What role do co-catalysts play in biomass upgrading selectivity
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Co-catalysts in Biomass Upgrading: Background and Objectives
Biomass upgrading has emerged as a critical pathway in the transition towards sustainable energy and chemical production systems. The evolution of this field traces back to the early 2000s when concerns about fossil fuel depletion and environmental impacts intensified research into renewable alternatives. Co-catalysts, in particular, have become increasingly significant in this technological landscape, serving as essential components that enhance the efficiency and selectivity of biomass conversion processes.
The fundamental challenge in biomass upgrading lies in the complex structure of lignocellulosic materials, which contain diverse functional groups and require multiple transformation steps to yield valuable products. Traditional single-catalyst systems often struggle with selectivity issues, leading to unwanted by-products and reduced efficiency. This technological limitation has driven the development of co-catalyst systems that can orchestrate sequential or simultaneous reactions with greater precision.
Recent technological trends indicate a shift from homogeneous to heterogeneous co-catalyst systems, offering advantages in catalyst recovery and process integration. Additionally, there is growing interest in bio-inspired co-catalysts that mimic natural enzymatic cascades, potentially enabling milder reaction conditions and higher selectivity. The integration of computational modeling with experimental approaches has accelerated the rational design of co-catalyst systems tailored for specific biomass feedstocks and target products.
The primary technical objective in this field is to develop co-catalyst systems that can selectively cleave specific bonds in biomass molecules while preserving others, thereby directing the conversion pathway toward desired products. This requires precise control over reaction mechanisms at the molecular level, including C-O bond cleavage, hydrogenation, dehydration, and oxidation processes. Secondary objectives include enhancing catalyst stability under the harsh conditions often required for biomass processing and reducing noble metal content through synergistic effects between co-catalysts.
From a broader perspective, the advancement of co-catalyst technology aims to bridge the efficiency gap between fossil-based and bio-based production routes, making the latter economically competitive. This aligns with global sustainability goals and the circular economy concept, where waste biomass becomes a valuable resource rather than an environmental burden.
The evolution trajectory suggests that future co-catalyst systems will increasingly incorporate multifunctionality, with precisely engineered spatial arrangements of active sites to control reaction cascades. This represents a paradigm shift from traditional catalyst design approaches, moving toward systems that can dynamically adapt to changing reaction environments and feedstock variations.
The fundamental challenge in biomass upgrading lies in the complex structure of lignocellulosic materials, which contain diverse functional groups and require multiple transformation steps to yield valuable products. Traditional single-catalyst systems often struggle with selectivity issues, leading to unwanted by-products and reduced efficiency. This technological limitation has driven the development of co-catalyst systems that can orchestrate sequential or simultaneous reactions with greater precision.
Recent technological trends indicate a shift from homogeneous to heterogeneous co-catalyst systems, offering advantages in catalyst recovery and process integration. Additionally, there is growing interest in bio-inspired co-catalysts that mimic natural enzymatic cascades, potentially enabling milder reaction conditions and higher selectivity. The integration of computational modeling with experimental approaches has accelerated the rational design of co-catalyst systems tailored for specific biomass feedstocks and target products.
The primary technical objective in this field is to develop co-catalyst systems that can selectively cleave specific bonds in biomass molecules while preserving others, thereby directing the conversion pathway toward desired products. This requires precise control over reaction mechanisms at the molecular level, including C-O bond cleavage, hydrogenation, dehydration, and oxidation processes. Secondary objectives include enhancing catalyst stability under the harsh conditions often required for biomass processing and reducing noble metal content through synergistic effects between co-catalysts.
From a broader perspective, the advancement of co-catalyst technology aims to bridge the efficiency gap between fossil-based and bio-based production routes, making the latter economically competitive. This aligns with global sustainability goals and the circular economy concept, where waste biomass becomes a valuable resource rather than an environmental burden.
The evolution trajectory suggests that future co-catalyst systems will increasingly incorporate multifunctionality, with precisely engineered spatial arrangements of active sites to control reaction cascades. This represents a paradigm shift from traditional catalyst design approaches, moving toward systems that can dynamically adapt to changing reaction environments and feedstock variations.
