How To Engineer Gas Diffusion Electrodes For Enhanced N2 Mass Transport
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gas Diffusion Electrodes Background and Objectives
Gas diffusion electrodes (GDEs) have emerged as critical components in electrochemical systems aimed at nitrogen reduction reaction (NRR) for ammonia synthesis. The development of these electrodes traces back to fuel cell technology in the mid-20th century, but their application for nitrogen fixation represents a relatively recent technological pivot. The evolution of GDEs has been marked by significant improvements in materials science, nanotechnology, and electrochemical engineering over the past two decades.
The fundamental challenge in nitrogen reduction lies in the exceptional stability of the N≡N triple bond, requiring 941 kJ/mol for dissociation. Traditional industrial ammonia production via the Haber-Bosch process operates under harsh conditions (400-500°C, 150-300 bar), consuming approximately 1-2% of global energy production. Electrochemical nitrogen reduction using GDEs presents a promising alternative pathway that can potentially operate under ambient conditions with renewable electricity.
Current technological trends in GDE development focus on hierarchical porous structures, advanced catalyst integration, and interface engineering. The field has witnessed a shift from conventional carbon-based GDEs to more sophisticated designs incorporating transition metal nitrides, single-atom catalysts, and defect-engineered materials specifically tailored for nitrogen activation and conversion.
The primary objective of engineering GDEs for enhanced N₂ mass transport is to overcome the inherent limitations of nitrogen's low solubility in aqueous electrolytes (0.66 mM at 25°C, 1 atm), which creates significant mass transport constraints. By facilitating efficient nitrogen delivery to catalytically active sites, properly engineered GDEs can substantially improve ammonia production rates and Faradaic efficiency.
Secondary objectives include developing electrodes with optimized three-phase boundaries where gas, liquid, and solid catalyst interfaces meet, enhancing hydrophobicity to prevent electrode flooding while maintaining ionic conductivity, and ensuring long-term operational stability under varying electrochemical conditions.
The technological goal extends beyond merely improving nitrogen transport to creating integrated electrode systems that can selectively reduce nitrogen while suppressing the competing hydrogen evolution reaction. This requires precise control over pore architecture, wettability gradients, and catalyst distribution within the electrode structure.
Future GDE development aims to achieve ammonia production rates exceeding 10⁻⁶ mol cm⁻² s⁻¹ with Faradaic efficiencies above 30% under ambient conditions, representing a significant advancement over current benchmarks. Achieving these metrics would position electrochemical nitrogen reduction as a viable alternative to the century-old Haber-Bosch process, particularly for distributed, renewable-powered ammonia production systems.
The fundamental challenge in nitrogen reduction lies in the exceptional stability of the N≡N triple bond, requiring 941 kJ/mol for dissociation. Traditional industrial ammonia production via the Haber-Bosch process operates under harsh conditions (400-500°C, 150-300 bar), consuming approximately 1-2% of global energy production. Electrochemical nitrogen reduction using GDEs presents a promising alternative pathway that can potentially operate under ambient conditions with renewable electricity.
Current technological trends in GDE development focus on hierarchical porous structures, advanced catalyst integration, and interface engineering. The field has witnessed a shift from conventional carbon-based GDEs to more sophisticated designs incorporating transition metal nitrides, single-atom catalysts, and defect-engineered materials specifically tailored for nitrogen activation and conversion.
The primary objective of engineering GDEs for enhanced N₂ mass transport is to overcome the inherent limitations of nitrogen's low solubility in aqueous electrolytes (0.66 mM at 25°C, 1 atm), which creates significant mass transport constraints. By facilitating efficient nitrogen delivery to catalytically active sites, properly engineered GDEs can substantially improve ammonia production rates and Faradaic efficiency.
Secondary objectives include developing electrodes with optimized three-phase boundaries where gas, liquid, and solid catalyst interfaces meet, enhancing hydrophobicity to prevent electrode flooding while maintaining ionic conductivity, and ensuring long-term operational stability under varying electrochemical conditions.
The technological goal extends beyond merely improving nitrogen transport to creating integrated electrode systems that can selectively reduce nitrogen while suppressing the competing hydrogen evolution reaction. This requires precise control over pore architecture, wettability gradients, and catalyst distribution within the electrode structure.
