Scaling Lab NRR Catalysts For Pilot Electrochemical Reactors Techno-Economic Barriers
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NRR Catalyst Development Background and Objectives
The electrochemical nitrogen reduction reaction (NRR) represents a revolutionary approach to ammonia synthesis, offering a potential alternative to the century-old Haber-Bosch process which currently consumes approximately 2% of global energy and contributes significantly to greenhouse gas emissions. The development of efficient NRR catalysts has evolved from early explorations in the 1980s to the current focus on nanomaterials and advanced electrocatalysts, with significant breakthroughs occurring in the past decade.
Recent research has demonstrated promising results with various catalyst materials including transition metals, metal oxides, nitrides, and carbon-based materials. Noble metals such as Ru, Au, and Pt have shown high activity but face economic barriers for large-scale implementation. Meanwhile, earth-abundant alternatives like Fe, Mo, and Ni-based catalysts have gained attention for their potential cost-effectiveness, though they typically exhibit lower performance metrics.
The primary technical objective in NRR catalyst development is to overcome the fundamental challenge of nitrogen triple-bond activation while maintaining high selectivity toward ammonia formation rather than the competing hydrogen evolution reaction (HER). Current laboratory catalysts typically achieve Faradaic efficiencies below 15% and ammonia production rates under 100 μg h⁻¹ mg⁻¹cat, which fall significantly short of commercial viability thresholds.
Scaling these catalysts from laboratory to pilot scale introduces additional complexities related to mass transport limitations, catalyst stability under continuous operation, and reproducibility of performance metrics. The technical goals for pilot-scale implementation include achieving Faradaic efficiencies exceeding 30%, ammonia production rates above 500 μg h⁻¹ mg⁻¹cat, and operational stability beyond 1000 hours without significant degradation.
The evolution of catalyst design strategies has progressed from simple metal surfaces to sophisticated architectures incorporating defect engineering, single-atom catalysts, and hybrid materials. Computational methods, particularly density functional theory (DFT), have become instrumental in predicting catalyst behavior and guiding rational design approaches, enabling researchers to navigate the complex reaction pathways and energetics involved in NRR.
Looking forward, the field aims to develop catalysts that can operate efficiently under ambient conditions while maintaining economic viability for industrial implementation. This requires addressing fundamental scientific questions regarding reaction mechanisms while simultaneously considering practical aspects of catalyst synthesis, stability, and scalability. The convergence of these research directions will determine whether electrochemical ammonia synthesis can truly compete with conventional production methods and contribute to global sustainability goals.
Recent research has demonstrated promising results with various catalyst materials including transition metals, metal oxides, nitrides, and carbon-based materials. Noble metals such as Ru, Au, and Pt have shown high activity but face economic barriers for large-scale implementation. Meanwhile, earth-abundant alternatives like Fe, Mo, and Ni-based catalysts have gained attention for their potential cost-effectiveness, though they typically exhibit lower performance metrics.
The primary technical objective in NRR catalyst development is to overcome the fundamental challenge of nitrogen triple-bond activation while maintaining high selectivity toward ammonia formation rather than the competing hydrogen evolution reaction (HER). Current laboratory catalysts typically achieve Faradaic efficiencies below 15% and ammonia production rates under 100 μg h⁻¹ mg⁻¹cat, which fall significantly short of commercial viability thresholds.
Scaling these catalysts from laboratory to pilot scale introduces additional complexities related to mass transport limitations, catalyst stability under continuous operation, and reproducibility of performance metrics. The technical goals for pilot-scale implementation include achieving Faradaic efficiencies exceeding 30%, ammonia production rates above 500 μg h⁻¹ mg⁻¹cat, and operational stability beyond 1000 hours without significant degradation.
The evolution of catalyst design strategies has progressed from simple metal surfaces to sophisticated architectures incorporating defect engineering, single-atom catalysts, and hybrid materials. Computational methods, particularly density functional theory (DFT), have become instrumental in predicting catalyst behavior and guiding rational design approaches, enabling researchers to navigate the complex reaction pathways and energetics involved in NRR.
