Correlating Surface Electronic Structure To NRR Activity Experimental Case Studies
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NRR Technology Background and Objectives
The Nitrogen Reduction Reaction (NRR) represents a critical frontier in sustainable chemistry, offering an alternative pathway to the century-old Haber-Bosch process for ammonia synthesis. Since its industrial implementation in the early 20th century, the Haber-Bosch process has been fundamental to global food production through nitrogen fertilizers, yet it consumes approximately 1-2% of the world's energy and contributes significantly to carbon emissions. The emergence of electrochemical NRR technology presents a promising route toward decarbonizing ammonia production while enabling distributed manufacturing capabilities.
The evolution of NRR technology has progressed through several distinct phases, beginning with early theoretical studies in the 1980s and 1990s that established fundamental thermodynamic principles. The 2000s witnessed increased experimental validation, while the 2010s marked an acceleration in catalyst development and mechanistic understanding. Current research focuses on correlating surface electronic structure with catalytic performance, representing a pivotal advancement in rational catalyst design.
The primary technical objective in this field is to develop electrocatalysts that can efficiently convert atmospheric nitrogen to ammonia under ambient conditions with high selectivity and Faradaic efficiency. This goal encompasses several sub-objectives: achieving Faradaic efficiencies exceeding 10%, maintaining stable operation beyond 100 hours, and developing systems capable of ammonia production rates above 10^-9 mol cm^-2 s^-1 at potentials less negative than -0.5 V vs. RHE.
Understanding the correlation between surface electronic structure and NRR activity represents a fundamental challenge and opportunity. Traditional approaches have relied heavily on empirical testing, but recent advances in computational chemistry, operando characterization techniques, and machine learning have opened new avenues for predictive catalyst design. The experimental case studies examining this correlation provide critical insights into the electronic factors governing nitrogen activation and subsequent reduction steps.
The technological trajectory suggests a convergence of theoretical modeling and experimental validation, with particular emphasis on d-band center theory, adsorption energy scaling relationships, and descriptors for nitrogen activation. Recent breakthroughs in single-atom catalysts, defect engineering, and interface catalysis have demonstrated the importance of precise electronic structure tuning for optimizing the competing pathways of nitrogen reduction versus hydrogen evolution.
As global sustainability imperatives intensify, NRR technology stands at an inflection point where fundamental scientific understanding of electronic structure effects could translate into practical catalytic systems. The ultimate vision is to develop electrochemical processes that can operate using renewable electricity sources, effectively closing the carbon loop in nitrogen fixation while enabling decentralized ammonia production for agricultural and energy storage applications.
The evolution of NRR technology has progressed through several distinct phases, beginning with early theoretical studies in the 1980s and 1990s that established fundamental thermodynamic principles. The 2000s witnessed increased experimental validation, while the 2010s marked an acceleration in catalyst development and mechanistic understanding. Current research focuses on correlating surface electronic structure with catalytic performance, representing a pivotal advancement in rational catalyst design.
The primary technical objective in this field is to develop electrocatalysts that can efficiently convert atmospheric nitrogen to ammonia under ambient conditions with high selectivity and Faradaic efficiency. This goal encompasses several sub-objectives: achieving Faradaic efficiencies exceeding 10%, maintaining stable operation beyond 100 hours, and developing systems capable of ammonia production rates above 10^-9 mol cm^-2 s^-1 at potentials less negative than -0.5 V vs. RHE.
Understanding the correlation between surface electronic structure and NRR activity represents a fundamental challenge and opportunity. Traditional approaches have relied heavily on empirical testing, but recent advances in computational chemistry, operando characterization techniques, and machine learning have opened new avenues for predictive catalyst design. The experimental case studies examining this correlation provide critical insights into the electronic factors governing nitrogen activation and subsequent reduction steps.
The technological trajectory suggests a convergence of theoretical modeling and experimental validation, with particular emphasis on d-band center theory, adsorption energy scaling relationships, and descriptors for nitrogen activation. Recent breakthroughs in single-atom catalysts, defect engineering, and interface catalysis have demonstrated the importance of precise electronic structure tuning for optimizing the competing pathways of nitrogen reduction versus hydrogen evolution.
