What catalysts drive efficiency in biomass upgrading to fuels
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomass Catalysis Background and Objectives
The conversion of biomass to fuels represents a critical pathway toward sustainable energy production, with catalysis serving as the cornerstone of efficient transformation processes. Biomass, as a renewable carbon source, has emerged as a promising alternative to fossil fuels, driven by increasing environmental concerns and the need for energy security. The historical trajectory of biomass conversion technologies spans from simple combustion methods to sophisticated catalytic processes that enable selective transformation of complex biomolecules into high-value fuels.
The evolution of biomass catalysis has been marked by significant milestones, including the development of acid-catalyzed hydrolysis in the early 20th century, followed by advances in heterogeneous catalysis during the 1970s oil crisis, and more recently, the emergence of nanoscale catalysts with unprecedented selectivity. Current research focuses on addressing the inherent challenges of biomass heterogeneity and oxygen-rich composition, which necessitate innovative catalytic approaches different from those used in traditional petrochemical processing.
The primary technical objectives in biomass catalysis center on developing catalysts that can efficiently cleave C-O bonds while preserving C-C bonds, operate effectively in aqueous environments, resist deactivation from impurities, and maintain stability under hydrothermal conditions. Additionally, there is a growing emphasis on designing multifunctional catalysts capable of performing sequential reactions in one-pot systems, thereby reducing separation costs and improving overall process economics.
Recent technological trends indicate a shift toward integrated catalytic systems that combine biological and thermochemical conversion routes, leveraging the strengths of each approach. The development of earth-abundant metal catalysts as alternatives to precious metals represents another significant trend, addressing concerns about resource scarcity and cost. Computational modeling and high-throughput experimentation have accelerated catalyst discovery, enabling rational design based on fundamental understanding of reaction mechanisms.
The ultimate goal of biomass catalysis research is to establish economically viable and environmentally sustainable processes that can compete with fossil-based technologies. This requires catalysts that not only exhibit high activity and selectivity but also demonstrate long-term stability under industrial conditions. Furthermore, these catalytic systems must be adaptable to various biomass feedstocks, including agricultural residues, forestry waste, and dedicated energy crops, to ensure broad applicability and resilience against supply fluctuations.
The evolution of biomass catalysis has been marked by significant milestones, including the development of acid-catalyzed hydrolysis in the early 20th century, followed by advances in heterogeneous catalysis during the 1970s oil crisis, and more recently, the emergence of nanoscale catalysts with unprecedented selectivity. Current research focuses on addressing the inherent challenges of biomass heterogeneity and oxygen-rich composition, which necessitate innovative catalytic approaches different from those used in traditional petrochemical processing.
The primary technical objectives in biomass catalysis center on developing catalysts that can efficiently cleave C-O bonds while preserving C-C bonds, operate effectively in aqueous environments, resist deactivation from impurities, and maintain stability under hydrothermal conditions. Additionally, there is a growing emphasis on designing multifunctional catalysts capable of performing sequential reactions in one-pot systems, thereby reducing separation costs and improving overall process economics.
Recent technological trends indicate a shift toward integrated catalytic systems that combine biological and thermochemical conversion routes, leveraging the strengths of each approach. The development of earth-abundant metal catalysts as alternatives to precious metals represents another significant trend, addressing concerns about resource scarcity and cost. Computational modeling and high-throughput experimentation have accelerated catalyst discovery, enabling rational design based on fundamental understanding of reaction mechanisms.
The ultimate goal of biomass catalysis research is to establish economically viable and environmentally sustainable processes that can compete with fossil-based technologies. This requires catalysts that not only exhibit high activity and selectivity but also demonstrate long-term stability under industrial conditions. Furthermore, these catalytic systems must be adaptable to various biomass feedstocks, including agricultural residues, forestry waste, and dedicated energy crops, to ensure broad applicability and resilience against supply fluctuations.
Market Analysis for Biomass-Derived Fuels
The global market for biomass-derived fuels has experienced significant growth over the past decade, driven by increasing environmental concerns and the push for renewable energy sources. Currently valued at approximately $120 billion, this market is projected to grow at a compound annual growth rate of 7.5% through 2030, reflecting the expanding interest in sustainable fuel alternatives.
Regionally, North America and Europe lead in market adoption, collectively accounting for over 60% of the global biomass-derived fuels market. This dominance stems from favorable regulatory frameworks, substantial government incentives, and established infrastructure. The European Union's Renewable Energy Directive II has particularly accelerated market growth by mandating increased renewable content in transportation fuels.
