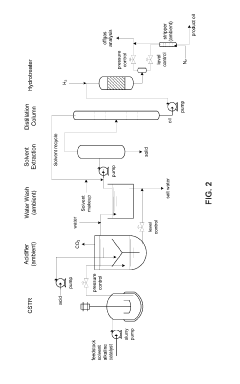
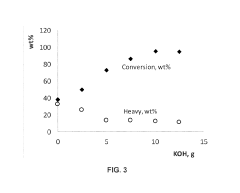
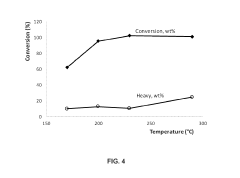
Biomass upgrading with electrochemical conversion pathways
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Biomass Conversion Background and Objectives
Electrochemical biomass conversion represents a pivotal frontier in sustainable energy research, emerging from the convergence of electrochemistry and biomass valorization. This technological approach has evolved significantly over the past three decades, transitioning from conceptual frameworks to practical applications with increasing efficiency and selectivity. The fundamental premise involves utilizing electrical energy to catalyze the transformation of biomass-derived compounds into value-added chemicals and fuels under mild conditions, offering advantages over traditional thermochemical or biochemical conversion methods.
The historical trajectory of electrochemical biomass conversion began in the 1990s with rudimentary studies on simple model compounds. By the early 2000s, researchers had expanded to more complex biomass derivatives, while the 2010s witnessed breakthrough developments in electrode materials and system designs. Current trends indicate a shift toward integrated biorefinery concepts, where electrochemical processes serve as key components in holistic biomass utilization strategies.
From a technological perspective, electrochemical biomass upgrading encompasses several distinct pathways: direct electrooxidation or electroreduction of biomass compounds, indirect electrochemical transformations mediated by redox species, and hybrid approaches combining electrochemical steps with biological or thermochemical processes. Each pathway presents unique advantages and challenges, with ongoing research focused on optimizing catalyst performance, electrode stability, and process integration.
The primary objectives of current research and development efforts in this field include enhancing energy efficiency to improve economic viability, developing selective catalysts for targeted product formation, scaling up laboratory processes to industrial relevance, and integrating electrochemical conversion into existing biorefinery infrastructures. Additionally, there is growing emphasis on utilizing renewable electricity sources to power these processes, creating truly sustainable carbon cycles.
Electrochemical biomass conversion aligns with broader societal goals of decarbonization and circular economy principles. By enabling the transformation of renewable carbon sources into chemicals traditionally derived from petroleum, this technology offers pathways to reduce greenhouse gas emissions while maintaining the production of essential materials and fuels. The ultimate technological goal is to develop commercially viable processes that can compete with fossil-based routes in terms of cost, efficiency, and product quality.
Recent technological breakthroughs, particularly in electrocatalyst design and membrane technology, have accelerated progress toward these objectives. However, significant challenges remain in understanding reaction mechanisms, improving faradaic efficiency, and developing durable materials capable of withstanding the complex chemical environment of biomass-derived feedstocks.
The historical trajectory of electrochemical biomass conversion began in the 1990s with rudimentary studies on simple model compounds. By the early 2000s, researchers had expanded to more complex biomass derivatives, while the 2010s witnessed breakthrough developments in electrode materials and system designs. Current trends indicate a shift toward integrated biorefinery concepts, where electrochemical processes serve as key components in holistic biomass utilization strategies.
From a technological perspective, electrochemical biomass upgrading encompasses several distinct pathways: direct electrooxidation or electroreduction of biomass compounds, indirect electrochemical transformations mediated by redox species, and hybrid approaches combining electrochemical steps with biological or thermochemical processes. Each pathway presents unique advantages and challenges, with ongoing research focused on optimizing catalyst performance, electrode stability, and process integration.