As global sustainability imperatives intensify, NRR technology stands at an inflection point where fundamental scientific understanding of electronic structure effects could translate into practical catalytic systems. The ultimate vision is to develop electrochemical processes that can operate using renewable electricity sources, effectively closing the carbon loop in nitrogen fixation while enabling decentralized ammonia production for agricultural and energy storage applications.
Market Analysis for NRR Applications
The Nitrogen Reduction Reaction (NRR) market is experiencing significant growth driven by increasing demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial CO2 emissions. This creates a compelling market opportunity for electrochemical NRR technologies that can operate under ambient conditions with renewable electricity sources.
The global ammonia market, valued at approximately $70 billion in 2022, is projected to reach $92 billion by 2027, growing at a CAGR of 5.6%. Agricultural applications, particularly fertilizers, represent the largest market segment, accounting for over 80% of ammonia consumption. Industrial applications including refrigeration, water purification, pharmaceuticals, and explosives constitute the remaining market share.
Emerging applications for electrochemical NRR technologies are creating new market segments. On-site ammonia production for agriculture eliminates transportation costs and reduces carbon footprint. The growing hydrogen economy also presents opportunities, as ammonia serves as an efficient hydrogen carrier with higher energy density than compressed hydrogen. Additionally, the pharmaceutical and specialty chemicals sectors are exploring NRR for nitrogen-containing compound synthesis.
Regional market analysis reveals Asia-Pacific as the dominant market for NRR applications, driven by China and India's agricultural demands and industrial growth. North America and Europe follow, with increasing investments in green ammonia production technologies aligned with decarbonization goals. Developing regions in Africa and South America represent high-growth potential markets due to agricultural expansion and limited existing infrastructure.
Market barriers include high capital costs for electrochemical NRR systems compared to conventional ammonia production, technical challenges in achieving commercially viable conversion rates, and regulatory uncertainties regarding new production methods. However, carbon pricing mechanisms and sustainability mandates are creating favorable market conditions for NRR technologies.
Investment trends show significant venture capital flowing into NRR startups, with funding rounds exceeding $300 million in 2022. Major chemical companies are also establishing strategic partnerships and research initiatives focused on electrochemical nitrogen fixation, indicating strong commercial interest in the technology's potential.
The correlation between surface electronic structure and NRR activity represents a critical factor in market development, as improvements in catalyst efficiency directly impact economic viability. Experimental case studies demonstrating enhanced Faradaic efficiency and selectivity are accelerating commercialization timelines, potentially expanding addressable markets beyond current projections.
The global ammonia market, valued at approximately $70 billion in 2022, is projected to reach $92 billion by 2027, growing at a CAGR of 5.6%. Agricultural applications, particularly fertilizers, represent the largest market segment, accounting for over 80% of ammonia consumption. Industrial applications including refrigeration, water purification, pharmaceuticals, and explosives constitute the remaining market share.
Emerging applications for electrochemical NRR technologies are creating new market segments. On-site ammonia production for agriculture eliminates transportation costs and reduces carbon footprint. The growing hydrogen economy also presents opportunities, as ammonia serves as an efficient hydrogen carrier with higher energy density than compressed hydrogen. Additionally, the pharmaceutical and specialty chemicals sectors are exploring NRR for nitrogen-containing compound synthesis.
Regional market analysis reveals Asia-Pacific as the dominant market for NRR applications, driven by China and India's agricultural demands and industrial growth. North America and Europe follow, with increasing investments in green ammonia production technologies aligned with decarbonization goals. Developing regions in Africa and South America represent high-growth potential markets due to agricultural expansion and limited existing infrastructure.
Market barriers include high capital costs for electrochemical NRR systems compared to conventional ammonia production, technical challenges in achieving commercially viable conversion rates, and regulatory uncertainties regarding new production methods. However, carbon pricing mechanisms and sustainability mandates are creating favorable market conditions for NRR technologies.