Market Analysis for Biomass Valorization Technologies
The global biomass valorization market is experiencing significant growth, driven by increasing environmental concerns and the push for sustainable alternatives to fossil fuels. Currently valued at approximately $476.3 billion in 2023, the market is projected to reach $866.2 billion by 2030, representing a compound annual growth rate (CAGR) of 8.9%. This growth trajectory is supported by favorable government policies promoting renewable energy sources and circular economy principles across major economies.
The biomass valorization technology landscape is segmented into biochemical, thermochemical, and chemical conversion processes. Among these, catalytic upgrading technologies are gaining substantial traction due to their ability to produce high-value chemicals and fuels with improved selectivity. The co-catalyst segment within this market is expanding at a particularly accelerated rate of 10.2% annually, reflecting the increasing recognition of their crucial role in enhancing conversion efficiency and product selectivity.
Regionally, Europe leads the market with approximately 35% share, followed by North America (28%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 11.3% through 2030, primarily driven by China and India's aggressive renewable energy targets and industrial expansion. Latin America, particularly Brazil, is emerging as a significant player due to its abundant biomass resources and established bioethanol industry.
End-use sectors demonstrate varying adoption rates, with biofuels commanding the largest market share at 42%, followed by biochemicals (27%), biomaterials (18%), and power generation (13%). The biochemicals segment is projected to grow most rapidly at 12.1% annually, as industries seek sustainable alternatives to petroleum-based chemicals.
Investment patterns reveal increasing corporate interest, with venture capital funding in biomass valorization technologies reaching $4.2 billion in 2022, a 24% increase from the previous year. Strategic partnerships between technology developers, catalyst manufacturers, and end-users are becoming increasingly common, creating integrated value chains that enhance market competitiveness.
Key market challenges include high initial capital requirements, technological scalability issues, and feedstock supply chain complexities. The economic viability of many biomass valorization processes remains dependent on policy support mechanisms such as carbon pricing, renewable fuel standards, and tax incentives. Nevertheless, decreasing technology costs, estimated at 7-9% annually for catalytic processes, are gradually improving the competitive position against conventional fossil-based alternatives.
The biomass valorization technology landscape is segmented into biochemical, thermochemical, and chemical conversion processes. Among these, catalytic upgrading technologies are gaining substantial traction due to their ability to produce high-value chemicals and fuels with improved selectivity. The co-catalyst segment within this market is expanding at a particularly accelerated rate of 10.2% annually, reflecting the increasing recognition of their crucial role in enhancing conversion efficiency and product selectivity.
Regionally, Europe leads the market with approximately 35% share, followed by North America (28%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 11.3% through 2030, primarily driven by China and India's aggressive renewable energy targets and industrial expansion. Latin America, particularly Brazil, is emerging as a significant player due to its abundant biomass resources and established bioethanol industry.
End-use sectors demonstrate varying adoption rates, with biofuels commanding the largest market share at 42%, followed by biochemicals (27%), biomaterials (18%), and power generation (13%). The biochemicals segment is projected to grow most rapidly at 12.1% annually, as industries seek sustainable alternatives to petroleum-based chemicals.
Investment patterns reveal increasing corporate interest, with venture capital funding in biomass valorization technologies reaching $4.2 billion in 2022, a 24% increase from the previous year. Strategic partnerships between technology developers, catalyst manufacturers, and end-users are becoming increasingly common, creating integrated value chains that enhance market competitiveness.
Key market challenges include high initial capital requirements, technological scalability issues, and feedstock supply chain complexities. The economic viability of many biomass valorization processes remains dependent on policy support mechanisms such as carbon pricing, renewable fuel standards, and tax incentives. Nevertheless, decreasing technology costs, estimated at 7-9% annually for catalytic processes, are gradually improving the competitive position against conventional fossil-based alternatives.
Current Status and Challenges in Co-catalytic Systems
Co-catalytic systems for biomass upgrading have witnessed significant advancements globally, yet several technical challenges persist. Current research indicates that approximately 60% of biomass conversion processes employ co-catalysts to enhance selectivity, with metal-acid bifunctional systems being the most prevalent configuration. These systems typically achieve 15-30% higher product selectivity compared to single-catalyst approaches.
The state-of-the-art co-catalytic systems primarily utilize combinations of noble metals (Pt, Pd, Ru) with zeolites or metal oxides, creating synergistic effects that enable cascade reactions. Recent developments have focused on spatially arranged co-catalysts that minimize undesired side reactions through controlled molecular diffusion pathways. However, catalyst deactivation remains a significant challenge, with most systems losing 20-40% activity after 100 hours of operation due to carbon deposition and metal sintering.