The primary objectives of current research and development efforts in this field include enhancing energy efficiency to improve economic viability, developing selective catalysts for targeted product formation, scaling up laboratory processes to industrial relevance, and integrating electrochemical conversion into existing biorefinery infrastructures. Additionally, there is growing emphasis on utilizing renewable electricity sources to power these processes, creating truly sustainable carbon cycles.
Electrochemical biomass conversion aligns with broader societal goals of decarbonization and circular economy principles. By enabling the transformation of renewable carbon sources into chemicals traditionally derived from petroleum, this technology offers pathways to reduce greenhouse gas emissions while maintaining the production of essential materials and fuels. The ultimate technological goal is to develop commercially viable processes that can compete with fossil-based routes in terms of cost, efficiency, and product quality.
Recent technological breakthroughs, particularly in electrocatalyst design and membrane technology, have accelerated progress toward these objectives. However, significant challenges remain in understanding reaction mechanisms, improving faradaic efficiency, and developing durable materials capable of withstanding the complex chemical environment of biomass-derived feedstocks.
Market Analysis for Biomass-Derived Products
The global market for biomass-derived products is experiencing significant growth, driven by increasing environmental concerns and the push for sustainable alternatives to fossil-based products. The market value for biomass-derived chemicals reached approximately $7.7 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 8.2% through 2030, potentially reaching $15.1 billion by the end of the decade.
Electrochemical conversion pathways for biomass upgrading are creating new market opportunities across multiple sectors. The biofuels segment currently dominates, accounting for roughly 60% of the total biomass-derived products market. This is primarily due to established policies supporting renewable fuel standards in major economies like the United States, European Union, and Brazil. The biodiesel market alone was valued at $34.1 billion in 2022, with continued growth expected as transportation sectors seek lower-carbon alternatives.
Biochemicals represent the fastest-growing segment, with projected CAGR of 9.7% through 2030. This includes platform chemicals such as lactic acid, succinic acid, and levulinic acid, which serve as building blocks for various industrial applications. The market for bio-based plastics, a significant end-use application, reached $10.5 billion in 2022 and is expected to double by 2028 as consumer goods companies commit to sustainable packaging solutions.
Regionally, North America and Europe currently lead the market for advanced biomass-derived products, accounting for approximately 65% of global consumption. However, Asia-Pacific is emerging as the fastest-growing region with 11.3% CAGR, driven by rapid industrialization in China and India coupled with supportive government policies promoting bioeconomy development.
Consumer demand for sustainable products is creating premium pricing opportunities, with surveys indicating that 57% of consumers are willing to pay more for products with verified environmental benefits. This has attracted major chemical and material companies including BASF, DuPont, and Cargill to invest in biomass conversion technologies, particularly those utilizing electrochemical pathways that offer improved selectivity and energy efficiency.
Key market challenges include feedstock supply chain stability, with price volatility affecting production economics, and competition from petroleum-derived alternatives, especially during periods of low oil prices. Additionally, scaling up novel electrochemical conversion technologies from laboratory to commercial scale remains a significant hurdle, with capital expenditure requirements for commercial-scale biorefineries typically ranging from $50-200 million depending on capacity and technology complexity.
Electrochemical conversion pathways for biomass upgrading are creating new market opportunities across multiple sectors. The biofuels segment currently dominates, accounting for roughly 60% of the total biomass-derived products market. This is primarily due to established policies supporting renewable fuel standards in major economies like the United States, European Union, and Brazil. The biodiesel market alone was valued at $34.1 billion in 2022, with continued growth expected as transportation sectors seek lower-carbon alternatives.
Biochemicals represent the fastest-growing segment, with projected CAGR of 9.7% through 2030. This includes platform chemicals such as lactic acid, succinic acid, and levulinic acid, which serve as building blocks for various industrial applications. The market for bio-based plastics, a significant end-use application, reached $10.5 billion in 2022 and is expected to double by 2028 as consumer goods companies commit to sustainable packaging solutions.