Looking forward, the field aims to develop catalysts that can operate efficiently under ambient conditions while maintaining economic viability for industrial implementation. This requires addressing fundamental scientific questions regarding reaction mechanisms while simultaneously considering practical aspects of catalyst synthesis, stability, and scalability. The convergence of these research directions will determine whether electrochemical ammonia synthesis can truly compete with conventional production methods and contribute to global sustainability goals.
Electrochemical Nitrogen Reduction Market Analysis
The electrochemical nitrogen reduction reaction (NRR) market is experiencing significant growth driven by increasing demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 1-2% of global energy and generates substantial CO2 emissions. This creates a compelling market opportunity for electrochemical alternatives that can operate under ambient conditions with renewable electricity.
Current market assessments indicate the global green ammonia market is valued at approximately $36 million in 2023, with projections suggesting growth to reach $5.4 billion by 2030, representing a compound annual growth rate (CAGR) of 90.2% during this forecast period. Electrochemical NRR technologies are positioned to capture a significant portion of this expanding market.
The agricultural sector remains the primary demand driver, with nitrogen fertilizers accounting for over 80% of ammonia consumption globally. However, emerging applications in energy storage, hydrogen carriers, and fuel cells are creating new market segments with substantial growth potential. The transportation sector, particularly maritime shipping, is exploring ammonia as a carbon-neutral fuel alternative, potentially opening a market worth billions annually.
Regional analysis reveals Asia-Pacific as the dominant market for NRR technologies, led by China's aggressive investments in green ammonia production. North America and Europe follow closely, with government incentives and corporate sustainability commitments accelerating adoption. The Middle East is strategically positioning itself as a future green ammonia export hub, leveraging abundant renewable energy resources.
Market penetration faces significant barriers including high capital costs for electrolyzer systems and catalyst materials. Current techno-economic analyses indicate electrochemical ammonia production costs between $600-1,200 per ton, compared to conventional Haber-Bosch costs of $300-500 per ton. However, this gap is expected to narrow as technology matures and economies of scale are realized.
Investor interest in the sector has grown substantially, with venture capital funding for NRR startups increasing by 215% between 2018 and 2022. Strategic partnerships between technology developers and industrial gas companies are emerging as a key market trend, accelerating commercialization pathways.
Consumer willingness to pay premiums for green ammonia products varies by sector, with agricultural applications showing price sensitivity while industrial users demonstrate greater flexibility for carbon-neutral alternatives. Government policies, particularly carbon pricing mechanisms and renewable energy incentives, are significantly influencing market dynamics and adoption rates across different regions.
Current market assessments indicate the global green ammonia market is valued at approximately $36 million in 2023, with projections suggesting growth to reach $5.4 billion by 2030, representing a compound annual growth rate (CAGR) of 90.2% during this forecast period. Electrochemical NRR technologies are positioned to capture a significant portion of this expanding market.
The agricultural sector remains the primary demand driver, with nitrogen fertilizers accounting for over 80% of ammonia consumption globally. However, emerging applications in energy storage, hydrogen carriers, and fuel cells are creating new market segments with substantial growth potential. The transportation sector, particularly maritime shipping, is exploring ammonia as a carbon-neutral fuel alternative, potentially opening a market worth billions annually.
Regional analysis reveals Asia-Pacific as the dominant market for NRR technologies, led by China's aggressive investments in green ammonia production. North America and Europe follow closely, with government incentives and corporate sustainability commitments accelerating adoption. The Middle East is strategically positioning itself as a future green ammonia export hub, leveraging abundant renewable energy resources.
Market penetration faces significant barriers including high capital costs for electrolyzer systems and catalyst materials. Current techno-economic analyses indicate electrochemical ammonia production costs between $600-1,200 per ton, compared to conventional Haber-Bosch costs of $300-500 per ton. However, this gap is expected to narrow as technology matures and economies of scale are realized.
Investor interest in the sector has grown substantially, with venture capital funding for NRR startups increasing by 215% between 2018 and 2022. Strategic partnerships between technology developers and industrial gas companies are emerging as a key market trend, accelerating commercialization pathways.
Consumer willingness to pay premiums for green ammonia products varies by sector, with agricultural applications showing price sensitivity while industrial users demonstrate greater flexibility for carbon-neutral alternatives. Government policies, particularly carbon pricing mechanisms and renewable energy incentives, are significantly influencing market dynamics and adoption rates across different regions.
