Suppressing Hydrogen Evolution Reaction In NRR Catalyst Design
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NRR Catalyst Evolution and Objectives
The evolution of nitrogen reduction reaction (NRR) catalysts represents one of the most significant pursuits in sustainable chemistry, aiming to revolutionize ammonia production beyond the century-old Haber-Bosch process. Since the early 2000s, electrocatalytic NRR has emerged as a promising alternative, offering the potential for ambient-condition ammonia synthesis powered by renewable electricity. This technological trajectory has accelerated dramatically in the past decade, with breakthrough developments in catalyst design principles and performance metrics.
The fundamental challenge in NRR catalyst development lies in the competing hydrogen evolution reaction (HER), which occurs at similar potential ranges and significantly reduces Faradaic efficiency for nitrogen conversion. Early catalysts (2010-2015) demonstrated proof-of-concept but suffered from single-digit Faradaic efficiencies due to overwhelming HER activity. The field subsequently evolved through several distinct phases: initial exploration of noble metal catalysts, transition to earth-abundant alternatives, and most recently, the development of advanced nanostructured and single-atom catalysts with engineered electronic structures.
Current technological objectives center on achieving selective nitrogen activation while suppressing the thermodynamically favored HER pathway. Specifically, the field aims to develop catalysts capable of Faradaic efficiencies exceeding 30% with ammonia production rates above 100 μg h⁻¹ mg⁻¹cat under ambient conditions. This represents approximately an order of magnitude improvement over current state-of-the-art systems. Additionally, catalyst stability must extend beyond 100 hours of continuous operation to approach commercial viability.
The evolution trajectory has been marked by several paradigm shifts in design philosophy. Initial approaches focused primarily on identifying materials with optimal nitrogen binding energy, following traditional descriptor-based catalyst design. However, recent advances have highlighted the critical importance of proton management strategies, including hydrophobic/hydrophilic interface engineering, proton-donor control, and creation of isolated nitrogen activation sites that minimize HER activity.
Looking forward, the field is converging on multifunctional catalyst systems that simultaneously address nitrogen activation, proton management, and electron transfer kinetics. Emerging objectives include the development of tandem catalytic systems that spatially separate HER and NRR active sites, the incorporation of molecular recognition elements to enhance N₂ concentration at catalyst surfaces, and the integration of advanced operando characterization techniques to elucidate reaction mechanisms under realistic conditions.
The ultimate goal remains the creation of sustainable, economically viable electrocatalytic ammonia synthesis technologies that can operate using intermittent renewable energy sources, potentially enabling distributed fertilizer production and energy storage applications while eliminating the carbon footprint associated with conventional ammonia synthesis.
The fundamental challenge in NRR catalyst development lies in the competing hydrogen evolution reaction (HER), which occurs at similar potential ranges and significantly reduces Faradaic efficiency for nitrogen conversion. Early catalysts (2010-2015) demonstrated proof-of-concept but suffered from single-digit Faradaic efficiencies due to overwhelming HER activity. The field subsequently evolved through several distinct phases: initial exploration of noble metal catalysts, transition to earth-abundant alternatives, and most recently, the development of advanced nanostructured and single-atom catalysts with engineered electronic structures.
Current technological objectives center on achieving selective nitrogen activation while suppressing the thermodynamically favored HER pathway. Specifically, the field aims to develop catalysts capable of Faradaic efficiencies exceeding 30% with ammonia production rates above 100 μg h⁻¹ mg⁻¹cat under ambient conditions. This represents approximately an order of magnitude improvement over current state-of-the-art systems. Additionally, catalyst stability must extend beyond 100 hours of continuous operation to approach commercial viability.
The evolution trajectory has been marked by several paradigm shifts in design philosophy. Initial approaches focused primarily on identifying materials with optimal nitrogen binding energy, following traditional descriptor-based catalyst design. However, recent advances have highlighted the critical importance of proton management strategies, including hydrophobic/hydrophilic interface engineering, proton-donor control, and creation of isolated nitrogen activation sites that minimize HER activity.
