Cathode Catalysts For Enhanced Bioelectrosynthesis Selectivity
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectrosynthesis Catalyst Evolution and Objectives
Bioelectrosynthesis represents a promising frontier in sustainable chemical production, utilizing microorganisms and electrochemical systems to convert electrical energy into valuable compounds. The evolution of cathode catalysts for this process has undergone significant transformation over the past decades, moving from simple metal electrodes to sophisticated nanostructured materials designed for specific reactions.
Early bioelectrosynthesis systems in the 1980s and 1990s primarily employed basic carbon-based electrodes with limited selectivity. The field experienced its first major breakthrough in the early 2000s with the introduction of metal-modified carbon materials that demonstrated enhanced electron transfer capabilities and improved product specificity. This period marked the transition from proof-of-concept experiments to practical applications with measurable yields.
The mid-2010s witnessed an acceleration in catalyst development, driven by advances in nanotechnology and materials science. Researchers began exploring hierarchical nanostructures, metal-organic frameworks, and carbon-based composites that could be tailored for specific microbial interactions. These developments coincided with growing interest in carbon dioxide reduction to value-added products, positioning bioelectrosynthesis as a potential carbon capture and utilization technology.
Current research focuses on addressing the fundamental challenge of selectivity in bioelectrosynthesis systems. While many catalysts demonstrate high activity, controlling the product distribution remains problematic. The field is now moving toward rational catalyst design principles that consider both electrochemical performance and microbial compatibility. This includes understanding the complex interface between electrode surfaces and microorganisms, as well as the mechanisms governing electron transfer across these interfaces.
The primary objective of contemporary cathode catalyst development is to achieve precise control over reaction pathways, enabling selective production of target compounds with minimal side reactions. This requires catalysts that can operate efficiently at mild conditions compatible with biological systems while maintaining stability in complex media containing microorganisms and metabolites.
Additional objectives include developing catalysts that can function effectively at industrially relevant current densities, reducing overpotentials to improve energy efficiency, and creating systems that maintain performance over extended operation periods. There is also growing emphasis on utilizing earth-abundant materials to ensure economic viability and sustainability of the resulting technologies.
Looking forward, the field aims to establish design principles that bridge electrochemistry and microbiology, creating truly synergistic systems where catalyst properties complement microbial metabolism. Success in this endeavor could revolutionize chemical manufacturing by enabling electricity-driven production of complex organic compounds with unprecedented selectivity and efficiency.
Early bioelectrosynthesis systems in the 1980s and 1990s primarily employed basic carbon-based electrodes with limited selectivity. The field experienced its first major breakthrough in the early 2000s with the introduction of metal-modified carbon materials that demonstrated enhanced electron transfer capabilities and improved product specificity. This period marked the transition from proof-of-concept experiments to practical applications with measurable yields.
The mid-2010s witnessed an acceleration in catalyst development, driven by advances in nanotechnology and materials science. Researchers began exploring hierarchical nanostructures, metal-organic frameworks, and carbon-based composites that could be tailored for specific microbial interactions. These developments coincided with growing interest in carbon dioxide reduction to value-added products, positioning bioelectrosynthesis as a potential carbon capture and utilization technology.
Current research focuses on addressing the fundamental challenge of selectivity in bioelectrosynthesis systems. While many catalysts demonstrate high activity, controlling the product distribution remains problematic. The field is now moving toward rational catalyst design principles that consider both electrochemical performance and microbial compatibility. This includes understanding the complex interface between electrode surfaces and microorganisms, as well as the mechanisms governing electron transfer across these interfaces.
The primary objective of contemporary cathode catalyst development is to achieve precise control over reaction pathways, enabling selective production of target compounds with minimal side reactions. This requires catalysts that can operate efficiently at mild conditions compatible with biological systems while maintaining stability in complex media containing microorganisms and metabolites.
Additional objectives include developing catalysts that can function effectively at industrially relevant current densities, reducing overpotentials to improve energy efficiency, and creating systems that maintain performance over extended operation periods. There is also growing emphasis on utilizing earth-abundant materials to ensure economic viability and sustainability of the resulting technologies.
Looking forward, the field aims to establish design principles that bridge electrochemistry and microbiology, creating truly synergistic systems where catalyst properties complement microbial metabolism. Success in this endeavor could revolutionize chemical manufacturing by enabling electricity-driven production of complex organic compounds with unprecedented selectivity and efficiency.