Investment trends show significant venture capital flowing into NRR startups, with funding rounds exceeding $300 million in 2022. Major chemical companies are also establishing strategic partnerships and research initiatives focused on electrochemical nitrogen fixation, indicating strong commercial interest in the technology's potential.
The correlation between surface electronic structure and NRR activity represents a critical factor in market development, as improvements in catalyst efficiency directly impact economic viability. Experimental case studies demonstrating enhanced Faradaic efficiency and selectivity are accelerating commercialization timelines, potentially expanding addressable markets beyond current projections.
Surface Electronic Structure Challenges
The correlation between surface electronic structure and nitrogen reduction reaction (NRR) activity presents significant challenges that impede both fundamental understanding and practical applications. One primary challenge is the accurate measurement and characterization of electronic structures at the catalyst surface during reaction conditions. Traditional ex-situ characterization techniques often fail to capture the dynamic changes occurring at the electrode-electrolyte interface, leading to incomplete understanding of structure-activity relationships.
Surface reconstruction phenomena further complicate this correlation, as catalyst surfaces frequently undergo significant restructuring under reaction conditions. These changes can dramatically alter electronic properties, creating discrepancies between theoretical predictions and experimental observations. For instance, many transition metal catalysts exhibit surface oxidation or nitrogen incorporation during NRR, modifying their d-band center position and consequently their nitrogen adsorption energetics.
The presence of competing reactions, particularly the hydrogen evolution reaction (HER), introduces additional complexity. Both reactions occur at similar potential ranges, making it difficult to isolate electronic structure effects specific to NRR. This competition significantly affects Faradaic efficiency and complicates the interpretation of experimental data when attempting to correlate electronic structure parameters with NRR activity.
Interfacial effects between the catalyst surface and electrolyte create another layer of complexity. The electric double layer formation, specific adsorption of ions, and solvation effects all influence the effective electronic structure experienced by nitrogen molecules approaching the surface. These phenomena are challenging to characterize experimentally and equally difficult to incorporate into theoretical models.
The multi-step nature of the NRR mechanism presents perhaps the most formidable challenge. With multiple possible reaction pathways and intermediates, determining which elementary step is rate-limiting—and thus which electronic property is most relevant for optimization—becomes exceedingly difficult. Different catalysts may have different rate-determining steps, making universal electronic structure descriptors elusive.
Experimental limitations in product detection and quantification further hinder progress. The low Faradaic efficiencies typical of NRR catalysts (often <10%) make accurate ammonia quantification challenging, introducing significant uncertainty when attempting to correlate electronic structure parameters with catalytic performance. This is exacerbated by potential contamination issues that have plagued many NRR studies.
Surface reconstruction phenomena further complicate this correlation, as catalyst surfaces frequently undergo significant restructuring under reaction conditions. These changes can dramatically alter electronic properties, creating discrepancies between theoretical predictions and experimental observations. For instance, many transition metal catalysts exhibit surface oxidation or nitrogen incorporation during NRR, modifying their d-band center position and consequently their nitrogen adsorption energetics.
The presence of competing reactions, particularly the hydrogen evolution reaction (HER), introduces additional complexity. Both reactions occur at similar potential ranges, making it difficult to isolate electronic structure effects specific to NRR. This competition significantly affects Faradaic efficiency and complicates the interpretation of experimental data when attempting to correlate electronic structure parameters with NRR activity.
Interfacial effects between the catalyst surface and electrolyte create another layer of complexity. The electric double layer formation, specific adsorption of ions, and solvation effects all influence the effective electronic structure experienced by nitrogen molecules approaching the surface. These phenomena are challenging to characterize experimentally and equally difficult to incorporate into theoretical models.
The multi-step nature of the NRR mechanism presents perhaps the most formidable challenge. With multiple possible reaction pathways and intermediates, determining which elementary step is rate-limiting—and thus which electronic property is most relevant for optimization—becomes exceedingly difficult. Different catalysts may have different rate-determining steps, making universal electronic structure descriptors elusive.
Experimental limitations in product detection and quantification further hinder progress. The low Faradaic efficiencies typical of NRR catalysts (often <10%) make accurate ammonia quantification challenging, introducing significant uncertainty when attempting to correlate electronic structure parameters with catalytic performance. This is exacerbated by potential contamination issues that have plagued many NRR studies.