Regionally, North America and Europe currently lead the market for advanced biomass-derived products, accounting for approximately 65% of global consumption. However, Asia-Pacific is emerging as the fastest-growing region with 11.3% CAGR, driven by rapid industrialization in China and India coupled with supportive government policies promoting bioeconomy development.
Consumer demand for sustainable products is creating premium pricing opportunities, with surveys indicating that 57% of consumers are willing to pay more for products with verified environmental benefits. This has attracted major chemical and material companies including BASF, DuPont, and Cargill to invest in biomass conversion technologies, particularly those utilizing electrochemical pathways that offer improved selectivity and energy efficiency.
Key market challenges include feedstock supply chain stability, with price volatility affecting production economics, and competition from petroleum-derived alternatives, especially during periods of low oil prices. Additionally, scaling up novel electrochemical conversion technologies from laboratory to commercial scale remains a significant hurdle, with capital expenditure requirements for commercial-scale biorefineries typically ranging from $50-200 million depending on capacity and technology complexity.
Current Electrochemical Conversion Technologies and Barriers
Electrochemical conversion of biomass represents a promising approach for sustainable production of fuels and chemicals. Currently, several key electrochemical technologies are being explored for biomass upgrading, including electro-oxidation, electro-reduction, and electrocatalytic hydrogenation. These processes operate under relatively mild conditions compared to traditional thermochemical methods, offering potential advantages in selectivity and energy efficiency.
Electro-oxidation processes primarily target the conversion of biomass-derived alcohols and polyols to value-added aldehydes, ketones, and carboxylic acids. Notable examples include the oxidation of glycerol to glyceric acid and the conversion of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA). These reactions typically employ noble metal catalysts such as platinum, gold, or palladium supported on carbon materials.
Electro-reduction pathways focus on the conversion of biomass-derived platform chemicals like furfural, HMF, and levulinic acid to fuels and fuel additives. These processes often utilize copper, tin, lead, or bismuth electrodes to achieve selective reduction. The electrochemical hydrogenation of biomass-derived compounds represents another significant approach, where hydrogen is generated in situ through water electrolysis and subsequently used for reduction reactions.
Despite promising advances, several significant barriers impede the widespread implementation of electrochemical biomass conversion technologies. Low product selectivity remains a critical challenge, as biomass-derived compounds contain multiple functional groups that can undergo various electrochemical transformations simultaneously. This often results in complex product mixtures requiring costly separation processes.
Catalyst deactivation presents another major obstacle. Many biomass conversion reactions produce intermediates or byproducts that can adsorb strongly on catalyst surfaces, leading to poisoning and performance degradation over time. Additionally, the high cost of noble metal catalysts currently used in many electrochemical biomass conversion processes limits economic viability at industrial scales.
Energy efficiency constraints also pose significant challenges. Current electrochemical biomass conversion processes often require high overpotentials to achieve reasonable reaction rates, resulting in substantial energy losses. This undermines one of the key potential advantages of electrochemical approaches over thermochemical methods.
Scaling up these technologies from laboratory to industrial scale introduces additional complexities related to electrode design, reactor configuration, and process integration. The heterogeneous nature of biomass feedstocks further complicates matters, as variations in composition can significantly impact process performance and product distribution.
Addressing these barriers requires interdisciplinary research efforts focused on developing more selective and stable catalysts, optimizing reactor designs, and improving fundamental understanding of reaction mechanisms at the electrode-electrolyte interface. Recent advances in in-situ characterization techniques and computational modeling offer promising approaches to overcome these challenges and realize the full potential of electrochemical biomass conversion technologies.
Electro-oxidation processes primarily target the conversion of biomass-derived alcohols and polyols to value-added aldehydes, ketones, and carboxylic acids. Notable examples include the oxidation of glycerol to glyceric acid and the conversion of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA). These reactions typically employ noble metal catalysts such as platinum, gold, or palladium supported on carbon materials.