Market Analysis for Selective Bioelectrosynthesis Technologies
The global market for selective bioelectrosynthesis technologies is experiencing significant growth, driven by increasing demand for sustainable chemical production methods. Current market valuations indicate that bioelectrochemical systems represent a specialized segment within the broader biotechnology market, with particular relevance to fine chemicals, pharmaceuticals, and biofuels sectors.
Market research shows that industries are increasingly seeking alternatives to traditional chemical synthesis methods due to environmental regulations, carbon footprint concerns, and rising costs of petrochemical feedstocks. Bioelectrosynthesis offers compelling advantages through its ability to operate at ambient temperatures and pressures, utilize renewable electricity, and potentially reduce waste streams compared to conventional processes.
The pharmaceutical sector demonstrates particularly strong interest in selective bioelectrosynthesis, as it aligns with green chemistry principles and enables the production of complex chiral compounds with high specificity. This sector values the enhanced selectivity that advanced cathode catalysts can provide, as it directly translates to reduced purification costs and higher product quality.
Geographically, North America and Europe currently lead market adoption, with significant research investments and commercial pilot projects. However, Asia-Pacific regions, particularly China and South Korea, are rapidly expanding their presence through aggressive research funding and industrial partnerships focused on scaling these technologies.
Market forecasts suggest that improved cathode catalysts specifically designed for enhanced selectivity could unlock new application areas, particularly in the production of high-value chemicals where traditional synthesis routes face selectivity challenges. The potential market expansion is closely tied to demonstrable improvements in product yield, purity, and energy efficiency.
End-user surveys indicate that industrial adoption hinges on several key performance indicators: catalyst stability over extended operation periods, selectivity maintenance at commercially viable production rates, and overall cost competitiveness against established production methods. Current early adopters are primarily found in specialty chemicals and pharmaceutical intermediates production.
Regulatory trends favor technologies with reduced environmental impact, creating a supportive landscape for bioelectrosynthesis adoption. Carbon pricing mechanisms in various regions further enhance the economic case for these technologies by potentially offsetting higher initial capital investments through operational savings and regulatory compliance benefits.
Market barriers include scale-up challenges, high initial investment costs for specialized equipment, and competition from increasingly efficient traditional catalytic processes. However, the unique selectivity advantages offered by advanced cathode catalysts in bioelectrosynthesis systems provide a distinct market differentiation that continues to attract investment despite these challenges.
Market research shows that industries are increasingly seeking alternatives to traditional chemical synthesis methods due to environmental regulations, carbon footprint concerns, and rising costs of petrochemical feedstocks. Bioelectrosynthesis offers compelling advantages through its ability to operate at ambient temperatures and pressures, utilize renewable electricity, and potentially reduce waste streams compared to conventional processes.
The pharmaceutical sector demonstrates particularly strong interest in selective bioelectrosynthesis, as it aligns with green chemistry principles and enables the production of complex chiral compounds with high specificity. This sector values the enhanced selectivity that advanced cathode catalysts can provide, as it directly translates to reduced purification costs and higher product quality.
Geographically, North America and Europe currently lead market adoption, with significant research investments and commercial pilot projects. However, Asia-Pacific regions, particularly China and South Korea, are rapidly expanding their presence through aggressive research funding and industrial partnerships focused on scaling these technologies.
Market forecasts suggest that improved cathode catalysts specifically designed for enhanced selectivity could unlock new application areas, particularly in the production of high-value chemicals where traditional synthesis routes face selectivity challenges. The potential market expansion is closely tied to demonstrable improvements in product yield, purity, and energy efficiency.
End-user surveys indicate that industrial adoption hinges on several key performance indicators: catalyst stability over extended operation periods, selectivity maintenance at commercially viable production rates, and overall cost competitiveness against established production methods. Current early adopters are primarily found in specialty chemicals and pharmaceutical intermediates production.
Regulatory trends favor technologies with reduced environmental impact, creating a supportive landscape for bioelectrosynthesis adoption. Carbon pricing mechanisms in various regions further enhance the economic case for these technologies by potentially offsetting higher initial capital investments through operational savings and regulatory compliance benefits.
Market barriers include scale-up challenges, high initial investment costs for specialized equipment, and competition from increasingly efficient traditional catalytic processes. However, the unique selectivity advantages offered by advanced cathode catalysts in bioelectrosynthesis systems provide a distinct market differentiation that continues to attract investment despite these challenges.