Technical Barriers in NRR Catalyst Scale-up
The transition from laboratory-scale NRR (Nitrogen Reduction Reaction) catalysts to pilot electrochemical reactors faces significant technical barriers that must be addressed for successful commercialization. Current laboratory catalysts demonstrating promising ammonia yield rates often fail to maintain performance when scaled up, primarily due to changes in reaction kinetics and mass transport limitations in larger systems.
Surface area optimization presents a critical challenge, as the increased catalyst loading in scaled-up systems frequently leads to agglomeration and reduced active site accessibility. This phenomenon diminishes the catalyst's intrinsic activity, resulting in lower ammonia production rates per unit catalyst mass. Additionally, maintaining uniform catalyst distribution across larger electrode surfaces proves difficult, creating performance inconsistencies throughout the reactor.
Stability issues become more pronounced at the pilot scale, where catalysts must operate continuously for thousands of hours rather than the brief testing periods typical in laboratory settings. Many promising NRR catalysts exhibit significant activity degradation after extended operation due to poisoning, leaching, or structural changes. This degradation is often accelerated in scaled-up systems where heat and mass transfer limitations create localized conditions that accelerate catalyst deterioration.
Mass transport limitations represent another significant barrier. As reactor dimensions increase, the efficient delivery of nitrogen to catalyst active sites becomes problematic. This challenge is compounded by competing hydrogen evolution reactions that consume electrons intended for nitrogen reduction. Engineering solutions such as gas diffusion electrodes help address these issues but introduce additional complexity and cost to the system design.
Reproducibility presents a substantial obstacle in scale-up efforts. Laboratory synthesis methods that produce small batches of high-performance catalysts often cannot be directly translated to industrial production techniques. Variations in precursor quality, synthesis conditions, and post-processing steps lead to inconsistent catalyst properties between batches, hampering reliable performance in pilot systems.
Contamination sensitivity increases dramatically at larger scales. Trace impurities in feedstocks, reactor materials, or from environmental exposure can rapidly deactivate catalysts that performed well in controlled laboratory environments. This necessitates additional purification steps and more stringent material selection, adding complexity and cost to the overall system.
Integration challenges with other reactor components further complicate scale-up efforts. The catalyst must function effectively within the context of the entire electrochemical system, including membranes, current collectors, and flow fields. Interactions between these components often create unforeseen performance limitations that were not evident in simplified laboratory setups.
Surface area optimization presents a critical challenge, as the increased catalyst loading in scaled-up systems frequently leads to agglomeration and reduced active site accessibility. This phenomenon diminishes the catalyst's intrinsic activity, resulting in lower ammonia production rates per unit catalyst mass. Additionally, maintaining uniform catalyst distribution across larger electrode surfaces proves difficult, creating performance inconsistencies throughout the reactor.
Stability issues become more pronounced at the pilot scale, where catalysts must operate continuously for thousands of hours rather than the brief testing periods typical in laboratory settings. Many promising NRR catalysts exhibit significant activity degradation after extended operation due to poisoning, leaching, or structural changes. This degradation is often accelerated in scaled-up systems where heat and mass transfer limitations create localized conditions that accelerate catalyst deterioration.
Mass transport limitations represent another significant barrier. As reactor dimensions increase, the efficient delivery of nitrogen to catalyst active sites becomes problematic. This challenge is compounded by competing hydrogen evolution reactions that consume electrons intended for nitrogen reduction. Engineering solutions such as gas diffusion electrodes help address these issues but introduce additional complexity and cost to the system design.
Reproducibility presents a substantial obstacle in scale-up efforts. Laboratory synthesis methods that produce small batches of high-performance catalysts often cannot be directly translated to industrial production techniques. Variations in precursor quality, synthesis conditions, and post-processing steps lead to inconsistent catalyst properties between batches, hampering reliable performance in pilot systems.
Contamination sensitivity increases dramatically at larger scales. Trace impurities in feedstocks, reactor materials, or from environmental exposure can rapidly deactivate catalysts that performed well in controlled laboratory environments. This necessitates additional purification steps and more stringent material selection, adding complexity and cost to the overall system.