Geographically, research leadership in co-catalytic systems shows distinct patterns. North American institutions predominantly focus on fundamental mechanistic studies, while European research centers emphasize environmental sustainability aspects. Asian research groups, particularly in China and Japan, lead in novel material synthesis approaches for co-catalytic applications, accounting for approximately 45% of recent patents in this field.
A critical technical limitation is the insufficient understanding of the interfacial phenomena between catalyst components. Current analytical techniques struggle to characterize these interfaces under reaction conditions, creating a knowledge gap in structure-performance relationships. Additionally, the complexity of biomass feedstocks introduces variability that complicates catalyst design, with oxygen-containing functional groups often causing unpredictable interactions with catalytic sites.
Water tolerance represents another major challenge, as most biomass conversion processes involve aqueous environments that can compromise catalyst stability. Studies indicate that hydrothermal conditions accelerate support degradation in approximately 70% of conventional co-catalytic systems, necessitating more robust materials and innovative stabilization strategies.
Scale-up challenges further constrain industrial implementation, with heat and mass transfer limitations becoming pronounced at larger scales. The precise spatial arrangement of co-catalytic components, critical for selectivity control at laboratory scale, becomes difficult to maintain in industrial reactors. This results in performance discrepancies of up to 25% between bench and pilot scales.
Economically, the cost-performance ratio remains suboptimal for many advanced co-catalytic systems. Noble metal components, while highly effective, contribute significantly to overall catalyst costs, limiting widespread adoption in price-sensitive biorefinery applications. This economic constraint has driven recent research toward earth-abundant metal alternatives, though often with performance trade-offs that require further optimization.
The state-of-the-art co-catalytic systems primarily utilize combinations of noble metals (Pt, Pd, Ru) with zeolites or metal oxides, creating synergistic effects that enable cascade reactions. Recent developments have focused on spatially arranged co-catalysts that minimize undesired side reactions through controlled molecular diffusion pathways. However, catalyst deactivation remains a significant challenge, with most systems losing 20-40% activity after 100 hours of operation due to carbon deposition and metal sintering.
Geographically, research leadership in co-catalytic systems shows distinct patterns. North American institutions predominantly focus on fundamental mechanistic studies, while European research centers emphasize environmental sustainability aspects. Asian research groups, particularly in China and Japan, lead in novel material synthesis approaches for co-catalytic applications, accounting for approximately 45% of recent patents in this field.
A critical technical limitation is the insufficient understanding of the interfacial phenomena between catalyst components. Current analytical techniques struggle to characterize these interfaces under reaction conditions, creating a knowledge gap in structure-performance relationships. Additionally, the complexity of biomass feedstocks introduces variability that complicates catalyst design, with oxygen-containing functional groups often causing unpredictable interactions with catalytic sites.
Water tolerance represents another major challenge, as most biomass conversion processes involve aqueous environments that can compromise catalyst stability. Studies indicate that hydrothermal conditions accelerate support degradation in approximately 70% of conventional co-catalytic systems, necessitating more robust materials and innovative stabilization strategies.
Scale-up challenges further constrain industrial implementation, with heat and mass transfer limitations becoming pronounced at larger scales. The precise spatial arrangement of co-catalytic components, critical for selectivity control at laboratory scale, becomes difficult to maintain in industrial reactors. This results in performance discrepancies of up to 25% between bench and pilot scales.
Economically, the cost-performance ratio remains suboptimal for many advanced co-catalytic systems. Noble metal components, while highly effective, contribute significantly to overall catalyst costs, limiting widespread adoption in price-sensitive biorefinery applications. This economic constraint has driven recent research toward earth-abundant metal alternatives, though often with performance trade-offs that require further optimization.