Looking forward, the field is converging on multifunctional catalyst systems that simultaneously address nitrogen activation, proton management, and electron transfer kinetics. Emerging objectives include the development of tandem catalytic systems that spatially separate HER and NRR active sites, the incorporation of molecular recognition elements to enhance N₂ concentration at catalyst surfaces, and the integration of advanced operando characterization techniques to elucidate reaction mechanisms under realistic conditions.
The ultimate goal remains the creation of sustainable, economically viable electrocatalytic ammonia synthesis technologies that can operate using intermittent renewable energy sources, potentially enabling distributed fertilizer production and energy storage applications while eliminating the carbon footprint associated with conventional ammonia synthesis.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability imperatives, with the market value projected to reach $70.3 billion by 2025, growing at a CAGR of 5.4%. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and generates substantial CO2 emissions, creating an urgent need for sustainable alternatives. The electrochemical nitrogen reduction reaction (NRR) represents a promising pathway for green ammonia synthesis, operating under ambient conditions with renewable electricity.
Market demand for sustainable ammonia is primarily driven by three sectors: agriculture, which accounts for 80% of ammonia consumption as fertilizer; industrial applications including cleaning products, refrigerants, and water purification; and emerging energy storage applications, where ammonia serves as a hydrogen carrier with an energy density of 22.5 MJ/kg.
Regional analysis reveals that Asia-Pacific dominates the ammonia market with 45% share, followed by Europe (25%) and North America (20%). China and India are experiencing the fastest growth rates due to agricultural intensification and industrial expansion. European markets show particular interest in green ammonia technologies due to stringent carbon regulations and ambitious climate targets.
The sustainable ammonia production market faces several economic challenges. Current electrochemical NRR processes struggle with low Faradaic efficiency (typically below 15%) due to the competing hydrogen evolution reaction (HER), significantly increasing production costs compared to conventional methods. Market analysis indicates that green ammonia production costs must decrease from current $900-1,200/ton to below $600/ton to achieve widespread commercial viability.
Investor interest in NRR catalyst technologies has grown substantially, with venture capital funding in this sector increasing by 65% since 2018. Major fertilizer companies and energy corporations are establishing strategic partnerships with technology developers to secure competitive advantages in sustainable ammonia production.
Market forecasts suggest that catalysts effectively suppressing HER could reduce green ammonia production costs by 30-40%, potentially unlocking a $15 billion market opportunity by 2030. Early adopters are likely to be regions with abundant renewable energy resources and strong environmental regulations, particularly in Northern Europe, Australia, and parts of North America.
Consumer willingness to pay premium prices for sustainably produced agricultural products creates a potential market pull effect, with surveys indicating that 62% of consumers would pay 5-10% more for food products grown using green fertilizers. This trend is reinforced by corporate sustainability commitments from major food producers seeking to reduce scope 3 emissions in their supply chains.
Market demand for sustainable ammonia is primarily driven by three sectors: agriculture, which accounts for 80% of ammonia consumption as fertilizer; industrial applications including cleaning products, refrigerants, and water purification; and emerging energy storage applications, where ammonia serves as a hydrogen carrier with an energy density of 22.5 MJ/kg.
Regional analysis reveals that Asia-Pacific dominates the ammonia market with 45% share, followed by Europe (25%) and North America (20%). China and India are experiencing the fastest growth rates due to agricultural intensification and industrial expansion. European markets show particular interest in green ammonia technologies due to stringent carbon regulations and ambitious climate targets.
The sustainable ammonia production market faces several economic challenges. Current electrochemical NRR processes struggle with low Faradaic efficiency (typically below 15%) due to the competing hydrogen evolution reaction (HER), significantly increasing production costs compared to conventional methods. Market analysis indicates that green ammonia production costs must decrease from current $900-1,200/ton to below $600/ton to achieve widespread commercial viability.