Integration challenges with other reactor components further complicate scale-up efforts. The catalyst must function effectively within the context of the entire electrochemical system, including membranes, current collectors, and flow fields. Interactions between these components often create unforeseen performance limitations that were not evident in simplified laboratory setups.
Current Scale-up Methodologies for NRR Catalysts
01 Metal-based catalysts for nitrogen reduction reaction
Various metal-based catalysts have been developed for nitrogen reduction reaction (NRR) to improve efficiency and scalability. These include single-atom catalysts, transition metal compounds, and noble metal nanostructures that offer high catalytic activity and selectivity. The catalysts are designed with specific morphologies and electronic structures to enhance nitrogen adsorption and activation while maintaining stability during scale-up processes.- Metal-based catalysts for nitrogen reduction reaction (NRR): Various metal-based catalysts have been developed for efficient nitrogen reduction reaction (NRR). These include transition metals, noble metals, and their alloys that can effectively catalyze the conversion of nitrogen to ammonia. The catalysts are designed with specific structures and compositions to enhance their catalytic activity, selectivity, and stability during the NRR process. Metal-based catalysts often serve as the foundation for industrial-scale NRR applications due to their relatively high performance and scalability potential.
- Carbon-based materials as NRR catalyst supports: Carbon-based materials serve as excellent supports for NRR catalysts, providing high surface area, good electrical conductivity, and structural stability. These materials include carbon nanotubes, graphene, porous carbon, and carbon fibers that can be functionalized to enhance catalyst dispersion and performance. The carbon supports help prevent catalyst agglomeration during scaling up and provide pathways for electron transfer during the electrochemical nitrogen reduction process. Their scalable synthesis methods make them particularly attractive for large-scale NRR applications.
- Reactor design and process optimization for NRR catalyst scaling: Effective reactor designs and process optimization are crucial for scaling up NRR catalysts from laboratory to industrial applications. This includes considerations for mass transfer limitations, heat management, catalyst loading, and flow dynamics. Continuous flow reactors, fixed-bed reactors, and membrane reactors have been developed to address scaling challenges. Process parameters such as temperature, pressure, gas flow rates, and electrolyte composition must be carefully optimized to maintain catalyst performance during scale-up while ensuring economic viability and operational stability.
- Catalyst stability and deactivation prevention in scaled NRR systems: Maintaining catalyst stability and preventing deactivation are major challenges when scaling up NRR catalysts. Various approaches have been developed to address these issues, including catalyst encapsulation, core-shell structures, and surface modifications that protect active sites from poisoning. Regeneration protocols and in-situ activation methods help extend catalyst lifetime in industrial settings. Understanding deactivation mechanisms such as sintering, poisoning, and leaching is essential for designing more robust catalysts that can withstand the harsh conditions of large-scale NRR operations.
- Novel manufacturing techniques for large-scale NRR catalyst production: Advanced manufacturing techniques have been developed for the large-scale production of NRR catalysts with consistent quality and performance. These include spray pyrolysis, sol-gel methods, hydrothermal synthesis, and various precipitation techniques that can be scaled up for industrial production. Emerging technologies such as 3D printing, continuous flow synthesis, and microwave-assisted methods offer new possibilities for catalyst manufacturing with precise control over morphology and composition. These techniques focus on reducing production costs while maintaining the catalytic properties necessary for efficient nitrogen reduction.