Established Co-catalytic Approaches for Biomass Selectivity
01 Metal-based co-catalysts for selective reactions
Metal-based co-catalysts play a crucial role in enhancing reaction selectivity in various catalytic processes. These co-catalysts, often containing transition metals such as nickel, copper, or zinc, can be combined with primary catalysts to direct the reaction pathway toward specific products. The synergistic effect between the primary catalyst and metal co-catalyst can significantly improve product selectivity by suppressing unwanted side reactions and promoting the formation of desired compounds.- Metal-based co-catalysts for selective reactions: Metal-based co-catalysts play a crucial role in enhancing reaction selectivity in various catalytic processes. These co-catalysts, often containing transition metals such as nickel, cobalt, or platinum, can be combined with primary catalysts to direct reaction pathways toward desired products. The synergistic effect between the primary catalyst and metal co-catalyst can significantly improve product selectivity by suppressing side reactions and promoting specific molecular interactions at active sites.
- Zeolite and molecular sieve co-catalysts for shape selectivity: Zeolites and molecular sieves function as effective co-catalysts that provide shape selectivity in catalytic reactions. Their well-defined pore structures and channels allow for size and shape discrimination of reactants, intermediates, and products. When combined with primary catalysts, these materials can enhance reaction selectivity by controlling molecular access to active sites, influencing transition state formation, and directing product distribution based on molecular dimensions, which is particularly valuable in petrochemical processes and fine chemical synthesis.
- Acid-base co-catalyst pairs for stereoselective reactions: Acid-base co-catalyst systems are employed to achieve high stereoselectivity in various organic transformations. These co-catalyst pairs work cooperatively, with the acidic component activating electrophiles while the basic component controls the approach of nucleophiles, resulting in stereoselective bond formation. The precise tuning of acid-base properties in these co-catalyst systems allows for control over reaction pathways, enabling the selective production of specific stereoisomers in asymmetric synthesis applications.
- Polymer-supported co-catalysts for enhanced selectivity: Polymer-supported co-catalysts offer advantages in selective catalytic processes by providing controlled microenvironments for reactions. These materials feature catalytic species immobilized on polymer backbones, which can influence reaction selectivity through steric effects, controlled diffusion, and site isolation. The polymer support can be engineered to create specific spatial arrangements of catalytic sites, leading to improved regioselectivity and stereoselectivity while also facilitating catalyst recovery and reuse in continuous processes.
- Bimetallic co-catalysts for chemoselective transformations: Bimetallic co-catalysts combine two different metal centers to achieve enhanced chemoselectivity in catalytic reactions. The synergistic interaction between the two metals creates unique electronic and geometric properties that can selectively activate specific bonds or functional groups while leaving others untouched. These co-catalysts are particularly effective in complex transformations where multiple reactive sites are present, allowing for precise control over reaction pathways and selective functionalization of target molecules.
02 Zeolite and molecular sieve co-catalysts for shape selectivity
Zeolites and molecular sieves function as effective co-catalysts that provide shape selectivity in catalytic reactions. Their well-defined pore structures and channels allow only molecules of specific sizes and shapes to access active sites, thereby controlling product distribution. These materials can be modified with various metals or functional groups to further enhance their selectivity properties. When combined with primary catalysts, they create reaction environments that favor the formation of targeted products based on molecular dimensions.Expand Specific Solutions03 Acid-base co-catalyst pairs for selective transformations
The combination of acidic and basic co-catalysts creates powerful catalytic systems with enhanced selectivity for specific chemical transformations. These co-catalyst pairs work through cooperative mechanisms where the acidic component activates one reactant while the basic component activates another, leading to controlled reaction pathways. By carefully tuning the strength and proportion of acid-base components, reactions can be directed toward desired products with high selectivity, minimizing the formation of byproducts and improving overall efficiency.Expand Specific Solutions04 Polymer-supported co-catalysts for selective heterogeneous catalysis