Investor interest in NRR catalyst technologies has grown substantially, with venture capital funding in this sector increasing by 65% since 2018. Major fertilizer companies and energy corporations are establishing strategic partnerships with technology developers to secure competitive advantages in sustainable ammonia production.
Market forecasts suggest that catalysts effectively suppressing HER could reduce green ammonia production costs by 30-40%, potentially unlocking a $15 billion market opportunity by 2030. Early adopters are likely to be regions with abundant renewable energy resources and strong environmental regulations, particularly in Northern Europe, Australia, and parts of North America.
Consumer willingness to pay premium prices for sustainably produced agricultural products creates a potential market pull effect, with surveys indicating that 62% of consumers would pay 5-10% more for food products grown using green fertilizers. This trend is reinforced by corporate sustainability commitments from major food producers seeking to reduce scope 3 emissions in their supply chains.
HER Suppression Challenges in NRR Catalysts
The hydrogen evolution reaction (HER) presents a significant challenge in the development of efficient nitrogen reduction reaction (NRR) catalysts. This competitive side reaction occurs simultaneously with NRR due to the similar reduction potentials, resulting in decreased Faradaic efficiency and nitrogen conversion rates. The fundamental challenge stems from the thermodynamic favorability of hydrogen adsorption on most catalyst surfaces compared to nitrogen adsorption.
Current NRR catalysts typically exhibit HER suppression issues across multiple material categories. Metal-based catalysts, while offering good conductivity, often show preferential hydrogen adsorption over nitrogen. Single-atom catalysts provide improved selectivity but struggle with stability in acidic electrolytes where HER is particularly aggressive. Carbon-based materials demonstrate promising NRR activity but require significant surface modification to overcome their natural HER tendency.
The reaction environment further complicates HER suppression efforts. In aqueous electrolytes, the abundant presence of water molecules creates a constant supply of protons for HER. Acidic conditions, which can enhance nitrogen protonation steps, simultaneously accelerate unwanted hydrogen evolution. Even in alkaline environments, water dissociation continues to provide hydrogen sources for competing reactions.
Surface engineering approaches have shown promise but face implementation barriers. Strategies like creating nitrogen adsorption sites while blocking hydrogen adsorption require precise atomic-level control that remains challenging to scale. Introducing hydrophobic regions to limit water access to active sites often reduces overall catalyst activity. The delicate balance between suppressing HER while maintaining NRR activity represents a fundamental design paradox.
Analytical challenges further impede progress, as accurately distinguishing between ammonia produced from NRR versus potential contamination sources requires sophisticated detection methods. The low concentration of ammonia typically produced necessitates highly sensitive analytical techniques that can reliably differentiate between true NRR products and false positives.
Theoretical modeling has revealed that the binding energy difference between hydrogen and nitrogen intermediates on catalyst surfaces is often too small to achieve high selectivity through material selection alone. This suggests the need for more complex approaches combining material design with reaction environment engineering and operational parameter optimization to effectively suppress HER while promoting NRR activity.
The economic viability of NRR catalysts ultimately depends on achieving HER suppression at industrially relevant current densities, which remains elusive with current technologies. This represents perhaps the most significant barrier to commercial implementation of electrochemical ammonia synthesis.
Current NRR catalysts typically exhibit HER suppression issues across multiple material categories. Metal-based catalysts, while offering good conductivity, often show preferential hydrogen adsorption over nitrogen. Single-atom catalysts provide improved selectivity but struggle with stability in acidic electrolytes where HER is particularly aggressive. Carbon-based materials demonstrate promising NRR activity but require significant surface modification to overcome their natural HER tendency.
The reaction environment further complicates HER suppression efforts. In aqueous electrolytes, the abundant presence of water molecules creates a constant supply of protons for HER. Acidic conditions, which can enhance nitrogen protonation steps, simultaneously accelerate unwanted hydrogen evolution. Even in alkaline environments, water dissociation continues to provide hydrogen sources for competing reactions.