Emerging economies, especially in Asia-Pacific and Latin America, represent the fastest-growing markets due to their abundant biomass resources and increasing energy demands. Countries like Brazil, with its successful sugarcane ethanol program, demonstrate how effectively biomass-derived fuels can be integrated into national energy strategies.
Market segmentation reveals that first-generation biofuels (primarily bioethanol and biodiesel) currently dominate with approximately 75% market share. However, advanced biofuels derived through catalytic processes are gaining traction, projected to grow at twice the rate of conventional biofuels over the next five years.
Consumer demand patterns indicate growing acceptance of biomass-derived fuels, particularly in transportation sectors seeking to reduce carbon footprints. The aviation industry has emerged as a significant potential market, with several major airlines committing to incorporating sustainable aviation fuels into their operations.
Key market drivers include stringent emission regulations, volatile fossil fuel prices, and increasing corporate sustainability commitments. The economic viability of biomass-derived fuels remains heavily dependent on catalyst efficiency, as processing costs represent 40-50% of total production expenses.
Market barriers include high initial capital requirements for biorefinery infrastructure, feedstock supply chain challenges, and competition from increasingly cost-effective electric vehicles. Additionally, the market faces technical challenges related to catalyst deactivation and process scalability.
Future market growth will likely be shaped by breakthroughs in catalyst technology that can significantly reduce production costs and improve fuel quality. Catalysts that enable efficient conversion of lignocellulosic biomass and agricultural waste represent particularly promising market opportunities, potentially unlocking vast new feedstock resources while addressing waste management challenges.
Regionally, North America and Europe lead in market adoption, collectively accounting for over 60% of the global biomass-derived fuels market. This dominance stems from favorable regulatory frameworks, substantial government incentives, and established infrastructure. The European Union's Renewable Energy Directive II has particularly accelerated market growth by mandating increased renewable content in transportation fuels.
Emerging economies, especially in Asia-Pacific and Latin America, represent the fastest-growing markets due to their abundant biomass resources and increasing energy demands. Countries like Brazil, with its successful sugarcane ethanol program, demonstrate how effectively biomass-derived fuels can be integrated into national energy strategies.
Market segmentation reveals that first-generation biofuels (primarily bioethanol and biodiesel) currently dominate with approximately 75% market share. However, advanced biofuels derived through catalytic processes are gaining traction, projected to grow at twice the rate of conventional biofuels over the next five years.
Consumer demand patterns indicate growing acceptance of biomass-derived fuels, particularly in transportation sectors seeking to reduce carbon footprints. The aviation industry has emerged as a significant potential market, with several major airlines committing to incorporating sustainable aviation fuels into their operations.
Key market drivers include stringent emission regulations, volatile fossil fuel prices, and increasing corporate sustainability commitments. The economic viability of biomass-derived fuels remains heavily dependent on catalyst efficiency, as processing costs represent 40-50% of total production expenses.
Market barriers include high initial capital requirements for biorefinery infrastructure, feedstock supply chain challenges, and competition from increasingly cost-effective electric vehicles. Additionally, the market faces technical challenges related to catalyst deactivation and process scalability.
Future market growth will likely be shaped by breakthroughs in catalyst technology that can significantly reduce production costs and improve fuel quality. Catalysts that enable efficient conversion of lignocellulosic biomass and agricultural waste represent particularly promising market opportunities, potentially unlocking vast new feedstock resources while addressing waste management challenges.
Current Catalyst Technologies and Challenges
The biomass-to-fuels conversion landscape currently employs several catalyst technologies, each with specific advantages and limitations. Noble metal catalysts, particularly platinum, palladium, and ruthenium, demonstrate exceptional activity for hydrogenation and deoxygenation reactions critical in biomass upgrading. These catalysts exhibit high selectivity and stability under various reaction conditions but face significant drawbacks including prohibitive costs and limited availability, restricting their large-scale industrial application.
Transition metal catalysts, especially nickel, copper, and iron-based systems, have emerged as cost-effective alternatives. While they generally show lower activity than noble metals, recent advancements in preparation methods have significantly improved their performance. Nickel-based catalysts, for instance, demonstrate promising results in hydrogenolysis reactions for lignin conversion, though they remain susceptible to deactivation through carbon deposition and sintering.