Future GDE development aims to achieve ammonia production rates exceeding 10⁻⁶ mol cm⁻² s⁻¹ with Faradaic efficiencies above 30% under ambient conditions, representing a significant advancement over current benchmarks. Achieving these metrics would position electrochemical nitrogen reduction as a viable alternative to the century-old Haber-Bosch process, particularly for distributed, renewable-powered ammonia production systems.
N2 Electroreduction Market Analysis
The global market for nitrogen electroreduction technology is experiencing significant growth, driven by increasing demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 2% of global energy production and generates substantial CO2 emissions. This creates a compelling market opportunity for electrochemical nitrogen reduction reaction (NRR) technologies that can operate at ambient conditions with renewable electricity.
The market for NRR technologies is currently segmented into research applications, pilot industrial implementations, and emerging commercial solutions. Research funding in this sector has seen a compound annual growth rate exceeding 15% over the past five years, with major investments coming from both public institutions and private corporations seeking to develop carbon-neutral fertilizer production capabilities.
Key market drivers include stringent environmental regulations, rising costs of carbon-intensive processes, and increasing adoption of distributed manufacturing models in the agricultural sector. The fertilizer industry, valued at over $170 billion globally, represents the primary target market, with additional opportunities in specialty chemicals and pharmaceutical intermediates production.
Regional analysis reveals that Asia-Pacific, particularly China, leads in research output and patent filings related to nitrogen electroreduction technologies. North America and Europe follow closely, with significant investments in startup companies developing scalable electrode technologies for enhanced nitrogen conversion efficiency.
Market challenges include the current low Faradaic efficiency of NRR processes compared to established technologies, high costs of catalyst materials, and scaling limitations. These factors have restricted widespread commercial adoption, creating a technology gap that innovative gas diffusion electrode designs could potentially address.
Industry forecasts suggest that improvements in nitrogen mass transport through advanced electrode engineering could reduce production costs by up to 40%, potentially unlocking a market segment worth billions in the green ammonia sector alone. Early adopters are likely to be decentralized agricultural operations and specialty chemical manufacturers seeking to reduce their carbon footprint.
Competitive analysis indicates that several technology startups have secured substantial funding to develop proprietary electrode designs specifically targeting enhanced N2 mass transport. Established chemical companies are also entering this space through strategic partnerships and acquisition of promising technologies, signaling strong market confidence in the commercial potential of electrochemical nitrogen reduction.
The market for NRR technologies is currently segmented into research applications, pilot industrial implementations, and emerging commercial solutions. Research funding in this sector has seen a compound annual growth rate exceeding 15% over the past five years, with major investments coming from both public institutions and private corporations seeking to develop carbon-neutral fertilizer production capabilities.
Key market drivers include stringent environmental regulations, rising costs of carbon-intensive processes, and increasing adoption of distributed manufacturing models in the agricultural sector. The fertilizer industry, valued at over $170 billion globally, represents the primary target market, with additional opportunities in specialty chemicals and pharmaceutical intermediates production.
Regional analysis reveals that Asia-Pacific, particularly China, leads in research output and patent filings related to nitrogen electroreduction technologies. North America and Europe follow closely, with significant investments in startup companies developing scalable electrode technologies for enhanced nitrogen conversion efficiency.
Market challenges include the current low Faradaic efficiency of NRR processes compared to established technologies, high costs of catalyst materials, and scaling limitations. These factors have restricted widespread commercial adoption, creating a technology gap that innovative gas diffusion electrode designs could potentially address.
Industry forecasts suggest that improvements in nitrogen mass transport through advanced electrode engineering could reduce production costs by up to 40%, potentially unlocking a market segment worth billions in the green ammonia sector alone. Early adopters are likely to be decentralized agricultural operations and specialty chemical manufacturers seeking to reduce their carbon footprint.
Competitive analysis indicates that several technology startups have secured substantial funding to develop proprietary electrode designs specifically targeting enhanced N2 mass transport. Established chemical companies are also entering this space through strategic partnerships and acquisition of promising technologies, signaling strong market confidence in the commercial potential of electrochemical nitrogen reduction.