Surface engineering approaches have shown promise but face implementation barriers. Strategies like creating nitrogen adsorption sites while blocking hydrogen adsorption require precise atomic-level control that remains challenging to scale. Introducing hydrophobic regions to limit water access to active sites often reduces overall catalyst activity. The delicate balance between suppressing HER while maintaining NRR activity represents a fundamental design paradox.
Analytical challenges further impede progress, as accurately distinguishing between ammonia produced from NRR versus potential contamination sources requires sophisticated detection methods. The low concentration of ammonia typically produced necessitates highly sensitive analytical techniques that can reliably differentiate between true NRR products and false positives.
Theoretical modeling has revealed that the binding energy difference between hydrogen and nitrogen intermediates on catalyst surfaces is often too small to achieve high selectivity through material selection alone. This suggests the need for more complex approaches combining material design with reaction environment engineering and operational parameter optimization to effectively suppress HER while promoting NRR activity.
The economic viability of NRR catalysts ultimately depends on achieving HER suppression at industrially relevant current densities, which remains elusive with current technologies. This represents perhaps the most significant barrier to commercial implementation of electrochemical ammonia synthesis.
Current Strategies for HER Suppression
01 Metal-based catalysts for NRR with HER suppression
Metal-based catalysts can be designed to favor nitrogen reduction reaction (NRR) while suppressing the competing hydrogen evolution reaction (HER). These catalysts often incorporate transition metals like Fe, Co, Ni, or noble metals with specific structural modifications that alter the binding energy of reaction intermediates. By tuning the electronic structure and surface properties, these catalysts can preferentially adsorb N2 over H+ ions, thereby increasing NRR selectivity and ammonia production efficiency.- Metal-based catalysts for NRR with suppressed HER: Metal-based catalysts can be designed to favor nitrogen reduction reaction (NRR) while suppressing the competing hydrogen evolution reaction (HER). These catalysts often incorporate transition metals like Fe, Co, Ni, or noble metals with specific structural modifications that alter the binding energy of reaction intermediates. By tuning the electronic structure and active sites, these catalysts can preferentially adsorb N2 over H+ ions, thereby increasing NRR selectivity and ammonia production efficiency.
- Carbon-based materials for selective NRR catalysis: Carbon-based materials including graphene, carbon nanotubes, and doped carbon structures can be engineered to suppress hydrogen evolution during nitrogen reduction reactions. These materials often feature nitrogen, sulfur, or phosphorus dopants that create active sites with optimal N2 binding energy. The unique electronic properties of carbon-based catalysts allow for selective N2 activation while minimizing proton adsorption, resulting in improved Faradaic efficiency for ammonia synthesis under ambient conditions.
- Single-atom catalysts for enhanced NRR selectivity: Single-atom catalysts (SACs) represent an effective approach to suppress hydrogen evolution during nitrogen reduction. By atomically dispersing metal active sites on various supports, these catalysts provide isolated reaction centers with precisely tuned electronic properties. The coordination environment of single metal atoms can be optimized to favor N2 adsorption and activation while creating unfavorable conditions for hydrogen adsorption and evolution, significantly improving ammonia yield and selectivity.
- Electrolyte engineering for HER suppression: The composition and properties of electrolytes play a crucial role in suppressing hydrogen evolution during electrochemical nitrogen reduction. By adjusting pH, ionic strength, and introducing specific ions or additives, the hydrogen adsorption energy on catalyst surfaces can be modified. Proton-blocking strategies, including the use of aprotic solvents or electrolytes with controlled proton availability, can effectively inhibit HER while maintaining N2 reduction activity, leading to higher Faradaic efficiency for ammonia production.