Bifunctional catalysts combining metal sites with acid/base functionalities represent a significant innovation in biomass processing. These systems can simultaneously facilitate multiple reaction pathways, enhancing overall process efficiency. Zeolite-supported metal catalysts exemplify this approach, where the zeolite framework provides shape selectivity and acid sites while metal nanoparticles catalyze hydrogenation reactions.
Heterogeneous catalysts dominate current industrial applications due to their separation advantages and reusability. However, homogeneous catalysts offer superior selectivity and operate under milder conditions, making them valuable for specific biomass transformations despite recovery challenges.
Several critical challenges persist in catalyst technology for biomass conversion. Catalyst deactivation remains a primary concern, occurring through mechanisms including coking, poisoning by heteroatoms (particularly sulfur and nitrogen), and metal leaching. Many biomass feedstocks contain impurities that rapidly degrade catalyst performance, necessitating costly pretreatment steps.
Selectivity challenges arise from biomass's complex, heterogeneous nature, which yields diverse reaction intermediates. Controlling reaction pathways to maximize desired products while minimizing side reactions represents a significant technical hurdle. Additionally, most current catalysts require high-pressure hydrogen, increasing operational costs and safety concerns.
Scale-up issues further complicate industrial implementation, as catalysts performing well in laboratory settings often show diminished efficiency at commercial scales. Heat and mass transfer limitations become particularly problematic in larger reactors, affecting reaction kinetics and catalyst lifetime.
Water tolerance represents another critical challenge, as many biomass conversion processes involve aqueous environments that can degrade conventional catalysts. Developing water-stable catalytic systems remains an active research area with significant potential impact on process economics.
Transition metal catalysts, especially nickel, copper, and iron-based systems, have emerged as cost-effective alternatives. While they generally show lower activity than noble metals, recent advancements in preparation methods have significantly improved their performance. Nickel-based catalysts, for instance, demonstrate promising results in hydrogenolysis reactions for lignin conversion, though they remain susceptible to deactivation through carbon deposition and sintering.
Bifunctional catalysts combining metal sites with acid/base functionalities represent a significant innovation in biomass processing. These systems can simultaneously facilitate multiple reaction pathways, enhancing overall process efficiency. Zeolite-supported metal catalysts exemplify this approach, where the zeolite framework provides shape selectivity and acid sites while metal nanoparticles catalyze hydrogenation reactions.
Heterogeneous catalysts dominate current industrial applications due to their separation advantages and reusability. However, homogeneous catalysts offer superior selectivity and operate under milder conditions, making them valuable for specific biomass transformations despite recovery challenges.
Several critical challenges persist in catalyst technology for biomass conversion. Catalyst deactivation remains a primary concern, occurring through mechanisms including coking, poisoning by heteroatoms (particularly sulfur and nitrogen), and metal leaching. Many biomass feedstocks contain impurities that rapidly degrade catalyst performance, necessitating costly pretreatment steps.
Selectivity challenges arise from biomass's complex, heterogeneous nature, which yields diverse reaction intermediates. Controlling reaction pathways to maximize desired products while minimizing side reactions represents a significant technical hurdle. Additionally, most current catalysts require high-pressure hydrogen, increasing operational costs and safety concerns.
Scale-up issues further complicate industrial implementation, as catalysts performing well in laboratory settings often show diminished efficiency at commercial scales. Heat and mass transfer limitations become particularly problematic in larger reactors, affecting reaction kinetics and catalyst lifetime.
Water tolerance represents another critical challenge, as many biomass conversion processes involve aqueous environments that can degrade conventional catalysts. Developing water-stable catalytic systems remains an active research area with significant potential impact on process economics.
State-of-the-Art Catalyst Solutions
01 Metal-based catalysts for biomass conversion
Various metal-based catalysts have been developed for efficient biomass upgrading processes. These catalysts, including noble metals (such as platinum, palladium) and transition metals (such as nickel, copper), facilitate the conversion of biomass into valuable chemicals and fuels. The catalysts can be used in different forms, such as supported on carriers or as nanoparticles, to enhance their catalytic activity and selectivity in biomass transformation reactions.- Metal-based catalysts for biomass conversion: Metal-based catalysts play a crucial role in biomass upgrading processes. Various metals such as nickel, platinum, palladium, and ruthenium are used as active components in catalysts to enhance the efficiency of biomass conversion. These catalysts facilitate reactions like hydrogenation, dehydration, and deoxygenation of biomass-derived compounds, leading to valuable products with higher energy density and improved properties.