Current GDE Technology Limitations
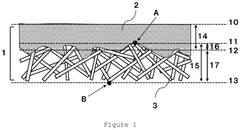
Despite significant advancements in gas diffusion electrode (GDE) technology, current implementations face several critical limitations that hinder optimal N₂ mass transport for electrochemical nitrogen reduction reaction (NRR) applications. The primary challenge lies in the inherent trade-off between porosity and conductivity. Existing GDEs typically utilize carbon-based materials with limited hydrophobicity, resulting in flooding issues during extended operation periods. This flooding significantly reduces the three-phase boundary essential for efficient gas-liquid-solid interactions, ultimately restricting nitrogen accessibility to catalytic sites.
The current pore structure design in commercial GDEs lacks optimization specifically for N₂ transport. Most GDEs were originally engineered for oxygen reduction reactions in fuel cells or CO₂ reduction, where the target molecules have different diffusion properties than nitrogen. The larger kinetic diameter of N₂ (3.64 Å) compared to O₂ (3.46 Å) creates additional mass transport limitations through conventional microporous structures, resulting in concentration polarization at high current densities.
Another significant limitation is the catalyst layer architecture in existing GDEs. The random distribution of catalytic sites and non-uniform coating techniques lead to inefficient utilization of expensive catalysts and inconsistent performance. Current manufacturing processes struggle to create precise catalyst gradients or controlled hierarchical structures that could potentially enhance nitrogen adsorption and subsequent reduction. The lack of directional porosity further exacerbates mass transport limitations, particularly in thicker electrode configurations.
The binder systems employed in conventional GDEs present additional challenges. PTFE and Nafion, the most commonly used binders, often create excessive hydrophobic or hydrophilic regions respectively, without achieving the optimal balance required for NRR. These binders can block active sites and create tortuous diffusion pathways that impede nitrogen transport to reaction zones. Furthermore, their degradation under operating conditions leads to progressive performance decline over extended operation periods.
Current GDE fabrication methods also suffer from scalability and reproducibility issues. Techniques like spray coating, doctor blading, and screen printing struggle to create consistent microstructures at scale. The resulting batch-to-batch variations in porosity, thickness, and catalyst distribution significantly impact performance metrics and complicate systematic optimization efforts. This manufacturing inconsistency represents a major barrier to commercial implementation of GDEs specifically engineered for enhanced N₂ mass transport.
Interface engineering between different GDE layers remains suboptimal in current technologies. The transition between the gas diffusion layer, microporous layer, and catalyst layer often creates bottlenecks for mass transport due to discontinuities in pore networks. These interface limitations are particularly problematic for nitrogen reduction, where maintaining consistent gas access while managing electrolyte distribution is critical for sustained performance.
The current pore structure design in commercial GDEs lacks optimization specifically for N₂ transport. Most GDEs were originally engineered for oxygen reduction reactions in fuel cells or CO₂ reduction, where the target molecules have different diffusion properties than nitrogen. The larger kinetic diameter of N₂ (3.64 Å) compared to O₂ (3.46 Å) creates additional mass transport limitations through conventional microporous structures, resulting in concentration polarization at high current densities.
Another significant limitation is the catalyst layer architecture in existing GDEs. The random distribution of catalytic sites and non-uniform coating techniques lead to inefficient utilization of expensive catalysts and inconsistent performance. Current manufacturing processes struggle to create precise catalyst gradients or controlled hierarchical structures that could potentially enhance nitrogen adsorption and subsequent reduction. The lack of directional porosity further exacerbates mass transport limitations, particularly in thicker electrode configurations.
The binder systems employed in conventional GDEs present additional challenges. PTFE and Nafion, the most commonly used binders, often create excessive hydrophobic or hydrophilic regions respectively, without achieving the optimal balance required for NRR. These binders can block active sites and create tortuous diffusion pathways that impede nitrogen transport to reaction zones. Furthermore, their degradation under operating conditions leads to progressive performance decline over extended operation periods.
Current GDE fabrication methods also suffer from scalability and reproducibility issues. Techniques like spray coating, doctor blading, and screen printing struggle to create consistent microstructures at scale. The resulting batch-to-batch variations in porosity, thickness, and catalyst distribution significantly impact performance metrics and complicate systematic optimization efforts. This manufacturing inconsistency represents a major barrier to commercial implementation of GDEs specifically engineered for enhanced N₂ mass transport.