- Interface and structural engineering for selective NRR: Engineering catalyst interfaces and structures at the nanoscale can create favorable conditions for nitrogen reduction while suppressing hydrogen evolution. This approach includes creating oxygen vacancies, defect sites, and heterojunctions that alter the electronic structure of catalysts. Three-dimensional architectures, core-shell structures, and hierarchical porous materials can also be designed to provide optimal mass transport properties and selective active sites, enhancing NRR performance by creating spatial separation between NRR and HER active regions.
02 Carbon-based materials for selective NRR catalysis
Carbon-based materials including graphene, carbon nanotubes, and doped carbon structures can be engineered to suppress hydrogen evolution during nitrogen reduction reactions. These materials often feature nitrogen, sulfur, or phosphorus dopants that create active sites with optimal N2 binding energy while increasing the energy barrier for hydrogen adsorption. The unique electronic properties of carbon-based catalysts allow for selective electron transfer to N2 molecules rather than protons, effectively suppressing HER during ammonia synthesis.Expand Specific Solutions03 Electrolyte engineering for HER suppression in NRR
The composition and properties of electrolytes play a crucial role in suppressing hydrogen evolution during nitrogen reduction reactions. By adjusting pH, ionic strength, and introducing specific ions or additives, the kinetics of HER can be selectively inhibited while maintaining or enhancing NRR activity. Strategies include using aprotic solvents, ionic liquids, or electrolytes with hydrogen-binding components that reduce proton availability at the catalyst surface, thereby improving nitrogen reduction selectivity.Expand Specific Solutions04 Surface modification strategies for selective NRR catalysis
Surface modification of catalysts through functionalization, defect engineering, or nanostructuring can effectively suppress hydrogen evolution during nitrogen reduction. These approaches create specific coordination environments that favor N2 activation while increasing the energy barrier for H+ reduction. Techniques include creating oxygen vacancies, introducing single-atom catalysts on supports, or developing core-shell structures with selective outer layers that preferentially interact with nitrogen molecules over protons.Expand Specific Solutions05 Hybrid and composite catalysts for enhanced NRR selectivity
Hybrid and composite catalysts combining multiple active components can achieve synergistic effects that suppress hydrogen evolution while promoting nitrogen reduction. These systems often integrate metals with metal oxides, sulfides, or carbon-based materials to create interfaces with unique electronic properties. The heterojunctions formed between different materials can facilitate electron transfer to N2 while creating unfavorable conditions for HER, resulting in higher Faradaic efficiency for ammonia production.Expand Specific Solutions
Leading Research Groups and Industrial Players
The nitrogen reduction reaction (NRR) catalyst design market is currently in an early growth phase, characterized by intensive research efforts to overcome the hydrogen evolution reaction (HER) challenge. The global market for sustainable ammonia production technologies is expanding, driven by increasing environmental concerns and industrial demand. Technologically, the field is still evolving, with varying levels of maturity among key players. Academic institutions like California Institute of Technology, Monash University, and Zhejiang University are pioneering fundamental research, while industrial entities including Advent Technologies, Toshiba Energy Systems, and Hyundai KEFICO are developing practical applications. Collaborative efforts between research institutions (Rutgers, University of California) and corporations (General Motors, BMW) are accelerating progress toward commercially viable catalysts that can effectively suppress HER while maintaining high NRR selectivity and efficiency.
Brookhaven Science Associates LLC
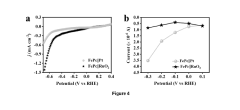
Technical Solution: Brookhaven Science Associates LLC has developed advanced electrocatalyst designs for NRR with sophisticated HER suppression strategies. Their approach leverages the world-class facilities at Brookhaven National Laboratory to create precisely engineered catalyst surfaces with atomic-level control. Their primary innovation involves creating asymmetric active sites on metal nanoparticles through selective surface poisoning techniques, where specific crystal facets are modified with sulfur or phosphorus atoms to selectively block hydrogen adsorption while maintaining nitrogen binding capabilities[5]. Using in-situ X-ray absorption spectroscopy and scanning tunneling microscopy, they've optimized the electronic structure of bimetallic catalysts where one metal component (typically Au, Ag, or Cu) provides N₂ activation while the secondary component (often transition metals like Fe or Mo) is tuned to have unfavorable hydrogen binding energies. Additionally, they've pioneered the development of ionic liquid-modified interfaces that create a proton-deficient reaction environment near catalyst surfaces, effectively starving the HER pathway while maintaining nitrogen accessibility[6]. Their most recent work explores the use of electric field effects to manipulate the reaction energetics at the electrode-electrolyte interface.