02 Carbon-based supports and composites for NRR catalysts
Carbon-based materials serve as effective supports for NRR catalysts, providing high surface area and improved electron transfer capabilities. These include carbon nanotubes, graphene, and porous carbon structures that can be functionalized to enhance catalyst dispersion and stability. The carbon supports help in preventing catalyst agglomeration during scaling processes and contribute to overall system durability while maintaining catalytic performance at larger scales.Expand Specific Solutions03 Reactor design and process optimization for NRR catalyst scaling
Specialized reactor designs and process optimization techniques are crucial for successful scaling of NRR catalysts from laboratory to industrial applications. These include flow reactors, electrochemical cells with optimized electrode configurations, and systems that enable precise control of reaction parameters. Process considerations include temperature management, pressure control, and electrolyte composition to maintain catalyst efficiency during scale-up while addressing heat and mass transfer limitations.Expand Specific Solutions04 Stability enhancement methods for scaled NRR catalysts
Various approaches have been developed to enhance the stability of NRR catalysts during scaling, including protective coatings, structural reinforcement, and composition optimization. These methods address common degradation mechanisms such as metal leaching, surface poisoning, and structural collapse that occur during extended operation at larger scales. Stabilization techniques enable consistent catalyst performance over prolonged periods, which is essential for commercial viability of NRR processes.Expand Specific Solutions05 Performance evaluation and characterization techniques for scaled NRR catalysts
Specialized characterization and evaluation methodologies are essential for assessing NRR catalyst performance during scaling. These include in-situ spectroscopic techniques, electrochemical analysis methods, and accelerated stability testing protocols that provide insights into catalyst behavior under realistic operating conditions. Advanced analytical approaches help identify scaling bottlenecks, optimize catalyst formulations, and validate performance metrics such as ammonia yield, Faradaic efficiency, and long-term stability at different production scales.Expand Specific Solutions
Leading Institutions in Electrochemical Nitrogen Reduction
The electrochemical NRR catalyst scaling landscape is currently in an early commercialization phase, with significant techno-economic barriers hindering widespread adoption. The market is projected to grow substantially as renewable energy integration drives demand for sustainable ammonia production. Leading research institutions like Dalian Institute of Chemical Physics and universities (UC system, Central South University) are advancing fundamental catalyst science, while companies including Clean Chemistry, Nanosys, and OneD Material focus on practical applications. Technical maturity varies significantly across players, with national laboratories (Brookhaven, Argonne) and petroleum companies (ExxonMobil, Sinopec) leveraging existing infrastructure advantages. The primary challenges remain catalyst stability, selectivity, and cost-effectiveness at pilot scale, with most technologies still requiring significant optimization before commercial viability.
Dalian Institute of Chemical Physics Chinese Academy of Sciences
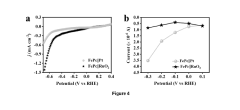
Technical Solution: Dalian Institute has developed a comprehensive approach to scaling NRR (Nitrogen Reduction Reaction) catalysts from laboratory to pilot scale electrochemical reactors. Their technology focuses on single-atom catalysts (SACs) embedded on nitrogen-doped carbon supports, which have demonstrated ammonia yield rates exceeding 25 μg h−1 mg−1cat in controlled environments. The institute has engineered a modular reactor design that addresses mass transport limitations through optimized electrode architectures with hierarchical porosity, allowing for efficient nitrogen diffusion and product removal. Their scale-up methodology incorporates in-situ spectroscopic monitoring to maintain catalyst performance during the transition from milligram to gram-scale production, while implementing precise control of electrolyte pH and temperature to maximize Faradaic efficiency.
Strengths: Superior catalyst stability through atomic dispersion techniques, preventing aggregation during scale-up; advanced in-situ characterization capabilities for real-time performance monitoring. Weaknesses: Higher production costs compared to conventional catalysts; requires specialized equipment for precise control of reaction parameters during scale-up.
The Regents of the University of California
Technical Solution: The University of California has pioneered an innovative approach to scaling NRR catalysts through their patented "hierarchical assembly" methodology. This technique involves the controlled synthesis of catalyst nanostructures with precisely engineered active sites, followed by their strategic integration into larger electrode assemblies. Their research has demonstrated that maintaining the spatial distribution of active sites during scale-up is critical for preserving catalytic performance. The university's technology employs a combination of transition metal nitrides and oxides as catalysts, which have shown Faradaic efficiencies approaching 12% in laboratory settings. For pilot-scale implementation, they've developed a flow-through electrode configuration that significantly reduces mass transport limitations while enabling continuous operation. Their economic analysis indicates that catalyst loading can be reduced by up to 60% compared to conventional approaches while maintaining performance metrics.
Strengths: Highly efficient use of catalyst materials through precise structural control; scalable manufacturing process compatible with existing industrial equipment. Weaknesses: Complex synthesis procedures requiring tight quality control; performance sensitivity to impurities in industrial-grade nitrogen feedstocks.