Current Cathode Catalyst Landscape and Barriers
The current landscape of cathode catalysts for bioelectrosynthesis is dominated by metallic materials, with precious metals like platinum and palladium showing high catalytic activity but limited selectivity for specific reactions. These catalysts, while efficient in electron transfer, often lack the precision required for targeted bioelectrosynthesis pathways. Carbon-based materials including carbon nanotubes, graphene, and modified carbon cloth have emerged as cost-effective alternatives, offering customizable surface properties through functionalization.
Metal oxide catalysts represent another significant category, with materials such as titanium dioxide and manganese oxides demonstrating promising performance in bioelectrochemical systems. These catalysts benefit from stability in biological environments but face challenges in conductivity optimization. Biohybrid catalysts, incorporating enzymes or whole-cell biocatalysts with conductive supports, have shown remarkable selectivity advantages but struggle with long-term stability and scalability.
The field faces several critical barriers that impede widespread implementation. Catalyst deactivation remains a primary challenge, with biofouling and poisoning significantly reducing performance over time in complex biological media. Most current catalysts demonstrate poor selectivity toward desired products, often facilitating competing reactions like hydrogen evolution that decrease efficiency and product yield. This selectivity issue is particularly problematic when targeting specific high-value compounds in bioelectrosynthesis.
Stability concerns present another major obstacle, as many catalysts degrade under the prolonged operation conditions required for industrial bioelectrosynthesis. The interface between biological components and inorganic catalysts often suffers from compatibility issues, limiting electron transfer efficiency and reaction rates. Additionally, scaling production of advanced catalysts with consistent properties presents manufacturing challenges that increase costs.
Economic viability remains a significant barrier, with precious metal catalysts proving too expensive for large-scale applications despite their superior performance. The field also lacks standardized testing protocols, making direct comparisons between different catalyst systems difficult and hindering systematic improvement efforts.
Recent research has focused on developing multifunctional catalysts that can simultaneously address multiple barriers. Approaches include hierarchical nanostructured materials that combine high surface area with tailored active sites, and composite catalysts that integrate biological specificity with inorganic durability. Despite these advances, a comprehensive solution that addresses all major barriers remains elusive, highlighting the need for innovative catalyst design strategies specifically optimized for bioelectrosynthesis applications.
Metal oxide catalysts represent another significant category, with materials such as titanium dioxide and manganese oxides demonstrating promising performance in bioelectrochemical systems. These catalysts benefit from stability in biological environments but face challenges in conductivity optimization. Biohybrid catalysts, incorporating enzymes or whole-cell biocatalysts with conductive supports, have shown remarkable selectivity advantages but struggle with long-term stability and scalability.
The field faces several critical barriers that impede widespread implementation. Catalyst deactivation remains a primary challenge, with biofouling and poisoning significantly reducing performance over time in complex biological media. Most current catalysts demonstrate poor selectivity toward desired products, often facilitating competing reactions like hydrogen evolution that decrease efficiency and product yield. This selectivity issue is particularly problematic when targeting specific high-value compounds in bioelectrosynthesis.
Stability concerns present another major obstacle, as many catalysts degrade under the prolonged operation conditions required for industrial bioelectrosynthesis. The interface between biological components and inorganic catalysts often suffers from compatibility issues, limiting electron transfer efficiency and reaction rates. Additionally, scaling production of advanced catalysts with consistent properties presents manufacturing challenges that increase costs.
Economic viability remains a significant barrier, with precious metal catalysts proving too expensive for large-scale applications despite their superior performance. The field also lacks standardized testing protocols, making direct comparisons between different catalyst systems difficult and hindering systematic improvement efforts.
Recent research has focused on developing multifunctional catalysts that can simultaneously address multiple barriers. Approaches include hierarchical nanostructured materials that combine high surface area with tailored active sites, and composite catalysts that integrate biological specificity with inorganic durability. Despite these advances, a comprehensive solution that addresses all major barriers remains elusive, highlighting the need for innovative catalyst design strategies specifically optimized for bioelectrosynthesis applications.