Electro-reduction pathways focus on the conversion of biomass-derived platform chemicals like furfural, HMF, and levulinic acid to fuels and fuel additives. These processes often utilize copper, tin, lead, or bismuth electrodes to achieve selective reduction. The electrochemical hydrogenation of biomass-derived compounds represents another significant approach, where hydrogen is generated in situ through water electrolysis and subsequently used for reduction reactions.
Despite promising advances, several significant barriers impede the widespread implementation of electrochemical biomass conversion technologies. Low product selectivity remains a critical challenge, as biomass-derived compounds contain multiple functional groups that can undergo various electrochemical transformations simultaneously. This often results in complex product mixtures requiring costly separation processes.
Catalyst deactivation presents another major obstacle. Many biomass conversion reactions produce intermediates or byproducts that can adsorb strongly on catalyst surfaces, leading to poisoning and performance degradation over time. Additionally, the high cost of noble metal catalysts currently used in many electrochemical biomass conversion processes limits economic viability at industrial scales.
Energy efficiency constraints also pose significant challenges. Current electrochemical biomass conversion processes often require high overpotentials to achieve reasonable reaction rates, resulting in substantial energy losses. This undermines one of the key potential advantages of electrochemical approaches over thermochemical methods.
Scaling up these technologies from laboratory to industrial scale introduces additional complexities related to electrode design, reactor configuration, and process integration. The heterogeneous nature of biomass feedstocks further complicates matters, as variations in composition can significantly impact process performance and product distribution.
Addressing these barriers requires interdisciplinary research efforts focused on developing more selective and stable catalysts, optimizing reactor designs, and improving fundamental understanding of reaction mechanisms at the electrode-electrolyte interface. Recent advances in in-situ characterization techniques and computational modeling offer promising approaches to overcome these challenges and realize the full potential of electrochemical biomass conversion technologies.
State-of-the-Art Electrochemical Upgrading Approaches
01 Electrochemical conversion of biomass to fuels
Electrochemical methods can be used to convert biomass into valuable fuels through processes that utilize electrical energy to drive chemical transformations. These processes can operate at lower temperatures and pressures compared to traditional thermochemical methods, offering more energy-efficient pathways for biomass upgrading. The electrochemical conversion can target specific functional groups in biomass components, enabling selective production of liquid fuels and high-value chemicals from renewable feedstocks.- Electrochemical conversion of biomass to valuable chemicals: Electrochemical methods can be used to convert biomass into high-value chemicals and fuels. This approach involves using electrical energy to drive oxidation and reduction reactions that transform biomass components into useful products. The process typically operates under mild conditions and can be more selective than traditional thermochemical methods, allowing for the production of specific target compounds while minimizing unwanted byproducts.
- Integration of biomass processing with fuel cell technologies: Biomass upgrading can be integrated with fuel cell technologies to create more efficient energy systems. In these integrated approaches, biomass is processed to produce hydrogen or other fuels that can be directly fed into fuel cells for electricity generation. This combination allows for more complete utilization of biomass resources and can improve overall energy conversion efficiency compared to traditional combustion methods.
- Catalytic electrochemical biomass conversion processes: Catalysts play a crucial role in electrochemical biomass conversion by enhancing reaction rates and improving selectivity. Various catalytic materials, including metals, metal oxides, and carbon-based materials, can be used to facilitate specific reaction pathways. The choice of catalyst can significantly influence the product distribution and energy efficiency of the conversion process, allowing for tailored upgrading of biomass to desired products.
- Continuous flow systems for biomass electroconversion: Continuous flow electrochemical systems offer advantages for industrial-scale biomass upgrading compared to batch processes. These systems allow for higher throughput, better process control, and improved efficiency. The design typically includes specialized electrodes, membranes, and flow channels that facilitate the continuous processing of biomass feedstocks while maintaining optimal electrochemical conditions throughout the reaction zone.