- Zeolite and molecular sieve catalysts: Zeolites and molecular sieves are important catalytic materials for biomass upgrading due to their unique porous structure and acidity. These catalysts provide shape selectivity and can be tailored with different pore sizes and acid strengths to target specific biomass conversion reactions. They are particularly effective in processes such as catalytic cracking, isomerization, and dehydration of biomass-derived compounds to produce biofuels and chemicals.
- Heterogeneous catalysts for hydrothermal processing: Heterogeneous catalysts designed for hydrothermal processing enable efficient biomass conversion under hot compressed water conditions. These catalysts are specifically developed to withstand harsh reaction environments including high temperatures, pressures, and the presence of water. They facilitate reactions such as hydrolysis, dehydration, and reforming of biomass components, allowing for the production of platform chemicals and liquid fuels from various biomass feedstocks.
- Bifunctional and multifunctional catalysts: Bifunctional and multifunctional catalysts combine multiple active sites to perform sequential or concurrent reactions in biomass upgrading processes. These catalysts typically incorporate both metal sites for hydrogenation/dehydrogenation and acid sites for cracking/isomerization. By integrating different functionalities into a single catalyst, reaction pathways can be optimized, reducing the number of processing steps and improving overall conversion efficiency of biomass to valuable products.
- Catalyst supports and promoters for enhanced stability: The development of specialized catalyst supports and promoters significantly enhances the stability and efficiency of biomass upgrading catalysts. Various support materials such as carbon, alumina, silica, and mixed oxides provide high surface area and mechanical strength while improving dispersion of active phases. Additionally, promoters can be incorporated to enhance catalyst activity, selectivity, and resistance to deactivation caused by water, acids, and other compounds present in biomass processing environments.
02 Zeolite and molecular sieve catalysts
Zeolites and molecular sieves serve as effective catalysts for biomass upgrading due to their unique porous structures and acidic properties. These materials provide shape selectivity and can be tailored with different pore sizes and acidity levels to target specific biomass conversion reactions. They are particularly useful in processes like catalytic cracking, dehydration, and isomerization of biomass-derived compounds, offering improved efficiency and product selectivity.Expand Specific Solutions03 Hydrothermal processing catalysts
Catalysts specifically designed for hydrothermal processing enable efficient biomass conversion under hot compressed water conditions. These specialized catalysts facilitate reactions like hydrolysis, dehydration, and reforming of biomass in aqueous environments. The hydrothermal approach allows for processing wet biomass without energy-intensive drying steps, and the catalysts are developed to withstand the harsh conditions while maintaining activity and stability.Expand Specific Solutions04 Biocatalysts and enzymatic systems
Biocatalysts and enzymatic systems offer environmentally friendly approaches to biomass upgrading. These biological catalysts operate under mild conditions and exhibit high specificity for targeted biomass conversion reactions. Engineered enzymes and microbial systems can break down complex biomass components like cellulose, hemicellulose, and lignin into simpler molecules. The development of robust biocatalysts with enhanced stability and activity has significantly improved the efficiency of biomass processing.Expand Specific Solutions05 Catalyst supports and promoters for enhanced efficiency
The efficiency of biomass upgrading catalysts can be significantly improved through the use of specialized supports and promoters. Various materials such as carbon, alumina, silica, and metal oxides serve as supports that enhance catalyst dispersion, stability, and accessibility. Additionally, promoters and dopants can modify the electronic and structural properties of catalysts, leading to improved activity, selectivity, and resistance to deactivation in biomass conversion processes.Expand Specific Solutions
Leading Companies and Research Institutions
Biomass upgrading to fuels is currently in a transitional phase from early commercialization to broader market adoption, with the global biofuels market projected to reach $218 billion by 2026. Technical maturity varies significantly across conversion pathways, with companies demonstrating different levels of innovation. Shell and KiOR lead in catalytic pyrolysis technologies, while RTI International and Fraunhofer-Gesellschaft advance thermochemical conversion methods. Academic institutions like Zhejiang University and Louisiana State University contribute fundamental research in catalyst development. Cool Planet and Virent are pioneering novel catalytic approaches for biomass-to-liquid fuels. The competitive landscape is characterized by strategic partnerships between research organizations and energy companies, with increasing focus on process intensification and catalyst stability to overcome efficiency barriers.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed advanced catalytic pyrolysis technologies for biomass conversion to fuels, focusing on zeolite-based catalysts that enhance the quality of bio-oil. Their IH² (Integrated Hydropyrolysis and Hydroconversion) process combines catalytic pyrolysis with hydroprocessing in a two-stage system. The first stage uses a proprietary catalyst to convert biomass directly to a partially deoxygenated product, while the second stage employs hydroprocessing catalysts to produce hydrocarbon fuels. This integrated approach achieves over 70% carbon efficiency and produces drop-in fuels compatible with existing infrastructure. Shell has also pioneered metal-modified zeolite catalysts that significantly reduce oxygen content in bio-oil while minimizing coke formation, addressing a key challenge in biomass conversion. Their research extends to bifunctional catalysts that combine acidic and metallic sites to simultaneously facilitate cracking and hydrogenation reactions, improving overall process efficiency.