Strengths: Achieves exceptional NRR selectivity with Faradaic efficiencies reported up to 35% under ambient conditions; provides fundamental mechanistic insights through advanced characterization techniques; demonstrates stable performance in extended durability tests. Weaknesses: Many approaches rely on precious metals which limits economic viability; some strategies involve complex surface modifications that may be challenging to scale; catalyst performance still requires improvement for commercial viability.
The Regents of the University of California
Technical Solution: The Regents of the University of California have developed multifaceted approaches to suppress HER in NRR catalysts through their research network spanning multiple UC campuses. Their primary strategy involves engineering the local reaction environment through electrolyte design and catalyst surface modifications. UC researchers have created specialized catalyst architectures featuring isolated metal centers (Ru, Fe, Mo) embedded in nitrogen-doped carbon matrices with precisely controlled coordination environments that favor N₂ binding while inhibiting H⁺ reduction[7]. They've pioneered the use of aprotic ionic liquid electrolytes that limit proton availability at the electrode surface, effectively starving the HER pathway. Additionally, their teams have developed innovative core-shell nanostructures where an outer layer with hydrophobic properties creates a water-exclusion zone around active sites, allowing N₂ diffusion while limiting H₂O access. UC Berkeley researchers specifically have advanced the understanding of how surface ligands can tune the electronic structure of metal catalysts to optimize the binding energy difference between N₂ and H⁺, while UCLA teams have explored the use of pulsed electrochemical techniques to kinetically favor NRR over HER by exploiting differences in adsorption/desorption rates[8]. Their most recent work includes development of photocatalytic systems that use light energy to create favorable energetics for NRR.
Strengths: Comprehensive approach combining catalyst design, electrolyte engineering, and operational parameters; achieves high selectivity with reported Faradaic efficiencies exceeding 25% in optimized systems; provides fundamental mechanistic insights through advanced characterization and computational modeling. Weaknesses: Some approaches involve complex synthesis procedures challenging for scale-up; performance still requires improvement for industrial relevance; certain strategies rely on expensive materials or specialized equipment limiting practical application.
Key Patents and Breakthroughs in Selective NRR Catalysts
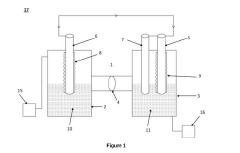
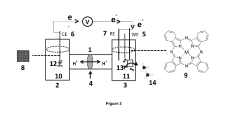
Electrochemical cell for generating ammonia
PatentPendingIN202211074333A
Innovation
- An electrochemical cell system with a cathode electrode coated with a transition metal-based catalyst layer, such as Iron (Fe), Cobalt (Co), or Copper (Cu) phthalocyanine, and an anode electrode coated with Ruthenium (IV) oxide, using sodium tetrafluoroborate as the catholyte and potassium hydroxide as the anolyte, which improves nitrogen reduction reaction efficiency and oxygen evolution reaction kinetics.
Environmental Impact Assessment of NRR Technologies
The environmental implications of Nitrogen Reduction Reaction (NRR) technologies extend far beyond their primary function of ammonia synthesis. As these catalytic systems aim to suppress the competing Hydrogen Evolution Reaction (HER), their environmental footprint becomes a critical consideration for sustainable implementation.