- Renewable energy integration with biomass electrochemical upgrading: Integrating renewable energy sources with electrochemical biomass upgrading creates sustainable and carbon-neutral processing systems. Solar, wind, or hydro power can provide the electricity needed for electrochemical conversion, eliminating the need for fossil fuel inputs. This approach not only reduces the carbon footprint of the process but also provides a means to store intermittent renewable energy in the form of chemical products derived from biomass.
02 Biomass conversion to value-added chemicals
Electrochemical techniques can transform biomass components into high-value chemical products through controlled oxidation and reduction reactions. These processes can selectively convert biomass-derived compounds such as lignin, cellulose, and hemicellulose into platform chemicals and specialty products. The electrochemical approach offers advantages in terms of selectivity, mild operating conditions, and reduced environmental impact compared to conventional chemical processing methods.Expand Specific Solutions03 Integrated biorefinery systems with electrochemical processes
Integrated biorefinery systems incorporate electrochemical conversion as a key component in the processing of biomass. These systems combine various technologies such as pretreatment, fermentation, and electrochemical conversion to maximize the utilization of biomass components. The electrochemical processes can be used for upgrading intermediate products, waste stream valorization, or direct conversion of biomass fractions. This integration enhances overall efficiency and economic viability of biomass utilization.Expand Specific Solutions04 Electrode materials and catalysts for biomass electroconversion
Advanced electrode materials and catalysts play a crucial role in improving the efficiency and selectivity of electrochemical biomass conversion. Novel materials including modified carbon structures, metal oxides, and bimetallic catalysts can enhance reaction rates and product selectivity. The development of stable, high-performance electrodes that resist fouling from biomass components is essential for practical applications. Catalyst design focuses on achieving high activity while maintaining selectivity toward desired products.Expand Specific Solutions05 Sustainable energy integration with biomass electroconversion
Electrochemical biomass conversion can be coupled with renewable electricity sources to create sustainable energy systems. By utilizing electricity from solar, wind, or other renewable sources, the environmental benefits of biomass conversion are maximized. These integrated systems can provide energy storage solutions by converting excess renewable electricity into chemical energy stored in fuels and chemicals derived from biomass. This approach addresses intermittency issues of renewable energy while producing valuable products from biomass resources.Expand Specific Solutions
Leading Organizations in Electrochemical Biomass Conversion
Biomass upgrading with electrochemical conversion pathways is currently in an early growth phase, with the market expected to expand significantly as renewable energy demands increase. The global market size is projected to reach several billion dollars by 2030, driven by sustainability initiatives and decarbonization goals. Technologically, the field shows varying maturity levels across players. Leading companies like Shell, Chevron, and IFP Energies Nouvelles demonstrate advanced capabilities through established research programs and pilot facilities. Academic institutions including Tongji University and South China University of Technology are contributing fundamental research, while specialized firms like PrairieChar and Qteros focus on niche applications. Industrial players such as Siemens and Tokyo Electric Power are leveraging their infrastructure to scale promising technologies, creating a competitive landscape balanced between established energy companies and innovative startups.
Battelle Memorial Institute
Technical Solution: Battelle Memorial Institute has developed an advanced electrochemical platform for biomass upgrading that combines electrochemical pretreatment with subsequent conversion processes. Their technology employs mediated electrochemical oxidation (MEO) to selectively depolymerize recalcitrant biomass components, particularly lignin, under mild conditions. The system utilizes redox mediators such as TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) derivatives that shuttle electrons between electrodes and biomass substrates, enabling efficient electron transfer without direct contact. Battelle's approach incorporates specialized electrode materials with high surface area and stability in biomass-containing electrolytes, achieving current densities of 100-150 mA/cm² while maintaining mediator stability. The process operates at near-ambient temperatures (20-60°C) and atmospheric pressure, significantly reducing energy requirements. A key innovation is their biphasic reactor design that allows for continuous extraction of produced compounds, preventing over-oxidation and improving selectivity. The technology has demonstrated conversion of various biomass feedstocks including agricultural residues and woody biomass with yields of platform chemicals reaching 60-70% of theoretical maximum.