Critical Patents in Electrochemical Reactor Design
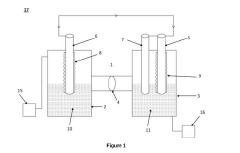
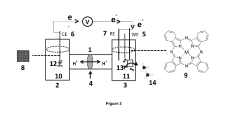
Electrochemical cell for generating ammonia
PatentPendingIN202211074333A
Innovation
- An electrochemical cell system with a cathode electrode coated with a transition metal-based catalyst layer, such as Iron (Fe), Cobalt (Co), or Copper (Cu) phthalocyanine, and an anode electrode coated with Ruthenium (IV) oxide, using sodium tetrafluoroborate as the catholyte and potassium hydroxide as the anolyte, which improves nitrogen reduction reaction efficiency and oxygen evolution reaction kinetics.
Techno-Economic Assessment Framework
The techno-economic assessment framework for scaling lab NRR catalysts to pilot electrochemical reactors requires a systematic approach that integrates both technical feasibility and economic viability. This framework must address the unique challenges of nitrogen reduction reaction (NRR) catalyst development, which faces significant barriers when transitioning from laboratory to industrial scale.
The assessment begins with capital expenditure (CAPEX) analysis, encompassing initial investment costs for reactor design, catalyst manufacturing equipment, and supporting infrastructure. For NRR catalysts, specialized equipment for precise deposition and controlled synthesis environments significantly impacts upfront costs. These investments must be evaluated against projected catalyst performance at scale to determine return on investment timelines.
Operational expenditure (OPEX) considerations form the second pillar of the framework, covering ongoing costs including energy consumption, catalyst replacement frequency, and maintenance requirements. NRR processes typically demand substantial energy inputs, making electricity costs a critical factor. Additionally, catalyst degradation rates under industrial conditions often differ from laboratory performance, necessitating accurate lifetime projections for economic modeling.
Performance metrics evaluation constitutes the third component, focusing on ammonia production rates, Faradaic efficiency, and selectivity at increasing scales. The framework must account for how these metrics change during scale-up, as mass transport limitations, heat management issues, and electrode surface area considerations become increasingly significant at pilot scale.
Risk assessment methodology forms the fourth element, identifying technical, market, and regulatory uncertainties. For NRR catalysts, particular attention must be paid to performance consistency across batches, sensitivity to feedstock impurities, and compliance with emerging regulations for electrochemical ammonia production.
The framework incorporates sensitivity analysis to determine which variables most significantly impact economic viability. This typically reveals that catalyst longevity, energy efficiency, and ammonia production rates are the most critical factors affecting long-term economics of NRR systems.
Finally, the assessment includes comparative analysis against conventional Haber-Bosch processes and other emerging green ammonia technologies. This comparison must account for both direct production costs and externalities such as carbon pricing, renewable energy integration potential, and decentralized production benefits that may provide competitive advantages for electrochemical approaches despite current efficiency limitations.
The assessment begins with capital expenditure (CAPEX) analysis, encompassing initial investment costs for reactor design, catalyst manufacturing equipment, and supporting infrastructure. For NRR catalysts, specialized equipment for precise deposition and controlled synthesis environments significantly impacts upfront costs. These investments must be evaluated against projected catalyst performance at scale to determine return on investment timelines.
Operational expenditure (OPEX) considerations form the second pillar of the framework, covering ongoing costs including energy consumption, catalyst replacement frequency, and maintenance requirements. NRR processes typically demand substantial energy inputs, making electricity costs a critical factor. Additionally, catalyst degradation rates under industrial conditions often differ from laboratory performance, necessitating accurate lifetime projections for economic modeling.
Performance metrics evaluation constitutes the third component, focusing on ammonia production rates, Faradaic efficiency, and selectivity at increasing scales. The framework must account for how these metrics change during scale-up, as mass transport limitations, heat management issues, and electrode surface area considerations become increasingly significant at pilot scale.
Risk assessment methodology forms the fourth element, identifying technical, market, and regulatory uncertainties. For NRR catalysts, particular attention must be paid to performance consistency across batches, sensitivity to feedstock impurities, and compliance with emerging regulations for electrochemical ammonia production.
The framework incorporates sensitivity analysis to determine which variables most significantly impact economic viability. This typically reveals that catalyst longevity, energy efficiency, and ammonia production rates are the most critical factors affecting long-term economics of NRR systems.