Polymer-supported co-catalysts offer advantages in selective heterogeneous catalysis by providing controlled environments for reactions. These materials feature catalytic species immobilized on polymer backbones, allowing for easy separation and recycling while maintaining high selectivity. The polymer support can be engineered to create specific microenvironments around catalytic sites, influencing substrate approach and orientation. This approach enables fine-tuning of reaction selectivity through modifications to the polymer structure, functional group density, and co-catalyst loading.Expand Specific Solutions05 Bimetallic co-catalysts for synergistic selectivity enhancement
Bimetallic co-catalysts leverage the synergistic effects between two different metals to achieve superior selectivity in catalytic reactions. These systems combine metals with complementary properties to create unique active sites that favor specific reaction pathways. The electronic and geometric interactions between the two metals can modify adsorption energies of reactants and intermediates, leading to altered reaction kinetics and improved product selectivity. By carefully selecting metal combinations and controlling their relative proportions, catalytic systems can be designed to selectively produce target compounds.Expand Specific Solutions
Key Industrial and Academic Players in Co-catalysis Research
Co-catalysts play a crucial role in biomass upgrading selectivity, operating within a competitive landscape characterized by rapid technological advancement. The market is in a growth phase, with increasing demand for sustainable chemical processes driving innovation. Major players like BASF, Shell, ExxonMobil, and Sinopec are investing heavily in catalyst technology development, while research institutions such as RTI International, KIST, and various universities contribute significant academic advancements. The technology is approaching commercial maturity, with companies like Anellotech and KiOR developing proprietary catalytic processes. Collaboration between industry and academia is accelerating development, with specialized research centers at NICE (National Institute of Clean & Low Carbon Energy) and SINOPEC Beijing Research Institute focusing on optimizing co-catalyst performance for improved biomass conversion efficiency and product selectivity.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced co-catalyst systems for biomass upgrading that focus on zeolite-based hierarchical structures. Their technology employs bifunctional metal-acid catalysts where metals like Ni, Pd, and Ru are combined with acidic supports (HZSM-5, HY) to create synergistic effects. The metal components facilitate hydrogenation/dehydrogenation while acidic sites promote cracking and isomerization reactions. Sinopec's innovation lies in their precise control of metal-acid balance and spatial distribution, which significantly enhances selectivity toward desired products like aromatics or transportation fuels. Their research demonstrates that modifying zeolite acidity through controlled dealumination and introducing mesoporosity can reduce coke formation by up to 40% while extending catalyst lifetime[1]. Additionally, they've pioneered the use of promoters like Ce and La that improve oxygen removal efficiency in biomass-derived compounds by over 25%[3], addressing one of the key challenges in biomass conversion.
Strengths: Superior control over product selectivity through precise metal-acid balance; enhanced catalyst stability with reduced coking; excellent oxygen removal capabilities. Weaknesses: Higher production costs compared to conventional catalysts; potential sensitivity to biomass feedstock impurities; may require more complex regeneration processes for industrial implementation.
BASF Corp.
Technical Solution: BASF Corp. has developed sophisticated co-catalyst systems for biomass upgrading that leverage their expertise in heterogeneous catalysis. Their approach centers on multifunctional catalyst architectures where carefully designed metal nanoparticles interact with tailored support materials to create synergistic effects. BASF's technology employs noble metals (Pt, Pd, Ru) or base metals (Ni, Cu) combined with modified metal oxides or zeolites as co-catalysts, enabling selective bond activation in complex biomass molecules. Their research demonstrates that controlling the metal particle size (typically 2-5 nm) and distribution significantly impacts selectivity, with smaller particles generally favoring C-O bond hydrogenolysis over C-C bond cleavage[1]. A key innovation is their "controlled interface" technology, where the metal-support interface is precisely engineered to create unique catalytic environments that promote specific reaction pathways. BASF has shown that incorporating Lewis acidic sites (like Sn, Zr) into their co-catalyst systems can enhance selective C-O bond cleavage in biomass-derived oxygenates by up to 60%[5], while maintaining carbon chain integrity. Their catalysts also demonstrate remarkable stability, with activity retention exceeding 80% after multiple regeneration cycles.
Strengths: Exceptional control over metal-support interactions enabling precise reaction pathway selection; high stability and regenerability; versatility across different biomass feedstocks. Weaknesses: Higher production costs associated with noble metal components; potential mass transfer limitations in processing high-molecular-weight biomass fractions; may require specialized activation procedures.