Interface engineering between different GDE layers remains suboptimal in current technologies. The transition between the gas diffusion layer, microporous layer, and catalyst layer often creates bottlenecks for mass transport due to discontinuities in pore networks. These interface limitations are particularly problematic for nitrogen reduction, where maintaining consistent gas access while managing electrolyte distribution is critical for sustained performance.
State-of-the-Art N2 Transport Solutions
01 Electrode structure optimization for N2 mass transport
Gas diffusion electrodes can be optimized structurally to enhance N2 mass transport. This includes designing porous structures with controlled pore size distribution, creating hierarchical porosity, and optimizing the thickness of diffusion layers. These structural modifications facilitate the efficient transport of N2 gas to active sites, reducing mass transport limitations and improving overall electrode performance in applications such as nitrogen reduction reactions.- Electrode structure optimization for N2 mass transport: Gas diffusion electrodes can be optimized structurally to enhance N2 mass transport. This includes designing porous structures with controlled pore size distribution, creating multi-layered electrodes with gradient porosity, and incorporating hydrophobic components to manage water content. These structural modifications create efficient pathways for nitrogen gas to reach active sites while maintaining proper electronic conductivity and mechanical stability.
- Catalyst design for nitrogen reduction reactions: Specialized catalysts can significantly improve N2 mass transport and activation in gas diffusion electrodes. These catalysts are designed with specific morphologies and compositions to lower the activation energy for nitrogen reduction. Metal-nitrogen-carbon (M-N-C) complexes, transition metal nitrides, and nanostructured materials with high surface area are particularly effective for facilitating nitrogen adsorption and subsequent electrochemical reactions.
- Interface engineering for gas-liquid-solid interactions: The interface between gas, liquid, and solid phases in gas diffusion electrodes is critical for N2 mass transport. Engineering this triple-phase boundary involves controlling the wettability of electrode surfaces, optimizing the electrolyte composition, and designing flow fields that enhance gas diffusion while maintaining ionic conductivity. These approaches minimize mass transport limitations and improve the overall efficiency of nitrogen-based electrochemical processes.
- Operating conditions optimization for N2 transport: Operating parameters significantly affect N2 mass transport in gas diffusion electrodes. Factors such as temperature, pressure, humidity, and flow rate can be optimized to enhance nitrogen diffusion through the electrode structure. Controlling these conditions helps balance the competing requirements of gas permeability, electronic conductivity, and electrochemical activity, leading to improved performance in applications like nitrogen reduction and ammonia synthesis.
- Advanced materials for enhanced N2 diffusion: Novel materials can significantly improve N2 mass transport properties in gas diffusion electrodes. These include carbon-based materials with tailored porosity, polymer composites with controlled hydrophobicity, and advanced ceramic supports with high thermal stability. Incorporating these materials into electrode designs creates optimized pathways for nitrogen diffusion while maintaining structural integrity and electrochemical performance under various operating conditions.
02 Catalyst design for improved N2 activation and transport
Specialized catalysts can be incorporated into gas diffusion electrodes to enhance N2 activation and transport. These catalysts often feature transition metals, metal-nitrogen complexes, or nanostructured materials that provide active sites for N2 adsorption and activation. By optimizing catalyst composition, loading, and distribution, the mass transport of N2 can be significantly improved, leading to higher reaction rates and efficiency in electrochemical nitrogen conversion processes.Expand Specific Solutions03 Interface engineering for enhanced gas-liquid-solid interactions
Engineering the interfaces within gas diffusion electrodes is crucial for optimizing N2 mass transport. This involves creating well-defined boundaries between gas, liquid, and solid phases to facilitate efficient N2 diffusion to reaction sites. Techniques include hydrophobic/hydrophilic treatment of electrode components, incorporation of ionomer materials, and design of triple-phase boundaries. These interface modifications help manage water content while maintaining gas permeability, thereby enhancing N2 mass transport through the electrode structure.Expand Specific Solutions04 Advanced materials for selective N2 transport
Novel materials can be incorporated into gas diffusion electrodes to enhance selective N2 transport. These include functionalized carbon supports, polymer membranes with tailored gas permeability, and composite materials with specific affinity for nitrogen molecules. By using materials that preferentially allow N2 transport while limiting competing species, the efficiency of nitrogen-based electrochemical reactions can be improved, addressing mass transport limitations that often occur in conventional electrode designs.Expand Specific Solutions05 Operating conditions optimization for N2 mass transport
The operating conditions of electrochemical systems significantly impact N2 mass transport in gas diffusion electrodes. Parameters such as pressure, temperature, humidity, and flow rate can be optimized to enhance nitrogen delivery to active sites. Strategies include pressurized operation to increase N2 concentration, temperature control to balance reaction kinetics with mass transport, and flow field design to ensure uniform gas distribution. These operational optimizations help overcome mass transport limitations and improve the overall performance of nitrogen-based electrochemical processes.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The gas diffusion electrode (GDE) technology for enhanced N2 mass transport is currently in an early growth phase, with increasing market interest driven by applications in green ammonia production and nitrogen reduction reactions. The global market for this technology is projected to expand significantly as industries seek sustainable nitrogen fixation methods, though current market size remains relatively modest. From a technical maturity perspective, the field shows varying development levels across key players. Industry leaders like Siemens Energy, Industrie De Nora, and BYD are advancing commercial applications, while research institutions such as Dalian Institute of Chemical Physics and Tsinghua University are pioneering fundamental breakthroughs in electrode design. Academic-industrial partnerships between companies like Toagosei, Mitsui Chemicals, and universities are accelerating technology transfer, though widespread commercialization remains several years away.