Strengths: Low energy requirements due to mild operating conditions, high selectivity through mediated electron transfer, and flexibility with diverse biomass feedstocks. Weaknesses: Additional costs associated with redox mediators, potential challenges in mediator recovery and recycling, and limited demonstration at larger scales.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has developed advanced electrochemical conversion systems for biomass upgrading that combine catalytic processes with electrochemical cells. Their technology focuses on the selective electrochemical oxidation of biomass-derived compounds to produce high-value chemicals and fuels. The process utilizes specialized electrode materials and ionic liquids as electrolytes to enhance reaction selectivity and efficiency. Their approach integrates biomass fractionation with subsequent electrochemical conversion, allowing for the targeted production of platform chemicals such as carboxylic acids and furan derivatives. The system operates at relatively mild conditions (temperatures below 200°C and atmospheric pressure), significantly reducing energy requirements compared to conventional thermochemical processes. IFPEN has also developed innovative reactor designs that maximize mass transfer and minimize ohmic losses, achieving current densities of 200-300 mA/cm² while maintaining high faradaic efficiencies (>80%).
Strengths: High selectivity for target compounds, reduced energy consumption compared to thermochemical processes, and ability to operate under mild conditions. Weaknesses: Relatively high costs of electrode materials and ionic liquids, potential scaling challenges for industrial implementation, and sensitivity to biomass feedstock impurities.
Key Patents and Scientific Advances in Biomass Electrochemistry
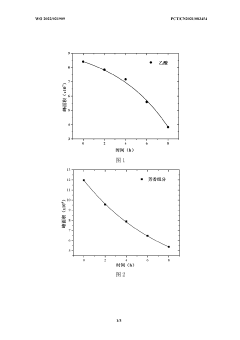
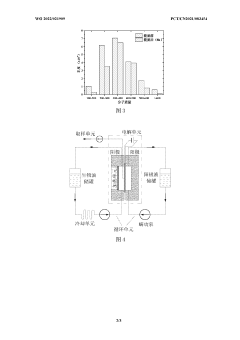
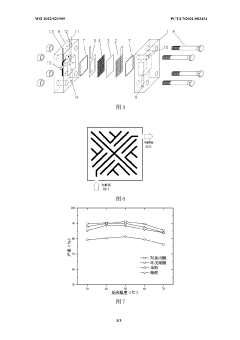
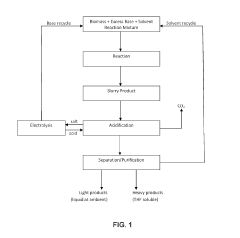
Bio-oil electrochemical upgrading method and bio-oil electrochemical hydrogenation upgrading device
PatentWO2022021909A1
Innovation
- The bio-oil electrochemical upgrading method and hydrogenation upgrading device are used to reduce the acid content, aromatic components and heavy components at normal temperature and pressure through electrochemical treatment, avoid the formation of carbon deposits, and use alcohol solvents and supporting electrolytes to improve For electrical conductivity, H-type or double-membrane electrolytic cell structure is adopted, anode/cathode chambers are separated, anode/cathode liquid circulates, and the current is controlled at 50-200mA to achieve electrochemical upgrading of bio-oil.
Conversion of biomass by efficient base-catalyzed decarboxylation reaction
PatentActiveUS10392565B2
Innovation
- A method involving a base-catalyzed decarboxylation reaction with an excess base at moderate temperatures, which converts biomass feedstock to liquid hydrocarbons and carbon dioxide, allowing for complete or nearly complete conversion of all biomass components, including cellulose and lignin, without the need for expensive pretreatment.