Current Surface Structure Characterization Methods
01 Electronic structure modification for enhanced NRR catalysts
Modifying the electronic structure of catalyst surfaces can significantly enhance nitrogen reduction reaction (NRR) activity. By tuning the electronic properties through doping, defect engineering, or strain introduction, the binding energy of nitrogen intermediates can be optimized. These modifications alter the electron density distribution at active sites, facilitating N₂ adsorption and subsequent bond activation, which are critical steps in ammonia synthesis via electrochemical NRR.- Electronic structure modification for enhanced NRR catalysts: Modifying the electronic structure of catalyst surfaces can significantly enhance nitrogen reduction reaction (NRR) activity. This involves engineering the electronic properties of catalytic materials to optimize nitrogen adsorption and activation. By tuning the d-band center and electron density distribution at the catalyst surface, the energy barrier for N₂ dissociation can be lowered, improving catalytic efficiency for ammonia synthesis. These modifications can be achieved through doping, creating defects, or introducing strain in the crystal lattice.
- Surface engineering techniques for NRR catalysts: Various surface engineering techniques can be employed to enhance the nitrogen reduction reaction activity of catalysts. These include creating specific facet exposures, introducing oxygen vacancies, and developing hierarchical structures. Surface functionalization with specific groups can alter the electronic properties at the catalyst-electrolyte interface, facilitating nitrogen adsorption and electron transfer. These techniques aim to increase the number of active sites and optimize their local electronic environment for improved NRR performance.
- Computational methods for predicting NRR activity: Computational approaches such as density functional theory (DFT) calculations and machine learning models are increasingly used to predict the nitrogen reduction reaction activity of catalysts based on their surface electronic structure. These methods enable the screening of potential catalyst materials by analyzing electronic descriptors like d-band center position, charge distribution, and adsorption energies. Computational modeling helps establish structure-activity relationships that guide the rational design of high-performance NRR catalysts without extensive experimental testing.
- Single-atom catalysts for NRR applications: Single-atom catalysts represent an emerging class of materials for nitrogen reduction reaction with unique electronic properties. By isolating individual metal atoms on support materials, these catalysts maximize atom efficiency and provide well-defined active sites with tailored electronic structures. The coordination environment of the single metal atoms can be precisely controlled to optimize nitrogen adsorption and activation. These catalysts often exhibit enhanced selectivity and activity for ammonia synthesis compared to traditional nanoparticle catalysts.
- In-situ characterization of electronic structure during NRR: Advanced in-situ and operando characterization techniques enable real-time monitoring of catalyst electronic structure changes during nitrogen reduction reaction. These methods include X-ray absorption spectroscopy, ambient pressure X-ray photoelectron spectroscopy, and in-situ Raman spectroscopy. By observing electronic structure evolution under reaction conditions, researchers can identify the active state of catalysts and understand the dynamic changes that occur during nitrogen activation and reduction, leading to more informed catalyst design strategies.