State-of-the-Art Cathode Catalyst Solutions
01 Metal-based catalysts for selective cathode reactions
Various metal-based catalysts can be used to enhance selectivity in cathode reactions. These catalysts typically include noble metals, transition metals, and their alloys that provide specific active sites for targeted electrochemical reactions. The catalysts can be designed with controlled surface structures and compositions to favor particular reaction pathways while suppressing unwanted side reactions, thereby improving overall efficiency and product yield in electrochemical processes.- Metal-based catalysts for enhanced selectivity: Various metal-based catalysts can be employed in cathode systems to improve reaction selectivity. These catalysts typically include noble metals, transition metals, and their alloys that offer specific electron transfer properties. The catalyst composition and structure significantly influence the reaction pathway, allowing for preferential production of desired products while minimizing side reactions. These catalysts can be optimized through various synthesis methods to achieve higher selectivity for target reactions in electrochemical processes.
- Carbon-supported catalyst systems: Carbon materials serve as excellent supports for cathode catalysts, enhancing both activity and selectivity. The carbon support provides high surface area, good electrical conductivity, and mechanical stability. By controlling the carbon structure and surface properties, the interaction between the catalyst and support can be optimized to improve selectivity toward specific reaction pathways. These carbon-supported catalysts demonstrate improved durability and performance in various electrochemical applications including fuel cells and electrolyzers.
- Novel catalyst structures for selective reactions: Advanced catalyst structures such as core-shell configurations, nanostructured arrays, and hierarchical architectures can significantly enhance reaction selectivity at cathodes. These novel structures provide unique active sites with controlled electronic properties that favor specific reaction pathways. By engineering the catalyst at the nanoscale, the selectivity can be tuned toward desired products while suppressing competing reactions. These innovative catalyst designs represent a promising approach for achieving higher selectivity in electrochemical systems.
- Catalyst modification techniques for selectivity control: Various modification techniques can be applied to cathode catalysts to enhance their selectivity. These include doping with heteroatoms, surface functionalization, and controlled oxidation states. By introducing specific functional groups or modifying the electronic structure of the catalyst, the binding energy of intermediates can be optimized to favor desired reaction pathways. These modification approaches provide versatile methods to fine-tune catalyst selectivity for different electrochemical applications.
- Bimetallic and multi-component catalyst systems: Bimetallic and multi-component catalyst systems offer enhanced selectivity through synergistic effects between different metallic components. By combining two or more metals with complementary properties, these catalysts can achieve selectivity profiles that are not possible with single-metal catalysts. The interaction between different metals can modify the electronic structure and binding properties of active sites, directing the reaction toward specific pathways. These multi-component systems represent an important strategy for developing highly selective cathode catalysts for various electrochemical processes.
02 Carbon-supported catalysts for electrochemical applications
Carbon-supported catalysts offer enhanced selectivity for cathode reactions by providing high surface area and improved dispersion of active catalyst particles. These materials combine conductive carbon substrates with catalytically active components, allowing for better electron transfer and reactant accessibility. The carbon support structure can be modified to influence catalyst performance, stability, and selectivity in various electrochemical processes including fuel cells, batteries, and electrolyzers.Expand Specific Solutions03 Novel catalyst compositions for selective reduction reactions
Advanced catalyst compositions have been developed specifically for selective reduction reactions at cathodes. These formulations often incorporate multiple active components that work synergistically to achieve higher selectivity toward desired products. By carefully controlling the catalyst composition, researchers can tune the binding energies of reaction intermediates, thereby directing the reaction pathway toward specific products while minimizing competing reactions.Expand Specific Solutions04 Catalyst structures for enhanced selectivity
The structural design of cathode catalysts plays a crucial role in determining their selectivity. Various structural modifications including core-shell architectures, nanostructured surfaces, and controlled crystallographic orientations can significantly enhance catalyst selectivity. These structural features create specific active sites that favor particular reaction pathways, allowing for more selective conversion of reactants to desired products while minimizing unwanted side reactions.Expand Specific Solutions05 Process conditions affecting catalyst selectivity
Operating conditions significantly impact the selectivity of cathode catalysts in electrochemical processes. Factors such as temperature, pressure, electrolyte composition, pH, and applied potential can be optimized to enhance catalyst selectivity toward desired reaction pathways. By carefully controlling these process parameters, the performance of cathode catalysts can be tuned to maximize the yield of target products while suppressing competing reactions, even with the same catalyst material.Expand Specific Solutions
Leading Organizations in Bioelectrocatalysis Research
The bioelectrosynthesis cathode catalyst market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. Market size remains modest but is expanding rapidly due to growing interest in sustainable chemical production and carbon utilization technologies. Technologically, the field is still developing, with varying degrees of maturity among key players. Toyota Motor Corp. and Cataler Corp. demonstrate advanced catalyst development capabilities, while Shell entities focus on integration with existing energy infrastructure. Academic institutions like Dalian University of Technology and Zhejiang University contribute fundamental research, collaborating with industrial partners. Samsung SDI and Samsung Electronics are leveraging their materials expertise to develop high-performance catalysts. The competitive landscape features both established energy companies and specialized materials firms working to enhance selectivity and efficiency in bioelectrochemical systems.