Industrie De Nora SpA
Technical Solution: De Nora has developed advanced gas diffusion electrodes (GDEs) with optimized three-phase interfaces specifically for enhanced N2 mass transport. Their proprietary design incorporates hierarchical porous structures with controlled pore size distribution (macro, meso, and micropores) that creates efficient pathways for nitrogen gas diffusion while maintaining optimal catalyst utilization. The company employs specialized hydrophobic PTFE treatments with precise loading gradients across the electrode thickness to balance gas permeability and electrolyte penetration[1]. Their latest generation electrodes feature advanced carbon-based supports with tailored surface chemistry and nitrogen-doped carbon materials that demonstrate improved nitrogen adsorption characteristics. De Nora has also pioneered the use of specialized flow-field designs that work synergistically with their GDEs to enhance nitrogen mass transport to active catalyst sites while minimizing concentration polarization effects[3].
Strengths: Industry-leading expertise in electrochemical technologies with over 100 years of experience; proprietary electrode manufacturing techniques allowing precise control of porosity and hydrophobicity; established global manufacturing infrastructure. Weaknesses: Higher production costs compared to conventional electrodes; technology primarily optimized for chlor-alkali applications requiring adaptation for nitrogen-specific processes.
Siemens AG
Technical Solution: Siemens has developed a comprehensive approach to gas diffusion electrode (GDE) engineering for enhanced N2 mass transport, leveraging their expertise in industrial electrochemical systems. Their technology centers on advanced electrode architectures featuring precisely engineered porosity gradients that optimize the balance between gas permeability and electrolyte penetration. Siemens' GDEs incorporate specialized hydrophobic treatments with controlled distribution throughout the electrode structure, creating dedicated gas channels that facilitate nitrogen transport while maintaining efficient three-phase boundaries[6]. The company has pioneered computational fluid dynamics modeling to optimize flow field designs that work synergistically with their electrode structures to minimize concentration polarization and enhance nitrogen availability at catalyst sites. Their manufacturing process employs proprietary techniques for precise control of pore structure and catalyst distribution, resulting in electrodes with superior long-term stability under industrial operating conditions[7]. Siemens has also developed innovative integration approaches that optimize the electrode-membrane interface to reduce mass transport limitations in complete electrochemical cell configurations.
Strengths: Extensive experience in industrial-scale electrochemical systems; sophisticated manufacturing capabilities allowing precise control of electrode properties; strong integration expertise for complete system optimization. Weaknesses: Solutions may be optimized for large-scale industrial applications with less focus on specialized research applications; potentially higher implementation costs compared to more targeted solutions.
Critical Patents in GDE Mass Transport Enhancement
Gas diffusion electrode, fuel cell, and transportation device
PatentPendingEP4503207A1
Innovation
- A gas diffusion electrode is designed with a microporous layer containing carbon black and graphite particles with an aspect ratio of 10 or more, applied to an electrically conductive porous substrate. The microporous layer has a specific thickness distribution and density range to enhance both electrical conductivity and gas diffusivity.