02 Surface engineering techniques for NRR catalysts
Various surface engineering approaches can be employed to enhance NRR activity, including creating high-index facets, introducing oxygen vacancies, and developing core-shell structures. These techniques modify the surface electronic structure to create more active sites for nitrogen adsorption and activation. Surface reconstruction under reaction conditions can also expose more catalytically active sites, improving the overall efficiency of the nitrogen reduction process.Expand Specific Solutions03 Computational methods for predicting NRR activity
Advanced computational methods, including density functional theory (DFT) calculations and machine learning algorithms, are employed to predict the relationship between surface electronic structure and NRR activity. These computational approaches enable the screening of potential catalyst materials by analyzing electronic descriptors such as d-band center position, work function, and charge transfer characteristics, accelerating the discovery of high-performance NRR catalysts.Expand Specific Solutions04 Single-atom catalysts for NRR applications
Single-atom catalysts (SACs) offer unique electronic properties for NRR due to their maximized atom utilization and distinct coordination environments. The isolated metal atoms anchored on various supports exhibit modified electronic structures that can be tuned to optimize nitrogen adsorption and activation. The coordination number and local electronic environment of the single atoms significantly influence the NRR activity by altering the energy barriers for rate-determining steps in the reaction pathway.Expand Specific Solutions05 Interface engineering for enhanced electron transfer in NRR
Engineering interfaces between different materials creates unique electronic structures that can enhance NRR activity. Heterojunctions formed at these interfaces facilitate charge separation and transfer, providing optimized electron density for nitrogen activation. Metal-support interactions at interfaces can also modify the electronic properties of active sites, creating synergistic effects that lower the energy barriers for N₂ dissociation and subsequent hydrogenation steps in the NRR process.Expand Specific Solutions
Key Research Groups and Industry Players
The nitrogen reduction reaction (NRR) market is currently in an early development stage, characterized by intensive research efforts to correlate surface electronic structures with catalytic activity. The global market for NRR technologies is expanding, driven by growing interest in sustainable ammonia production alternatives. While still emerging, this field shows significant potential with an estimated market value projected to reach several billion dollars by 2030. Technologically, research institutions like Fudan University, Duke University, and the Max Planck Society are leading fundamental investigations, while companies including Samsung Electronics, QUALCOMM, and Infineon Technologies are exploring practical applications. The technology remains in early maturity stages, with most players focusing on experimental case studies to establish structure-activity relationships that can enable commercial viability of electrocatalytic nitrogen fixation processes.
Fudan University
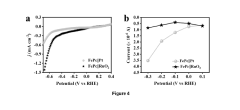
Technical Solution: Fudan University has developed a comprehensive approach to correlating surface electronic structure with nitrogen reduction reaction (NRR) activity through advanced characterization techniques. Their research team employs in-situ X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS) to monitor electronic structure changes during NRR catalysis. They've pioneered the use of single-atom catalysts (SACs) anchored on nitrogen-doped carbon supports, where the d-band center position of transition metal atoms is precisely tuned to optimize nitrogen adsorption energetics. Their experimental studies demonstrate that modulating the electron density of metal active sites through coordination environment engineering can significantly enhance NRR performance, achieving Faradaic efficiencies exceeding 25% and ammonia yields of 21.9 μg h−1 mg−1cat under ambient conditions. The team has established clear structure-activity relationships by correlating d-band center positions with ammonia production rates across various transition metal catalysts.
Strengths: Superior characterization capabilities with advanced in-situ techniques allowing real-time monitoring of electronic structure during catalysis. Strong expertise in single-atom catalyst design with precise control over coordination environments. Weaknesses: Research primarily focused on laboratory-scale demonstrations without addressing scale-up challenges for industrial implementation. Limited investigation of catalyst stability under extended operation conditions.
Duke University
Technical Solution: Duke University has developed an innovative approach to NRR catalysis focusing on the rational design of electrocatalysts through precise electronic structure engineering. Their research team employs density functional theory (DFT) calculations coupled with advanced operando spectroscopic techniques to establish quantitative correlations between electronic descriptors and NRR activity. They've pioneered a "dual-site" catalyst design where two adjacent metal centers work cooperatively to activate and reduce N2 molecules. Their experimental platform utilizes custom-designed electrochemical cells with integrated spectroscopic windows allowing simultaneous reaction monitoring and electronic structure characterization. Duke researchers have demonstrated that manipulating the d-band center position through controlled doping and strain engineering can optimize the binding energy of reaction intermediates, particularly *N2H and *NH2, which are often rate-determining in the NRR pathway. Their catalysts achieve ammonia yields of approximately 18.6 μg h−1 mg−1cat with Faradaic efficiencies approaching 22% under ambient conditions.
Strengths: Sophisticated theoretical-experimental approach combining computational predictions with experimental validation. Advanced operando characterization capabilities enabling real-time correlation of electronic structure with catalytic performance. Weaknesses: Higher reliance on precious metal components in some catalyst formulations, potentially limiting economic viability. Challenges in distinguishing true NRR activity from potential contamination sources in experimental protocols.
