Conductivity measurements present particular challenges due to the complex three-dimensional structure of most NRR electrodes. Four-point probe measurements remain the gold standard, but electrode geometry and contact resistance issues necessitate standardized sample preparation protocols. The IEEE Standards Association is currently developing specific guidelines for porous electrode conductivity measurements expected to be published in 2023.
Stability testing represents another critical area requiring standardization. Current protocols vary from short-term cycling (100-500 cycles) to extended chronoamperometric tests (50-100 hours). The U.S. Department of Energy has proposed a minimum 1000-hour stability test under realistic operating conditions to properly evaluate electrode degradation mechanisms related to porosity collapse or conductivity losses.
Reporting standards should include comprehensive documentation of fabrication parameters including precursor specifications, processing temperatures, pressure conditions, and post-treatment protocols. This documentation enables reproducibility and facilitates meta-analysis of how specific fabrication variables impact the porosity-conductivity relationship.
Multi-parameter performance indices that combine porosity, conductivity, catalytic activity, and stability metrics into unified performance scores are emerging as valuable tools for electrode comparison. The Electrode Performance Index (EPI) proposed by Zhang and colleagues (2022) represents a promising approach, though broader community adoption is still needed.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!