Strengths: Shell's IH² technology achieves higher carbon efficiency than conventional pyrolysis methods and produces fuels that require minimal additional refining. Their catalyst formulations demonstrate excellent stability and reduced coking compared to traditional zeolites. Weaknesses: The process requires significant hydrogen input, increasing operational costs, and the catalysts may be susceptible to deactivation from biomass impurities like alkali metals and nitrogen compounds.
KiOR, Inc.
Technical Solution: KiOR developed a proprietary Biomass Fluid Catalytic Cracking (BFCC) technology that adapts petroleum refining catalytic cracking processes for biomass conversion. Their approach utilized modified zeolite catalysts with controlled mesoporosity to overcome diffusion limitations inherent in biomass molecules. The process operated at moderate temperatures (400-500°C) and atmospheric pressure, making it less energy-intensive than competing technologies. KiOR's catalysts featured rare earth metal modifications that enhanced selectivity toward hydrocarbon production while minimizing oxygenated compounds. The technology employed a circulating fluidized bed reactor system that allowed continuous catalyst regeneration, addressing the rapid deactivation issues common in biomass processing. This system achieved conversion of woody biomass to a bio-oil intermediate with significantly reduced oxygen content (10-20% vs. 35-40% in conventional pyrolysis), which could then be upgraded to transportation fuels through conventional hydroprocessing steps with less hydrogen consumption than competing processes.
Strengths: KiOR's technology operated at lower pressure and with less hydrogen than many competing processes, potentially reducing capital and operating costs. The continuous catalyst regeneration system addressed catalyst deactivation issues. Weaknesses: Despite technical innovations, KiOR faced challenges with catalyst stability and product consistency at commercial scale, ultimately leading to the company's bankruptcy in 2014. The process still required a secondary hydroprocessing step to produce finished fuels.
Key Catalyst Innovations and Patents
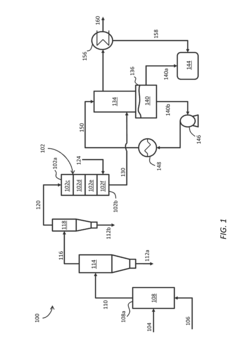
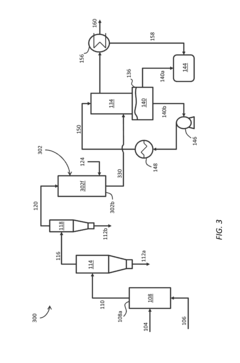
In-Situ Upgrading Of Biomass Pyrolysis Vapor Using Water-Gas Shift And Hydroprocessing
PatentInactiveUS20140230317A1
Innovation
- In-situ upgrading of biomass pyrolysis vapor using a multi-layer catalyst bed or cascaded catalytic reactors, incorporating cracking, water-gas shift, hydrotreating, and acid catalysts to reduce oxygen and water content, and acidity, allowing direct conversion into a refined biooil that can be combined with crude oil to produce gasoline.
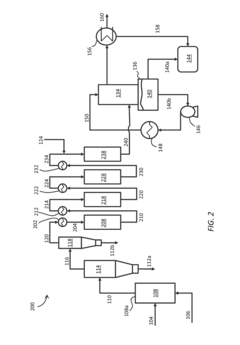
Mesoporous Zeolite-Containing Catalysts For The Thermoconversion Of Biomass And For Upgrading Bio-Oils
PatentInactiveUS20160017238A1
Innovation
- Development of a catalyst system with a hierarchical microporous-mesoporous structure comprising zeolites and a non-zeolitic matrix, optimized to enhance accessibility and attrition resistance, utilizing a method that includes modifying zeolites, forming a slurry precursor mixture, shaping, and thermally treating to create a mesoporous-macroporous structure.