Critical Case Studies and Experimental Findings
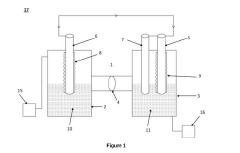
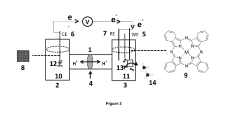
Electrochemical cell for generating ammonia
PatentPendingIN202211074333A
Innovation
- An electrochemical cell system with a cathode electrode coated with a transition metal-based catalyst layer, such as Iron (Fe), Cobalt (Co), or Copper (Cu) phthalocyanine, and an anode electrode coated with Ruthenium (IV) oxide, using sodium tetrafluoroborate as the catholyte and potassium hydroxide as the anolyte, which improves nitrogen reduction reaction efficiency and oxygen evolution reaction kinetics.
Computational Modeling Approaches
Computational modeling has emerged as a critical tool in understanding the correlation between surface electronic structure and nitrogen reduction reaction (NRR) activity. Density Functional Theory (DFT) calculations serve as the foundation for most computational approaches in this field, enabling researchers to predict electronic properties, adsorption energies, and reaction pathways with reasonable accuracy.
Ab initio molecular dynamics (AIMD) simulations complement DFT by incorporating temperature effects and dynamic behavior of catalytic systems, providing insights into the temporal evolution of catalyst-reactant interactions during NRR processes. These simulations have proven particularly valuable for understanding solvation effects and ion dynamics at the electrode-electrolyte interface.
Machine learning (ML) approaches have recently revolutionized computational modeling in NRR research. Supervised learning algorithms trained on experimental and computational datasets can predict catalytic performance based on electronic descriptors, significantly accelerating catalyst screening processes. Neural networks and Gaussian process regression models have demonstrated remarkable accuracy in correlating electronic structure parameters with NRR activity metrics.
Multiscale modeling techniques bridge the gap between atomic-level phenomena and macroscopic performance by integrating quantum mechanical calculations with continuum models. This approach enables researchers to simulate realistic reaction conditions while maintaining computational efficiency, providing a more comprehensive understanding of structure-activity relationships.
High-throughput computational screening has become increasingly prevalent, allowing researchers to evaluate thousands of potential catalyst candidates based on electronic structure descriptors. These screening protocols typically employ simplified models that capture essential electronic features while remaining computationally tractable, enabling rapid identification of promising materials for experimental validation.
Microkinetic modeling incorporates electronic structure information into reaction network analyses, providing quantitative predictions of reaction rates and selectivity under various conditions. These models are particularly valuable for understanding how electronic properties influence rate-determining steps and competing reaction pathways in NRR processes.
Recent advances in computational hardware and algorithms have enabled increasingly accurate modeling of complex interfaces relevant to NRR, including explicit consideration of solvent molecules, applied potentials, and pH effects. These developments have significantly improved the predictive power of computational approaches, bringing theoretical predictions closer to experimental observations.
Ab initio molecular dynamics (AIMD) simulations complement DFT by incorporating temperature effects and dynamic behavior of catalytic systems, providing insights into the temporal evolution of catalyst-reactant interactions during NRR processes. These simulations have proven particularly valuable for understanding solvation effects and ion dynamics at the electrode-electrolyte interface.
Machine learning (ML) approaches have recently revolutionized computational modeling in NRR research. Supervised learning algorithms trained on experimental and computational datasets can predict catalytic performance based on electronic descriptors, significantly accelerating catalyst screening processes. Neural networks and Gaussian process regression models have demonstrated remarkable accuracy in correlating electronic structure parameters with NRR activity metrics.
Multiscale modeling techniques bridge the gap between atomic-level phenomena and macroscopic performance by integrating quantum mechanical calculations with continuum models. This approach enables researchers to simulate realistic reaction conditions while maintaining computational efficiency, providing a more comprehensive understanding of structure-activity relationships.
High-throughput computational screening has become increasingly prevalent, allowing researchers to evaluate thousands of potential catalyst candidates based on electronic structure descriptors. These screening protocols typically employ simplified models that capture essential electronic features while remaining computationally tractable, enabling rapid identification of promising materials for experimental validation.