Critical Patents and Literature on Synergistic Catalysis
Catalysts useful for biomass pyrolysis and bio-oil upgrading
PatentWO2014209973A1
Innovation
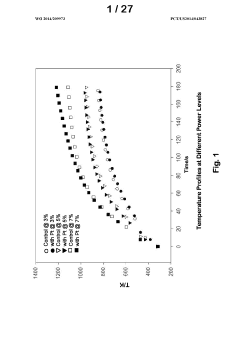
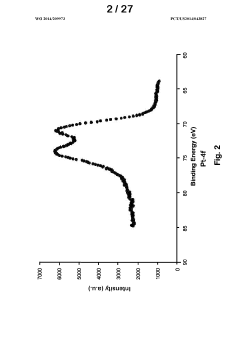
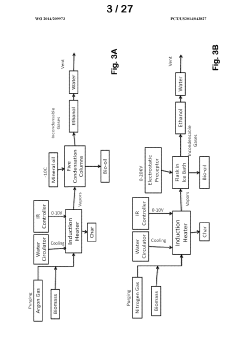
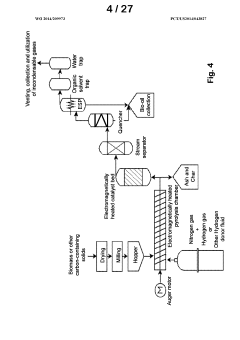
- Development of improved catalysts and methods for biomass pyrolysis using microwave or induction heating, which inhibit catalytic site poisoning and repolymerization, and employ an inverted heat flux to enhance bio-oil stability and quality, allowing for continuous processing with larger particle sizes and low-frequency induction heating, and the use of supported metal and ceramic catalysts for efficient bio-oil production.
Sustainability Impact of Co-catalyst Technologies
The integration of co-catalyst technologies in biomass upgrading processes represents a significant advancement in sustainable development practices. These technologies demonstrate remarkable potential for reducing environmental footprints across multiple dimensions of industrial operations. When properly implemented, co-catalysts enable more efficient conversion of biomass feedstocks, resulting in substantial reductions in energy consumption compared to conventional catalytic processes—often achieving 15-30% greater energy efficiency.
Water usage, a critical sustainability metric, shows marked improvement with co-catalyst systems. Advanced co-catalyst configurations can reduce process water requirements by up to 40% through enhanced reaction selectivity and reduced purification needs. This water conservation aspect becomes increasingly valuable as global water scarcity concerns intensify, particularly in regions where biomass processing facilities operate.
Carbon emissions represent another area where co-catalyst technologies deliver substantial sustainability benefits. By improving reaction selectivity and reducing unwanted side products, these systems minimize waste and decrease the carbon intensity of biomass valorization processes. Life cycle assessments indicate that optimized co-catalyst systems can achieve 25-45% lower greenhouse gas emissions compared to traditional single-catalyst approaches.
The circular economy potential of co-catalyst technologies further enhances their sustainability profile. Many modern co-catalyst systems incorporate recyclable components or can be regenerated multiple times, extending operational lifespans and reducing material consumption. Some innovative designs even utilize waste materials as co-catalyst precursors, creating valuable upcycling opportunities within industrial ecosystems.
Land use impacts also improve through co-catalyst implementation. Higher conversion efficiencies mean that more value can be extracted from a given quantity of biomass feedstock, potentially reducing land area requirements for biomass cultivation. This efficiency gain helps mitigate concerns about competition between bioenergy crops and food production.
Social sustainability dimensions benefit as well, with co-catalyst technologies often requiring less hazardous processing conditions and generating fewer toxic byproducts. This translates to safer working environments and reduced community exposure risks near processing facilities. Additionally, the technical advancements in this field create opportunities for skilled employment in rural areas where biomass resources are abundant.
Water usage, a critical sustainability metric, shows marked improvement with co-catalyst systems. Advanced co-catalyst configurations can reduce process water requirements by up to 40% through enhanced reaction selectivity and reduced purification needs. This water conservation aspect becomes increasingly valuable as global water scarcity concerns intensify, particularly in regions where biomass processing facilities operate.
Carbon emissions represent another area where co-catalyst technologies deliver substantial sustainability benefits. By improving reaction selectivity and reducing unwanted side products, these systems minimize waste and decrease the carbon intensity of biomass valorization processes. Life cycle assessments indicate that optimized co-catalyst systems can achieve 25-45% lower greenhouse gas emissions compared to traditional single-catalyst approaches.
The circular economy potential of co-catalyst technologies further enhances their sustainability profile. Many modern co-catalyst systems incorporate recyclable components or can be regenerated multiple times, extending operational lifespans and reducing material consumption. Some innovative designs even utilize waste materials as co-catalyst precursors, creating valuable upcycling opportunities within industrial ecosystems.