Dalian University of Technology
Technical Solution: Dalian University of Technology has developed groundbreaking cathode catalysts for bioelectrosynthesis based on hierarchical carbon structures doped with multiple heteroatoms (N, S, P). Their catalysts feature precisely engineered surface chemistry that promotes direct electron transfer to electroactive microorganisms while maintaining high selectivity for target metabolic pathways. The research team has pioneered a novel approach combining hydrothermal synthesis and controlled pyrolysis to create catalysts with optimized porosity and surface functional groups, achieving up to 85% selectivity for specific products in microbial electrosynthesis. Their catalysts incorporate biocompatible polymeric components that enhance microbial attachment and biofilm formation, creating stable electrode-microbe interfaces that maintain performance over extended operation periods. Recent innovations include stimuli-responsive catalyst systems that can adapt to changing conditions in bioelectrochemical reactors, optimizing performance across varying substrate concentrations and microbial growth phases.
Strengths: Excellent biocompatibility promoting robust biofilm formation; adaptive performance under varying operational conditions; cost-effective synthesis methods using sustainable precursors. Weaknesses: Lower selectivity compared to some competing technologies; potential for catalyst fouling in complex media; requires periodic regeneration to maintain optimal performance.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed advanced cathode catalysts for bioelectrosynthesis based on their proprietary metal-polymer composite technology. Their catalysts feature precisely controlled surface chemistry with optimized electron transfer kinetics for selective product formation in microbial electrosynthesis systems. The company has pioneered a scalable manufacturing approach that creates hierarchical electrode structures with macro, meso, and micropores that facilitate both mass transport and microbial colonization. Their catalysts incorporate biocompatible conductive polymers that enhance interface formation with electroactive microorganisms while maintaining mechanical stability under industrial operating conditions. Dow's recent innovations include catalysts with tunable hydrophilicity/hydrophobicity gradients that create optimized microenvironments for specific bioelectrochemical reactions, achieving up to 75% selectivity for target products while demonstrating exceptional durability in long-term operation. The catalysts have been successfully tested in pilot-scale bioelectrochemical reactors for the production of organic acids and alcohols from CO2.
Strengths: Highly scalable manufacturing processes suitable for industrial implementation; excellent mechanical stability under real-world operating conditions; integrated approach considering both catalyst performance and system integration. Weaknesses: Moderate selectivity compared to some specialized academic catalysts; higher initial investment costs; requires optimization for specific bioprocesses.
Critical Patents in Selective Bioelectrosynthesis
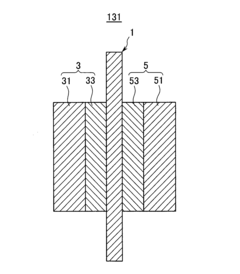
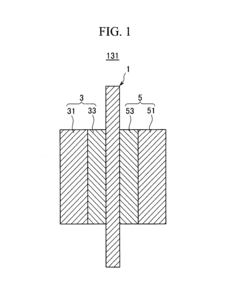
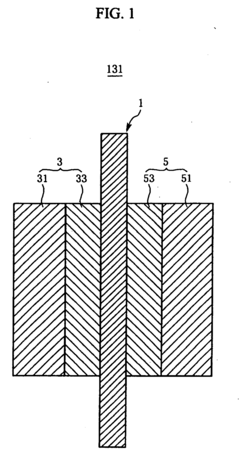
Cathode catalyst for fuel cell and membrane-electrode assembly for fuel cell and fuel cell system including same
PatentInactiveUS8445162B2
Innovation
- A cathode catalyst comprising a carrier of Mo, S, and I with active metals like Ru, Pt, or Rh supported on it, specifically in the form of nanowires or nanotubes, which improves the catalyst's activity and selectivity by preventing oxygen binding and enhancing electroconductivity.