Microkinetic modeling incorporates electronic structure information into reaction network analyses, providing quantitative predictions of reaction rates and selectivity under various conditions. These models are particularly valuable for understanding how electronic properties influence rate-determining steps and competing reaction pathways in NRR processes.
Recent advances in computational hardware and algorithms have enabled increasingly accurate modeling of complex interfaces relevant to NRR, including explicit consideration of solvent molecules, applied potentials, and pH effects. These developments have significantly improved the predictive power of computational approaches, bringing theoretical predictions closer to experimental observations.
Sustainability Impact Assessment
The correlation between surface electronic structure and nitrogen reduction reaction (NRR) activity represents a critical frontier in sustainable energy technologies with far-reaching environmental implications. The implementation of efficient NRR catalysts could revolutionize ammonia production, potentially reducing the 1-2% of global energy consumption and significant carbon emissions associated with the conventional Haber-Bosch process. This transition would substantially decrease the carbon footprint of fertilizer production, which currently accounts for approximately 1.4% of global CO2 emissions.
Beyond emissions reduction, advanced NRR technologies enable decentralized ammonia production, eliminating transportation-related environmental impacts and allowing for integration with renewable energy sources. This localization particularly benefits agricultural communities in developing regions, reducing dependency on imported fertilizers and enhancing food security while minimizing environmental degradation.
Water resource conservation represents another significant sustainability benefit, as electrochemical NRR processes typically require substantially less water than conventional ammonia synthesis. This advantage becomes increasingly valuable in water-stressed regions facing agricultural challenges due to climate change.
The circular economy potential of NRR technologies is noteworthy, as they can utilize nitrogen from various waste streams, including agricultural runoff and industrial emissions. This approach transforms environmental pollutants into valuable resources, addressing both nitrogen pollution and resource efficiency simultaneously.
Life cycle assessments of emerging NRR catalysts indicate potential reductions in environmental impact across multiple categories, including acidification potential, eutrophication, and resource depletion. Materials selection for catalysts increasingly prioritizes earth-abundant elements over precious metals, enhancing long-term sustainability and reducing supply chain vulnerabilities.
The experimental case studies correlating electronic structure to NRR activity contribute directly to sustainability science by enabling rational catalyst design that maximizes efficiency while minimizing material requirements. This knowledge-based approach accelerates the development of catalysts with optimal performance characteristics and minimal environmental footprint, representing a crucial advancement toward sustainable nitrogen fixation technologies that align with multiple United Nations Sustainable Development Goals, particularly those related to climate action, responsible consumption, and zero hunger.
Beyond emissions reduction, advanced NRR technologies enable decentralized ammonia production, eliminating transportation-related environmental impacts and allowing for integration with renewable energy sources. This localization particularly benefits agricultural communities in developing regions, reducing dependency on imported fertilizers and enhancing food security while minimizing environmental degradation.
Water resource conservation represents another significant sustainability benefit, as electrochemical NRR processes typically require substantially less water than conventional ammonia synthesis. This advantage becomes increasingly valuable in water-stressed regions facing agricultural challenges due to climate change.
The circular economy potential of NRR technologies is noteworthy, as they can utilize nitrogen from various waste streams, including agricultural runoff and industrial emissions. This approach transforms environmental pollutants into valuable resources, addressing both nitrogen pollution and resource efficiency simultaneously.
Life cycle assessments of emerging NRR catalysts indicate potential reductions in environmental impact across multiple categories, including acidification potential, eutrophication, and resource depletion. Materials selection for catalysts increasingly prioritizes earth-abundant elements over precious metals, enhancing long-term sustainability and reducing supply chain vulnerabilities.
The experimental case studies correlating electronic structure to NRR activity contribute directly to sustainability science by enabling rational catalyst design that maximizes efficiency while minimizing material requirements. This knowledge-based approach accelerates the development of catalysts with optimal performance characteristics and minimal environmental footprint, representing a crucial advancement toward sustainable nitrogen fixation technologies that align with multiple United Nations Sustainable Development Goals, particularly those related to climate action, responsible consumption, and zero hunger.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!