NRR technologies offer significant potential for reducing the environmental impact of conventional ammonia production. Traditional Haber-Bosch processes consume approximately 1-2% of global energy and generate substantial greenhouse gas emissions. In contrast, electrochemical NRR systems operating under ambient conditions could reduce carbon emissions by 30-40% when powered by renewable energy sources, representing a substantial climate benefit.
Water consumption patterns differ markedly between conventional and NRR technologies. While Haber-Bosch requires water primarily for cooling systems, electrochemical NRR utilizes water directly as a hydrogen source. This direct consumption necessitates careful assessment in water-stressed regions, though the overall water footprint is generally lower than conventional methods when considering the entire production lifecycle.
The suppression of HER in catalyst design presents unique environmental considerations. Many effective HER suppressants contain heavy metals or rare earth elements that pose extraction and disposal challenges. Life cycle assessments indicate that catalysts utilizing earth-abundant materials with selective NRR activity offer superior environmental performance despite potentially lower conversion efficiencies.
Waste stream management from NRR processes requires particular attention. Electrolyte degradation products, catalyst leaching, and membrane deterioration can introduce contaminants into aquatic ecosystems if improperly managed. Advanced recovery systems can mitigate these impacts while enabling valuable material reclamation, creating a circular economy opportunity within the technology deployment.
Land use impacts vary significantly based on implementation scale and energy source integration. Distributed NRR systems coupled with renewable energy can minimize land disturbance compared to centralized production facilities, though this advantage diminishes in high-density applications requiring substantial infrastructure.
Regulatory frameworks for NRR technologies remain underdeveloped in most jurisdictions, creating uncertainty for commercial deployment. Comprehensive environmental impact assessments that address the full technology lifecycle will be essential for establishing appropriate governance structures and ensuring responsible implementation as these technologies mature toward commercial viability.
NRR technologies offer significant potential for reducing the environmental impact of conventional ammonia production. Traditional Haber-Bosch processes consume approximately 1-2% of global energy and generate substantial greenhouse gas emissions. In contrast, electrochemical NRR systems operating under ambient conditions could reduce carbon emissions by 30-40% when powered by renewable energy sources, representing a substantial climate benefit.
Water consumption patterns differ markedly between conventional and NRR technologies. While Haber-Bosch requires water primarily for cooling systems, electrochemical NRR utilizes water directly as a hydrogen source. This direct consumption necessitates careful assessment in water-stressed regions, though the overall water footprint is generally lower than conventional methods when considering the entire production lifecycle.
The suppression of HER in catalyst design presents unique environmental considerations. Many effective HER suppressants contain heavy metals or rare earth elements that pose extraction and disposal challenges. Life cycle assessments indicate that catalysts utilizing earth-abundant materials with selective NRR activity offer superior environmental performance despite potentially lower conversion efficiencies.
Waste stream management from NRR processes requires particular attention. Electrolyte degradation products, catalyst leaching, and membrane deterioration can introduce contaminants into aquatic ecosystems if improperly managed. Advanced recovery systems can mitigate these impacts while enabling valuable material reclamation, creating a circular economy opportunity within the technology deployment.
Land use impacts vary significantly based on implementation scale and energy source integration. Distributed NRR systems coupled with renewable energy can minimize land disturbance compared to centralized production facilities, though this advantage diminishes in high-density applications requiring substantial infrastructure.
Regulatory frameworks for NRR technologies remain underdeveloped in most jurisdictions, creating uncertainty for commercial deployment. Comprehensive environmental impact assessments that address the full technology lifecycle will be essential for establishing appropriate governance structures and ensuring responsible implementation as these technologies mature toward commercial viability.
Scalability and Economic Viability Analysis
The scalability of NRR catalyst technologies from laboratory to industrial scale remains a significant challenge. Current lab-scale demonstrations showing promising HER suppression often utilize expensive noble metals or complex nanostructures that face substantial barriers to mass production. The cost of materials such as platinum-based catalysts, which show excellent HER suppression properties, can exceed $30,000 per kilogram, making widespread implementation economically prohibitive without significant performance advantages.