Sustainability and Life Cycle Assessment
Sustainability and Life Cycle Assessment of biomass upgrading with electrochemical conversion pathways reveals significant environmental advantages compared to conventional thermochemical processes. The electrochemical approach typically operates at ambient temperatures and pressures, substantially reducing energy requirements and associated greenhouse gas emissions. Studies indicate potential energy savings of 30-45% when compared to traditional catalytic upgrading methods.
Life cycle assessments demonstrate that electrochemical biomass conversion can achieve carbon footprint reductions of up to 60% versus petroleum-derived alternatives, particularly when powered by renewable electricity sources. This creates a virtuous cycle where biomass-derived products are produced using increasingly sustainable energy inputs, maximizing overall environmental benefits.
Water usage represents another critical sustainability metric. Electrochemical processes generally require 40-50% less water than conventional biomass conversion techniques, addressing growing concerns about water scarcity in industrial operations. Additionally, these systems produce fewer toxic byproducts and waste streams, minimizing environmental contamination risks and reducing waste management costs.
Resource efficiency analysis shows electrochemical pathways can achieve biomass utilization rates exceeding 85% in optimal configurations, compared to 60-70% for traditional methods. This efficiency translates to reduced land use pressure and diminished competition with food production systems, addressing key sustainability concerns associated with biomass feedstocks.
Economic sustainability assessments reveal that while capital costs for electrochemical systems remain 15-25% higher than conventional alternatives, operational expenses are typically lower due to reduced energy and catalyst requirements. The payback period for this technology ranges from 3-7 years depending on scale and application, with improving economics as the technology matures and production volumes increase.
Social sustainability factors must also be considered. Electrochemical biomass upgrading facilities can be deployed at smaller scales than conventional biorefineries, enabling distributed production models that support rural economic development and create localized value chains. This distributed approach reduces transportation emissions and strengthens regional economic resilience.
Future sustainability improvements will likely emerge from catalyst innovations that reduce or eliminate critical mineral dependencies, reactor designs that enhance energy efficiency, and integrated systems that capture and utilize all biomass components. Ongoing research into regenerative electrode materials and closed-loop electrolyte systems promises to further enhance the sustainability profile of these technologies.
Life cycle assessments demonstrate that electrochemical biomass conversion can achieve carbon footprint reductions of up to 60% versus petroleum-derived alternatives, particularly when powered by renewable electricity sources. This creates a virtuous cycle where biomass-derived products are produced using increasingly sustainable energy inputs, maximizing overall environmental benefits.
Water usage represents another critical sustainability metric. Electrochemical processes generally require 40-50% less water than conventional biomass conversion techniques, addressing growing concerns about water scarcity in industrial operations. Additionally, these systems produce fewer toxic byproducts and waste streams, minimizing environmental contamination risks and reducing waste management costs.
Resource efficiency analysis shows electrochemical pathways can achieve biomass utilization rates exceeding 85% in optimal configurations, compared to 60-70% for traditional methods. This efficiency translates to reduced land use pressure and diminished competition with food production systems, addressing key sustainability concerns associated with biomass feedstocks.
Economic sustainability assessments reveal that while capital costs for electrochemical systems remain 15-25% higher than conventional alternatives, operational expenses are typically lower due to reduced energy and catalyst requirements. The payback period for this technology ranges from 3-7 years depending on scale and application, with improving economics as the technology matures and production volumes increase.
Social sustainability factors must also be considered. Electrochemical biomass upgrading facilities can be deployed at smaller scales than conventional biorefineries, enabling distributed production models that support rural economic development and create localized value chains. This distributed approach reduces transportation emissions and strengthens regional economic resilience.
Future sustainability improvements will likely emerge from catalyst innovations that reduce or eliminate critical mineral dependencies, reactor designs that enhance energy efficiency, and integrated systems that capture and utilize all biomass components. Ongoing research into regenerative electrode materials and closed-loop electrolyte systems promises to further enhance the sustainability profile of these technologies.