Sustainability and Life Cycle Assessment
The sustainability of biomass upgrading processes is increasingly becoming a critical factor in determining the viability of biofuel technologies. Life Cycle Assessment (LCA) provides a comprehensive framework for evaluating the environmental impacts of catalytic processes used in biomass conversion to fuels, from raw material extraction through production, use, and disposal.
Catalyst selection significantly influences the sustainability profile of biomass upgrading pathways. Metal-based catalysts, particularly those containing platinum group metals (PGMs), often demonstrate superior efficiency but present sustainability challenges due to resource scarcity and energy-intensive mining processes. Recent LCA studies indicate that noble metal catalysts can contribute up to 40% of the total environmental impact in certain biomass conversion processes, highlighting the importance of catalyst optimization.
Alternative catalytic materials such as transition metal carbides, nitrides, and earth-abundant metal oxides are emerging as more sustainable options. These materials typically require less energy to produce and utilize more abundant elements, though they may currently offer lower catalytic performance. The trade-off between catalytic efficiency and environmental impact necessitates a holistic approach to catalyst development and selection.
Process intensification strategies incorporating catalyst innovations can significantly enhance sustainability metrics. Multifunctional catalysts that enable one-pot reactions reduce separation steps and associated energy requirements, potentially decreasing the carbon footprint by 15-30% compared to conventional multi-step processes. Similarly, continuous flow systems utilizing heterogeneous catalysts often demonstrate improved atom economy and reduced waste generation compared to batch processes.
Catalyst stability and recyclability represent crucial factors in sustainability assessments. Extended catalyst lifetimes directly correlate with reduced environmental impacts, as fewer replacement cycles are needed. Recent advances in catalyst regeneration techniques have shown promise in extending effective catalyst lifetimes by 2-3 times, substantially improving life cycle performance.
Water and solvent usage in catalytic biomass upgrading processes presents another significant sustainability consideration. Aqueous-phase processing using water-tolerant catalysts can eliminate the need for energy-intensive drying steps and organic solvents, potentially reducing process energy requirements by up to 50% in certain applications. However, catalyst deactivation in aqueous environments remains a challenge requiring further research.
The integration of renewable energy sources for powering catalytic processes represents a promising approach to further enhance sustainability. Electrocatalytic and photocatalytic systems powered by renewable electricity or direct solar energy could potentially reduce the carbon intensity of biomass upgrading processes by 60-80% compared to conventional thermochemical approaches, though these technologies remain largely at the laboratory scale.
Catalyst selection significantly influences the sustainability profile of biomass upgrading pathways. Metal-based catalysts, particularly those containing platinum group metals (PGMs), often demonstrate superior efficiency but present sustainability challenges due to resource scarcity and energy-intensive mining processes. Recent LCA studies indicate that noble metal catalysts can contribute up to 40% of the total environmental impact in certain biomass conversion processes, highlighting the importance of catalyst optimization.
Alternative catalytic materials such as transition metal carbides, nitrides, and earth-abundant metal oxides are emerging as more sustainable options. These materials typically require less energy to produce and utilize more abundant elements, though they may currently offer lower catalytic performance. The trade-off between catalytic efficiency and environmental impact necessitates a holistic approach to catalyst development and selection.
Process intensification strategies incorporating catalyst innovations can significantly enhance sustainability metrics. Multifunctional catalysts that enable one-pot reactions reduce separation steps and associated energy requirements, potentially decreasing the carbon footprint by 15-30% compared to conventional multi-step processes. Similarly, continuous flow systems utilizing heterogeneous catalysts often demonstrate improved atom economy and reduced waste generation compared to batch processes.
Catalyst stability and recyclability represent crucial factors in sustainability assessments. Extended catalyst lifetimes directly correlate with reduced environmental impacts, as fewer replacement cycles are needed. Recent advances in catalyst regeneration techniques have shown promise in extending effective catalyst lifetimes by 2-3 times, substantially improving life cycle performance.
Water and solvent usage in catalytic biomass upgrading processes presents another significant sustainability consideration. Aqueous-phase processing using water-tolerant catalysts can eliminate the need for energy-intensive drying steps and organic solvents, potentially reducing process energy requirements by up to 50% in certain applications. However, catalyst deactivation in aqueous environments remains a challenge requiring further research.