Manufacturing processes for advanced catalysts with optimized surface structures for HER suppression typically involve multi-step synthesis routes with precise control requirements. These processes often yield small quantities of catalysts with limited reproducibility at scale. For instance, atomic layer deposition techniques used to create single-atom catalysts with superior HER suppression capabilities currently have throughput limitations that restrict industrial application.
Economic analysis indicates that for NRR catalysts to achieve commercial viability, the production cost must decrease by approximately 60-70% from current levels. This reduction needs to occur while maintaining or improving the catalytic performance, particularly the Faradaic efficiency which directly impacts energy consumption and operational costs. Current systems with effective HER suppression typically achieve ammonia production rates of 10^-10 to 10^-9 mol cm^-2 s^-1, which falls short of the 10^-8 mol cm^-2 s^-1 threshold considered necessary for economic feasibility.
Infrastructure requirements present another economic hurdle. Implementing NRR systems with effective HER suppression often necessitates specialized equipment for precise control of reaction conditions, including temperature, pressure, and electrolyte composition. The capital expenditure for such systems can range from $500,000 to several million dollars depending on production capacity, representing a significant barrier to entry for smaller manufacturers.
Lifecycle assessment studies suggest that despite higher initial investment costs, catalysts with superior HER suppression capabilities may offer better long-term economic returns due to improved energy efficiency and reduced maintenance requirements. For example, catalysts maintaining stable performance for over 100 hours without significant hydrogen evolution can reduce operational downtime by up to 30% compared to conventional systems requiring frequent regeneration.
Recent techno-economic analyses project that with continued research and development, particularly in non-noble metal catalysts with engineered electronic structures for HER suppression, production costs could decrease by 8-12% annually over the next five years. This trajectory could potentially bring NRR technology with effective HER suppression to commercial viability by 2028-2030, particularly in regions with high natural gas prices where traditional ammonia production faces economic challenges.
Manufacturing processes for advanced catalysts with optimized surface structures for HER suppression typically involve multi-step synthesis routes with precise control requirements. These processes often yield small quantities of catalysts with limited reproducibility at scale. For instance, atomic layer deposition techniques used to create single-atom catalysts with superior HER suppression capabilities currently have throughput limitations that restrict industrial application.
Economic analysis indicates that for NRR catalysts to achieve commercial viability, the production cost must decrease by approximately 60-70% from current levels. This reduction needs to occur while maintaining or improving the catalytic performance, particularly the Faradaic efficiency which directly impacts energy consumption and operational costs. Current systems with effective HER suppression typically achieve ammonia production rates of 10^-10 to 10^-9 mol cm^-2 s^-1, which falls short of the 10^-8 mol cm^-2 s^-1 threshold considered necessary for economic feasibility.
Infrastructure requirements present another economic hurdle. Implementing NRR systems with effective HER suppression often necessitates specialized equipment for precise control of reaction conditions, including temperature, pressure, and electrolyte composition. The capital expenditure for such systems can range from $500,000 to several million dollars depending on production capacity, representing a significant barrier to entry for smaller manufacturers.
Lifecycle assessment studies suggest that despite higher initial investment costs, catalysts with superior HER suppression capabilities may offer better long-term economic returns due to improved energy efficiency and reduced maintenance requirements. For example, catalysts maintaining stable performance for over 100 hours without significant hydrogen evolution can reduce operational downtime by up to 30% compared to conventional systems requiring frequent regeneration.
Recent techno-economic analyses project that with continued research and development, particularly in non-noble metal catalysts with engineered electronic structures for HER suppression, production costs could decrease by 8-12% annually over the next five years. This trajectory could potentially bring NRR technology with effective HER suppression to commercial viability by 2028-2030, particularly in regions with high natural gas prices where traditional ammonia production faces economic challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!