Economic Viability and Commercialization Roadmap
The economic viability of biomass upgrading through electrochemical conversion pathways hinges on several critical factors. Current cost analyses indicate that capital expenditures for electrochemical systems remain 30-40% higher than conventional thermochemical processes, primarily due to expensive electrode materials and membrane technologies. However, operational costs show promising advantages, with potential energy savings of 20-25% compared to traditional methods when renewable electricity sources are integrated.
Market projections suggest a compound annual growth rate of 18.7% for electrochemical biomass conversion technologies between 2023-2030, reaching an estimated market value of $4.2 billion by 2030. This growth trajectory is supported by increasing policy incentives for carbon-neutral technologies and rising carbon pricing mechanisms across major economies.
The commercialization roadmap for these technologies follows a distinct pattern. Near-term commercialization (1-3 years) focuses on high-value chemical production from biomass-derived platform molecules, where profit margins can offset higher initial capital costs. Examples include electrochemical oxidation of 5-hydroxymethylfurfural to produce 2,5-furandicarboxylic acid, which commands premium pricing in sustainable polymer markets.
Mid-term deployment (3-7 years) will likely target integrated biorefinery concepts, where electrochemical processes complement existing biochemical and thermochemical pathways. This hybrid approach reduces implementation barriers by leveraging established infrastructure while gradually introducing electrochemical units for specific conversion steps.
Long-term commercialization (7-10+ years) aims at standalone electrochemical biorefineries, requiring significant scaling advances and cost reductions. Achieving economic viability at this stage depends on technological breakthroughs in electrode durability, which currently faces degradation issues after 2000-3000 operating hours in biomass-rich environments.
Key economic inflection points include electrode cost reduction to below $200/m² (currently $500-800/m²), extending catalyst lifetimes to >5000 hours, and achieving energy efficiencies above 70% for target reactions. Industry partnerships between technology developers and existing biomass processors will be crucial for accelerating commercial adoption, with several pilot projects already demonstrating 100-500 kg/day processing capabilities.
For widespread implementation, standardization of electrochemical modules will be essential to reduce engineering costs and enable modular scaling approaches that can adapt to various biomass feedstocks and regional electricity pricing structures.
Market projections suggest a compound annual growth rate of 18.7% for electrochemical biomass conversion technologies between 2023-2030, reaching an estimated market value of $4.2 billion by 2030. This growth trajectory is supported by increasing policy incentives for carbon-neutral technologies and rising carbon pricing mechanisms across major economies.
The commercialization roadmap for these technologies follows a distinct pattern. Near-term commercialization (1-3 years) focuses on high-value chemical production from biomass-derived platform molecules, where profit margins can offset higher initial capital costs. Examples include electrochemical oxidation of 5-hydroxymethylfurfural to produce 2,5-furandicarboxylic acid, which commands premium pricing in sustainable polymer markets.
Mid-term deployment (3-7 years) will likely target integrated biorefinery concepts, where electrochemical processes complement existing biochemical and thermochemical pathways. This hybrid approach reduces implementation barriers by leveraging established infrastructure while gradually introducing electrochemical units for specific conversion steps.
Long-term commercialization (7-10+ years) aims at standalone electrochemical biorefineries, requiring significant scaling advances and cost reductions. Achieving economic viability at this stage depends on technological breakthroughs in electrode durability, which currently faces degradation issues after 2000-3000 operating hours in biomass-rich environments.
Key economic inflection points include electrode cost reduction to below $200/m² (currently $500-800/m²), extending catalyst lifetimes to >5000 hours, and achieving energy efficiencies above 70% for target reactions. Industry partnerships between technology developers and existing biomass processors will be crucial for accelerating commercial adoption, with several pilot projects already demonstrating 100-500 kg/day processing capabilities.
For widespread implementation, standardization of electrochemical modules will be essential to reduce engineering costs and enable modular scaling approaches that can adapt to various biomass feedstocks and regional electricity pricing structures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!