Finally, the assessment includes comparative analysis against conventional Haber-Bosch processes and other emerging green ammonia technologies. This comparison must account for both direct production costs and externalities such as carbon pricing, renewable energy integration potential, and decentralized production benefits that may provide competitive advantages for electrochemical approaches despite current efficiency limitations.
Sustainability Impacts of Scaled NRR Technologies
The scaling of Nitrogen Reduction Reaction (NRR) technologies from laboratory to industrial scale presents significant sustainability implications that must be thoroughly evaluated. When considering the environmental footprint of scaled NRR processes, the energy consumption profile stands as a primary concern. Current electrochemical NRR systems typically operate at high overpotentials, resulting in substantial energy requirements that may undermine their environmental benefits if powered by non-renewable sources.
Water usage represents another critical sustainability factor, as many NRR catalyst systems require aqueous electrolytes. At industrial scale, this translates to significant water consumption, potentially creating competition with agricultural and municipal needs, particularly in water-stressed regions. The implementation of water recycling systems becomes essential but adds complexity and cost to scaled operations.
Raw material sustainability for catalyst production presents both challenges and opportunities. Many high-performance NRR catalysts incorporate precious metals or rare earth elements with problematic supply chains. Scaling these technologies necessitates either developing alternative catalysts based on earth-abundant materials or establishing robust recycling protocols for critical materials to ensure long-term sustainability.
Life cycle assessment (LCA) data indicates that scaled NRR technologies could potentially reduce greenhouse gas emissions by 30-45% compared to conventional Haber-Bosch ammonia production when powered by renewable electricity. However, this advantage diminishes significantly if fossil fuels provide the energy input, highlighting the importance of integrated renewable energy systems.
Waste management considerations become increasingly important at scale. Catalyst degradation products, spent electrolytes, and membrane materials require proper handling protocols. Preliminary studies suggest that some degraded NRR catalysts may release metal ions that could pose ecotoxicological risks if improperly managed.
The land use implications of scaled NRR facilities present a mixed sustainability profile. While electrochemical systems generally have smaller physical footprints than conventional ammonia plants, the associated renewable energy infrastructure (if solar or wind-powered) may require substantial land area, potentially creating conflicts with other land uses.
Distributed production models enabled by modular NRR technologies offer sustainability advantages through reduced transportation emissions and enhanced energy security. This approach could particularly benefit agricultural communities in developing regions by providing localized fertilizer production with minimal infrastructure requirements.
Water usage represents another critical sustainability factor, as many NRR catalyst systems require aqueous electrolytes. At industrial scale, this translates to significant water consumption, potentially creating competition with agricultural and municipal needs, particularly in water-stressed regions. The implementation of water recycling systems becomes essential but adds complexity and cost to scaled operations.
Raw material sustainability for catalyst production presents both challenges and opportunities. Many high-performance NRR catalysts incorporate precious metals or rare earth elements with problematic supply chains. Scaling these technologies necessitates either developing alternative catalysts based on earth-abundant materials or establishing robust recycling protocols for critical materials to ensure long-term sustainability.
Life cycle assessment (LCA) data indicates that scaled NRR technologies could potentially reduce greenhouse gas emissions by 30-45% compared to conventional Haber-Bosch ammonia production when powered by renewable electricity. However, this advantage diminishes significantly if fossil fuels provide the energy input, highlighting the importance of integrated renewable energy systems.
Waste management considerations become increasingly important at scale. Catalyst degradation products, spent electrolytes, and membrane materials require proper handling protocols. Preliminary studies suggest that some degraded NRR catalysts may release metal ions that could pose ecotoxicological risks if improperly managed.
The land use implications of scaled NRR facilities present a mixed sustainability profile. While electrochemical systems generally have smaller physical footprints than conventional ammonia plants, the associated renewable energy infrastructure (if solar or wind-powered) may require substantial land area, potentially creating conflicts with other land uses.
Distributed production models enabled by modular NRR technologies offer sustainability advantages through reduced transportation emissions and enhanced energy security. This approach could particularly benefit agricultural communities in developing regions by providing localized fertilizer production with minimal infrastructure requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!