Gas diffusion electrode material, process for producing the same, and process for producing gas diffusion electrode
PatentWO2000029643A1
Innovation
- The method involves dispersing hydrophobic carbon black and PTFE particles to achieve a narrow particle size distribution of less than 1 micron, using a jet mill and self-assembly with a surfactant and alcohol to form a self-assembled film, and incorporating hydrophilic fine particles to prevent silver sintering during the pressing process, thereby enhancing the three-phase interface and electrode performance.
Materials Science Advancements for GDE Performance
Recent advancements in materials science have significantly contributed to enhancing the performance of Gas Diffusion Electrodes (GDEs) for nitrogen reduction reactions. The development of novel nanomaterials with tailored porosity has emerged as a critical factor in optimizing N2 mass transport. Carbon-based materials, including carbon nanotubes (CNTs), graphene, and mesoporous carbon, have demonstrated exceptional properties for GDE fabrication due to their high surface area, excellent electrical conductivity, and tunable pore structures.
Hierarchical porous structures incorporating both macro and micropores have proven particularly effective for N2 mass transport. These structures facilitate rapid gas diffusion through macropores while providing abundant active sites in micropores for electrochemical reactions. The integration of hydrophobic and hydrophilic domains within these materials creates an optimal three-phase boundary essential for efficient gas-liquid-solid interactions in electrochemical nitrogen reduction.
Metal-organic frameworks (MOFs) and their derivatives represent another promising material class for GDEs. Their crystalline structures with well-defined pore architectures can be precisely engineered to enhance N2 adsorption and activation. MOF-derived carbon materials maintain high porosity while gaining improved electrical conductivity, making them excellent candidates for electrochemical applications requiring efficient gas transport.
Surface modification techniques have evolved to optimize the wettability of GDE materials. Controlled fluorination and silane treatments enable the creation of materials with gradient hydrophobicity, which helps maintain an ideal balance between gas permeability and electrolyte accessibility. These modifications prevent electrode flooding while ensuring sufficient ionic conductivity throughout the electrode structure.
Composite materials combining conductive substrates with catalytically active components have shown remarkable synergistic effects. For instance, nitrogen-doped carbon materials decorated with transition metal nanoparticles exhibit enhanced N2 adsorption capabilities and lower activation barriers for N-N bond cleavage. The strategic incorporation of these dopants creates defect sites that serve as preferential adsorption centers for nitrogen molecules.
Advanced polymer binders have been developed to improve the mechanical stability and longevity of GDEs while maintaining optimal porosity. Fluorinated polymers and ion-conducting polymers provide mechanical reinforcement without significantly compromising gas transport pathways. These binders help maintain electrode integrity during long-term operation under the harsh conditions typical of nitrogen reduction processes.
Computational materials design has accelerated the discovery of optimal GDE structures. Molecular dynamics simulations and density functional theory calculations now enable researchers to predict gas diffusion behavior in complex porous networks before experimental validation, significantly reducing development time and resources required for material optimization.
Hierarchical porous structures incorporating both macro and micropores have proven particularly effective for N2 mass transport. These structures facilitate rapid gas diffusion through macropores while providing abundant active sites in micropores for electrochemical reactions. The integration of hydrophobic and hydrophilic domains within these materials creates an optimal three-phase boundary essential for efficient gas-liquid-solid interactions in electrochemical nitrogen reduction.
Metal-organic frameworks (MOFs) and their derivatives represent another promising material class for GDEs. Their crystalline structures with well-defined pore architectures can be precisely engineered to enhance N2 adsorption and activation. MOF-derived carbon materials maintain high porosity while gaining improved electrical conductivity, making them excellent candidates for electrochemical applications requiring efficient gas transport.
Surface modification techniques have evolved to optimize the wettability of GDE materials. Controlled fluorination and silane treatments enable the creation of materials with gradient hydrophobicity, which helps maintain an ideal balance between gas permeability and electrolyte accessibility. These modifications prevent electrode flooding while ensuring sufficient ionic conductivity throughout the electrode structure.
Composite materials combining conductive substrates with catalytically active components have shown remarkable synergistic effects. For instance, nitrogen-doped carbon materials decorated with transition metal nanoparticles exhibit enhanced N2 adsorption capabilities and lower activation barriers for N-N bond cleavage. The strategic incorporation of these dopants creates defect sites that serve as preferential adsorption centers for nitrogen molecules.