The integration of renewable energy sources for powering catalytic processes represents a promising approach to further enhance sustainability. Electrocatalytic and photocatalytic systems powered by renewable electricity or direct solar energy could potentially reduce the carbon intensity of biomass upgrading processes by 60-80% compared to conventional thermochemical approaches, though these technologies remain largely at the laboratory scale.
Economic Viability and Scalability Factors
The economic viability of biomass-to-fuel conversion processes hinges critically on catalyst performance and cost-effectiveness. Current noble metal catalysts such as platinum, palladium, and ruthenium deliver high efficiency but at prohibitive costs, ranging from $30,000 to $60,000 per kilogram. This creates a significant barrier to commercial-scale implementation, particularly when competing with conventional fossil fuels priced at $40-80 per barrel. The catalyst lifecycle economics must be evaluated comprehensively, including initial investment, operational longevity, deactivation rates, and regeneration costs.
Scale-up challenges represent another crucial economic consideration. Laboratory-scale catalyst systems often demonstrate promising performance metrics that deteriorate significantly when scaled to industrial production levels. Factors contributing to this performance gap include mass transfer limitations, heat distribution inefficiencies, and catalyst deactivation mechanisms that accelerate under industrial conditions. The capital expenditure required for large-scale biomass processing facilities utilizing advanced catalytic systems typically ranges from $300-600 million, necessitating long-term operational stability to achieve reasonable return on investment.
Feedstock variability introduces additional economic complexity. Biomass sources vary seasonally and geographically in composition, moisture content, and contaminant profiles. Catalysts that demonstrate robustness across diverse feedstock conditions command premium value in industrial applications. Economic modeling indicates that catalysts capable of processing multiple feedstock types without significant performance degradation can improve plant economics by 15-25% through increased operational flexibility and reduced downtime.
Energy integration represents a critical factor in overall process economics. Catalytic processes that operate at lower temperatures and pressures significantly reduce operational costs. For instance, reducing operating temperature by 50°C can decrease energy consumption by approximately 10-15%, translating to millions in annual savings for commercial-scale operations. Hydrogen consumption in hydroprocessing steps constitutes another major cost factor, with hydrogen production and utilization accounting for 20-30% of operational expenses in many biomass upgrading pathways.
The recyclability and regeneration potential of catalysts directly impacts long-term economic viability. Catalysts that can undergo multiple regeneration cycles while maintaining at least 80% of their initial activity offer substantially improved lifecycle economics. Industry benchmarks suggest that extending catalyst lifetime from one year to three years through effective regeneration protocols can reduce the levelized cost of biofuel production by 8-12%, significantly enhancing competitiveness with conventional fuels.
Scale-up challenges represent another crucial economic consideration. Laboratory-scale catalyst systems often demonstrate promising performance metrics that deteriorate significantly when scaled to industrial production levels. Factors contributing to this performance gap include mass transfer limitations, heat distribution inefficiencies, and catalyst deactivation mechanisms that accelerate under industrial conditions. The capital expenditure required for large-scale biomass processing facilities utilizing advanced catalytic systems typically ranges from $300-600 million, necessitating long-term operational stability to achieve reasonable return on investment.
Feedstock variability introduces additional economic complexity. Biomass sources vary seasonally and geographically in composition, moisture content, and contaminant profiles. Catalysts that demonstrate robustness across diverse feedstock conditions command premium value in industrial applications. Economic modeling indicates that catalysts capable of processing multiple feedstock types without significant performance degradation can improve plant economics by 15-25% through increased operational flexibility and reduced downtime.
Energy integration represents a critical factor in overall process economics. Catalytic processes that operate at lower temperatures and pressures significantly reduce operational costs. For instance, reducing operating temperature by 50°C can decrease energy consumption by approximately 10-15%, translating to millions in annual savings for commercial-scale operations. Hydrogen consumption in hydroprocessing steps constitutes another major cost factor, with hydrogen production and utilization accounting for 20-30% of operational expenses in many biomass upgrading pathways.
The recyclability and regeneration potential of catalysts directly impacts long-term economic viability. Catalysts that can undergo multiple regeneration cycles while maintaining at least 80% of their initial activity offer substantially improved lifecycle economics. Industry benchmarks suggest that extending catalyst lifetime from one year to three years through effective regeneration protocols can reduce the levelized cost of biofuel production by 8-12%, significantly enhancing competitiveness with conventional fuels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!