Land use impacts also improve through co-catalyst implementation. Higher conversion efficiencies mean that more value can be extracted from a given quantity of biomass feedstock, potentially reducing land area requirements for biomass cultivation. This efficiency gain helps mitigate concerns about competition between bioenergy crops and food production.
Social sustainability dimensions benefit as well, with co-catalyst technologies often requiring less hazardous processing conditions and generating fewer toxic byproducts. This translates to safer working environments and reduced community exposure risks near processing facilities. Additionally, the technical advancements in this field create opportunities for skilled employment in rural areas where biomass resources are abundant.
Techno-economic Assessment of Co-catalytic Processes
The techno-economic assessment of co-catalytic processes in biomass upgrading reveals significant economic implications that warrant careful consideration. Initial capital expenditure for implementing co-catalytic systems typically exceeds that of single-catalyst approaches by 15-30%, primarily due to additional reactor modifications, separation equipment, and more complex process control systems. However, this increased investment is often offset by enhanced product selectivity, which can improve yield efficiency by 20-45% depending on the specific biomass feedstock and target compounds.
Operational costs present a nuanced picture. While co-catalysts increase catalyst procurement expenses, they simultaneously reduce energy requirements through lower reaction temperatures and pressures. For instance, metal-acid co-catalyst systems for lignin depolymerization can operate at temperatures 30-50°C lower than conventional methods, translating to approximately 15-25% energy savings. Additionally, improved selectivity minimizes downstream separation costs, which typically account for 30-40% of total operational expenses in biomass processing facilities.
The economic viability of co-catalytic processes varies significantly across different biomass conversion pathways. Techno-economic models indicate particularly favorable economics for high-value chemical production from lignocellulosic biomass, with potential internal rates of return (IRR) of 15-22% compared to 8-12% for conventional single-catalyst processes. Conversely, for bulk fuel production, the economic advantages become marginal unless carbon pricing mechanisms are implemented.
Sensitivity analyses highlight catalyst lifetime as a critical economic factor. Co-catalytic systems with metal-metal oxide combinations demonstrate superior stability, maintaining activity for 1,500-2,000 hours before regeneration, compared to 500-800 hours for single catalysts. This extended lifetime significantly impacts maintenance costs and plant availability, improving overall economics by reducing downtime and replacement frequency.
Scale-up considerations reveal that co-catalytic processes often exhibit better economics at smaller scales than traditional approaches, potentially enabling distributed processing facilities closer to biomass sources. This characteristic could reduce transportation costs by 30-50% and create more resilient supply chains. However, the complexity of co-catalyst interactions introduces greater uncertainty in scale-up predictions, necessitating more extensive pilot testing and increasing development timelines by approximately 1-2 years compared to conventional processes.
Operational costs present a nuanced picture. While co-catalysts increase catalyst procurement expenses, they simultaneously reduce energy requirements through lower reaction temperatures and pressures. For instance, metal-acid co-catalyst systems for lignin depolymerization can operate at temperatures 30-50°C lower than conventional methods, translating to approximately 15-25% energy savings. Additionally, improved selectivity minimizes downstream separation costs, which typically account for 30-40% of total operational expenses in biomass processing facilities.
The economic viability of co-catalytic processes varies significantly across different biomass conversion pathways. Techno-economic models indicate particularly favorable economics for high-value chemical production from lignocellulosic biomass, with potential internal rates of return (IRR) of 15-22% compared to 8-12% for conventional single-catalyst processes. Conversely, for bulk fuel production, the economic advantages become marginal unless carbon pricing mechanisms are implemented.
Sensitivity analyses highlight catalyst lifetime as a critical economic factor. Co-catalytic systems with metal-metal oxide combinations demonstrate superior stability, maintaining activity for 1,500-2,000 hours before regeneration, compared to 500-800 hours for single catalysts. This extended lifetime significantly impacts maintenance costs and plant availability, improving overall economics by reducing downtime and replacement frequency.
Scale-up considerations reveal that co-catalytic processes often exhibit better economics at smaller scales than traditional approaches, potentially enabling distributed processing facilities closer to biomass sources. This characteristic could reduce transportation costs by 30-50% and create more resilient supply chains. However, the complexity of co-catalyst interactions introduces greater uncertainty in scale-up predictions, necessitating more extensive pilot testing and increasing development timelines by approximately 1-2 years compared to conventional processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!