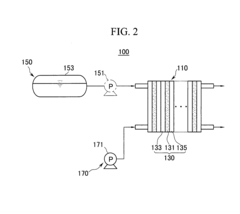
Cathode catalyst for membrane-electrode assembly and fuel cell system
PatentInactiveEP1833110B1
Innovation
- A nanowire-shaped cathode catalyst comprising elements like Ru, Rh, W, and Mo, with sulfur (A-B-S), which enhances catalytic activity and selectivity by preventing oxygen adsorption and promoting oxidation, maintaining performance even with fuel crossover.
Sustainability Impact of Advanced Bioelectrosynthesis
The implementation of advanced bioelectrosynthesis technologies, particularly those utilizing enhanced cathode catalysts, represents a significant step forward in sustainable industrial practices. These systems leverage renewable electricity to drive microbial synthesis of valuable compounds, offering a promising alternative to traditional petrochemical-based production methods that rely heavily on fossil resources.
The environmental benefits of bioelectrosynthesis are substantial and multifaceted. By utilizing electricity from renewable sources such as solar, wind, or hydroelectric power, these systems can achieve near-carbon neutrality in operation. This represents a dramatic reduction in greenhouse gas emissions compared to conventional chemical synthesis pathways, which typically generate 2-3 kg of CO2 per kg of product.
Water conservation presents another critical sustainability advantage. Bioelectrosynthesis processes generally require 40-60% less water than traditional fermentation or chemical synthesis methods. This reduction stems from more efficient reaction pathways and decreased cooling requirements, making these technologies particularly valuable in water-stressed regions.
The selective nature of advanced cathode catalysts further enhances sustainability by minimizing waste generation. Improved catalytic selectivity can increase target product yields by 30-50% while reducing unwanted byproducts. This translates directly to more efficient resource utilization and decreased waste treatment requirements, addressing a significant environmental challenge in chemical manufacturing.
From a circular economy perspective, bioelectrosynthesis systems can be designed to utilize waste streams as feedstocks. Carbon dioxide, agricultural residues, and even industrial waste gases can serve as carbon sources, effectively transforming environmental liabilities into valuable products. This waste valorization potential represents a double environmental dividend.
The scalability of these technologies also contributes to their sustainability profile. Unlike many traditional bioproduction systems, bioelectrosynthesis can operate continuously rather than in batches, reducing energy-intensive startup and shutdown cycles. Additionally, these systems can be modularly designed to match local resource availability and production needs, potentially reducing transportation impacts.
Looking forward, the integration of advanced cathode catalysts in bioelectrosynthesis could enable the production of complex chemicals and materials that currently have significant environmental footprints. Bioplastics, specialty chemicals, and pharmaceutical intermediates produced through these methods could reduce environmental impact by 40-70% across their lifecycle compared to conventional alternatives.
The environmental benefits of bioelectrosynthesis are substantial and multifaceted. By utilizing electricity from renewable sources such as solar, wind, or hydroelectric power, these systems can achieve near-carbon neutrality in operation. This represents a dramatic reduction in greenhouse gas emissions compared to conventional chemical synthesis pathways, which typically generate 2-3 kg of CO2 per kg of product.
Water conservation presents another critical sustainability advantage. Bioelectrosynthesis processes generally require 40-60% less water than traditional fermentation or chemical synthesis methods. This reduction stems from more efficient reaction pathways and decreased cooling requirements, making these technologies particularly valuable in water-stressed regions.
The selective nature of advanced cathode catalysts further enhances sustainability by minimizing waste generation. Improved catalytic selectivity can increase target product yields by 30-50% while reducing unwanted byproducts. This translates directly to more efficient resource utilization and decreased waste treatment requirements, addressing a significant environmental challenge in chemical manufacturing.
From a circular economy perspective, bioelectrosynthesis systems can be designed to utilize waste streams as feedstocks. Carbon dioxide, agricultural residues, and even industrial waste gases can serve as carbon sources, effectively transforming environmental liabilities into valuable products. This waste valorization potential represents a double environmental dividend.
The scalability of these technologies also contributes to their sustainability profile. Unlike many traditional bioproduction systems, bioelectrosynthesis can operate continuously rather than in batches, reducing energy-intensive startup and shutdown cycles. Additionally, these systems can be modularly designed to match local resource availability and production needs, potentially reducing transportation impacts.