Advanced polymer binders have been developed to improve the mechanical stability and longevity of GDEs while maintaining optimal porosity. Fluorinated polymers and ion-conducting polymers provide mechanical reinforcement without significantly compromising gas transport pathways. These binders help maintain electrode integrity during long-term operation under the harsh conditions typical of nitrogen reduction processes.
Computational materials design has accelerated the discovery of optimal GDE structures. Molecular dynamics simulations and density functional theory calculations now enable researchers to predict gas diffusion behavior in complex porous networks before experimental validation, significantly reducing development time and resources required for material optimization.
Sustainability Impact of Improved Nitrogen Fixation
The advancement of nitrogen fixation technologies through improved gas diffusion electrodes represents a significant opportunity for global sustainability. Enhanced N2 mass transport in electrochemical systems could revolutionize fertilizer production, currently dominated by the energy-intensive Haber-Bosch process which consumes approximately 1-2% of global energy and generates substantial CO2 emissions.
Improved nitrogen fixation technologies would dramatically reduce the carbon footprint of agricultural inputs. Current estimates suggest that transitioning from conventional Haber-Bosch to optimized electrochemical nitrogen fixation could potentially reduce associated greenhouse gas emissions by 30-60%, depending on the renewable energy integration level and system efficiency.
Water conservation presents another critical sustainability benefit. Traditional ammonia production requires significant water resources for cooling and processing. Electrochemical systems with enhanced N2 mass transport properties could operate at ambient conditions with substantially lower water requirements, potentially reducing water usage by 40-70% compared to conventional methods.
Land use efficiency would also improve through decentralized production capabilities. Enhanced gas diffusion electrodes enable smaller-scale, distributed ammonia production facilities closer to agricultural application sites, reducing transportation emissions and infrastructure requirements while promoting local agricultural resilience and food security in remote regions.
Resource circularity represents a transformative potential of this technology. Advanced gas diffusion electrodes could be designed to utilize waste nitrogen compounds from agricultural runoff or industrial processes as feedstock, creating circular nitrogen economy systems that mitigate environmental pollution while reducing dependency on virgin nitrogen extraction.
Biodiversity protection would benefit from precision application of locally-produced nitrogen fertilizers. Reduced nitrogen runoff from more efficient fertilizer use would help mitigate eutrophication of water bodies, protecting aquatic ecosystems and maintaining biodiversity in agricultural landscapes.
Economic sustainability would be enhanced through reduced energy costs and price volatility. Decentralized production using renewable energy could stabilize fertilizer prices in developing agricultural economies, reducing dependency on imported fertilizers and strengthening local agricultural value chains while creating new green technology employment opportunities.
Improved nitrogen fixation technologies would dramatically reduce the carbon footprint of agricultural inputs. Current estimates suggest that transitioning from conventional Haber-Bosch to optimized electrochemical nitrogen fixation could potentially reduce associated greenhouse gas emissions by 30-60%, depending on the renewable energy integration level and system efficiency.
Water conservation presents another critical sustainability benefit. Traditional ammonia production requires significant water resources for cooling and processing. Electrochemical systems with enhanced N2 mass transport properties could operate at ambient conditions with substantially lower water requirements, potentially reducing water usage by 40-70% compared to conventional methods.
Land use efficiency would also improve through decentralized production capabilities. Enhanced gas diffusion electrodes enable smaller-scale, distributed ammonia production facilities closer to agricultural application sites, reducing transportation emissions and infrastructure requirements while promoting local agricultural resilience and food security in remote regions.
Resource circularity represents a transformative potential of this technology. Advanced gas diffusion electrodes could be designed to utilize waste nitrogen compounds from agricultural runoff or industrial processes as feedstock, creating circular nitrogen economy systems that mitigate environmental pollution while reducing dependency on virgin nitrogen extraction.
Biodiversity protection would benefit from precision application of locally-produced nitrogen fertilizers. Reduced nitrogen runoff from more efficient fertilizer use would help mitigate eutrophication of water bodies, protecting aquatic ecosystems and maintaining biodiversity in agricultural landscapes.
Economic sustainability would be enhanced through reduced energy costs and price volatility. Decentralized production using renewable energy could stabilize fertilizer prices in developing agricultural economies, reducing dependency on imported fertilizers and strengthening local agricultural value chains while creating new green technology employment opportunities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!