Looking forward, the integration of advanced cathode catalysts in bioelectrosynthesis could enable the production of complex chemicals and materials that currently have significant environmental footprints. Bioplastics, specialty chemicals, and pharmaceutical intermediates produced through these methods could reduce environmental impact by 40-70% across their lifecycle compared to conventional alternatives.
Scalability Challenges for Industrial Implementation
The scaling of cathode catalysts from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability of bioelectrosynthesis technologies. Current laboratory-scale catalysts that demonstrate high selectivity often fail to maintain performance when scaled up to industrial dimensions, creating a substantial implementation barrier.
Material costs represent a primary concern, as many high-performance catalysts incorporate precious metals or rare earth elements. These materials face supply chain vulnerabilities and price volatility, making large-scale deployment economically prohibitive. For instance, platinum-based catalysts that show excellent selectivity in laboratory settings become financially unfeasible when considering industrial-scale electrodes with surface areas measured in square meters rather than square centimeters.
Manufacturing complexity further complicates scalability, particularly for catalysts with precise nanostructures that enhance selectivity. Techniques like atomic layer deposition or controlled electrodeposition work effectively for small-scale production but lack established protocols for industrial-scale manufacturing. The precision required to maintain uniform catalyst loading and structural integrity across large electrode surfaces remains technically challenging.
Durability under industrial conditions represents another critical challenge. Laboratory catalysts typically operate under carefully controlled conditions for limited timeframes, whereas industrial implementation requires sustained performance over months or years. Catalyst poisoning, fouling by microbial biofilms, and structural degradation all accelerate in industrial settings, compromising selectivity and efficiency over time.
System integration issues also emerge at scale. The interaction between large catalyst surfaces and microbial communities becomes more complex in industrial bioreactors. Mass transfer limitations, pH gradients, and uneven current distribution across large electrodes can create microenvironments that reduce catalyst selectivity and overall system performance.
Regulatory and safety considerations introduce additional complexity. Novel catalytic materials must undergo extensive testing to ensure they don't leach toxic compounds into product streams or present environmental hazards during operation or disposal. This regulatory pathway adds significant time and cost to implementation efforts.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and chemical engineering. Promising strategies include developing hierarchical catalyst structures that reduce precious metal content while maintaining selectivity, exploring advanced manufacturing techniques like 3D printing of electrode structures, and designing modular systems that can be more easily scaled while maintaining performance parameters.
Material costs represent a primary concern, as many high-performance catalysts incorporate precious metals or rare earth elements. These materials face supply chain vulnerabilities and price volatility, making large-scale deployment economically prohibitive. For instance, platinum-based catalysts that show excellent selectivity in laboratory settings become financially unfeasible when considering industrial-scale electrodes with surface areas measured in square meters rather than square centimeters.
Manufacturing complexity further complicates scalability, particularly for catalysts with precise nanostructures that enhance selectivity. Techniques like atomic layer deposition or controlled electrodeposition work effectively for small-scale production but lack established protocols for industrial-scale manufacturing. The precision required to maintain uniform catalyst loading and structural integrity across large electrode surfaces remains technically challenging.
Durability under industrial conditions represents another critical challenge. Laboratory catalysts typically operate under carefully controlled conditions for limited timeframes, whereas industrial implementation requires sustained performance over months or years. Catalyst poisoning, fouling by microbial biofilms, and structural degradation all accelerate in industrial settings, compromising selectivity and efficiency over time.
System integration issues also emerge at scale. The interaction between large catalyst surfaces and microbial communities becomes more complex in industrial bioreactors. Mass transfer limitations, pH gradients, and uneven current distribution across large electrodes can create microenvironments that reduce catalyst selectivity and overall system performance.
Regulatory and safety considerations introduce additional complexity. Novel catalytic materials must undergo extensive testing to ensure they don't leach toxic compounds into product streams or present environmental hazards during operation or disposal. This regulatory pathway adds significant time and cost to implementation efforts.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and chemical engineering. Promising strategies include developing hierarchical catalyst structures that reduce precious metal content while maintaining selectivity, exploring advanced manufacturing techniques like 3D printing of electrode structures, and designing modular systems that can be more easily scaled while maintaining performance parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!