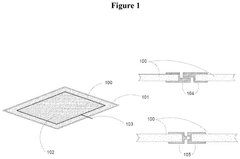
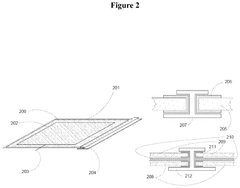
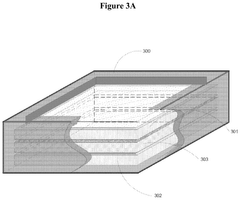
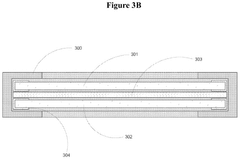
Electrode Materials For High-Performance Bioelectrochemical Systems
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioelectrochemical Electrode Materials Background and Objectives
Bioelectrochemical systems (BES) represent a revolutionary intersection of microbiology, electrochemistry, and materials science, offering sustainable solutions for energy generation, waste treatment, and resource recovery. The evolution of BES technology traces back to the early 20th century with rudimentary microbial fuel cells, but significant advancements have emerged only in the past two decades with improved understanding of extracellular electron transfer mechanisms and electrode materials development.
The technological trajectory of electrode materials has progressed from simple carbon-based substrates to sophisticated nanostructured composites. Early systems utilized plain carbon rods or sheets, while contemporary research focuses on hierarchical architectures combining multiple functional components to enhance performance metrics. This evolution reflects the growing recognition that electrode materials serve as the critical interface between biological systems and electrical circuits, directly influencing system efficiency and operational stability.
Current research trends indicate a shift toward biomimetic approaches, where electrode designs draw inspiration from natural biological interfaces. Concurrently, there is increasing emphasis on sustainable and scalable manufacturing processes to facilitate industrial adoption. The integration of advanced characterization techniques, including in-situ spectroscopy and high-resolution microscopy, has accelerated understanding of electrode-microbe interactions at the molecular level.
The primary technical objectives for high-performance bioelectrochemical electrode materials encompass several dimensions. First, enhancing biocompatibility to support robust microbial colonization while minimizing biofouling effects that diminish long-term performance. Second, optimizing electrical conductivity to facilitate efficient electron transfer between microorganisms and electrode surfaces. Third, developing architectures with high specific surface area to maximize microbial attachment and catalytic activity per unit volume.
Additional objectives include improving mechanical stability for extended operational lifetimes, particularly in harsh environmental conditions; developing cost-effective fabrication methods suitable for large-scale production; and ensuring environmental sustainability throughout the material lifecycle. The ideal electrode material must balance these sometimes competing requirements while maintaining economic viability for commercial applications.
The technological horizon suggests potential breakthroughs in self-healing electrode materials, stimuli-responsive surfaces that adapt to changing microbial communities, and hybrid systems incorporating both biological and abiotic catalysts. These advances could dramatically expand the application scope of bioelectrochemical systems beyond current limitations, potentially revolutionizing sectors including wastewater treatment, biosensing, and renewable energy production.
The technological trajectory of electrode materials has progressed from simple carbon-based substrates to sophisticated nanostructured composites. Early systems utilized plain carbon rods or sheets, while contemporary research focuses on hierarchical architectures combining multiple functional components to enhance performance metrics. This evolution reflects the growing recognition that electrode materials serve as the critical interface between biological systems and electrical circuits, directly influencing system efficiency and operational stability.
Current research trends indicate a shift toward biomimetic approaches, where electrode designs draw inspiration from natural biological interfaces. Concurrently, there is increasing emphasis on sustainable and scalable manufacturing processes to facilitate industrial adoption. The integration of advanced characterization techniques, including in-situ spectroscopy and high-resolution microscopy, has accelerated understanding of electrode-microbe interactions at the molecular level.
The primary technical objectives for high-performance bioelectrochemical electrode materials encompass several dimensions. First, enhancing biocompatibility to support robust microbial colonization while minimizing biofouling effects that diminish long-term performance. Second, optimizing electrical conductivity to facilitate efficient electron transfer between microorganisms and electrode surfaces. Third, developing architectures with high specific surface area to maximize microbial attachment and catalytic activity per unit volume.
Additional objectives include improving mechanical stability for extended operational lifetimes, particularly in harsh environmental conditions; developing cost-effective fabrication methods suitable for large-scale production; and ensuring environmental sustainability throughout the material lifecycle. The ideal electrode material must balance these sometimes competing requirements while maintaining economic viability for commercial applications.
The technological horizon suggests potential breakthroughs in self-healing electrode materials, stimuli-responsive surfaces that adapt to changing microbial communities, and hybrid systems incorporating both biological and abiotic catalysts. These advances could dramatically expand the application scope of bioelectrochemical systems beyond current limitations, potentially revolutionizing sectors including wastewater treatment, biosensing, and renewable energy production.
Market Analysis for High-Performance BES Applications
The bioelectrochemical systems (BES) market is experiencing significant growth driven by increasing environmental concerns and the push for sustainable technologies. The global BES market was valued at approximately $32 million in 2022 and is projected to reach $75 million by 2028, representing a compound annual growth rate (CAGR) of 15.3%. This growth trajectory is primarily fueled by applications in wastewater treatment, bioenergy production, and biosensing technologies.
Wastewater treatment represents the largest application segment, accounting for nearly 45% of the total BES market. The increasing stringent regulations on water quality and discharge standards across developed economies have accelerated the adoption of BES technologies. Industrial sectors, particularly food and beverage, chemical, and pharmaceutical industries, are increasingly implementing BES solutions to treat their effluents while simultaneously generating electricity.
The bioenergy production segment is expected to witness the fastest growth rate of 18.7% during the forecast period. This is attributed to the rising demand for renewable energy sources and the ability of BES to convert organic waste into electricity. Government initiatives promoting clean energy technologies and subsidies for renewable energy projects are further propelling market expansion in this segment.
Geographically, North America dominates the BES market with a share of 38%, followed by Europe at 32%. The Asia-Pacific region, however, is anticipated to register the highest growth rate of 17.5% during the forecast period, primarily due to rapid industrialization, urbanization, and increasing environmental awareness in countries like China, Japan, and South Korea.
Key customer segments include municipal wastewater treatment facilities, industrial waste management companies, research institutions, and renewable energy developers. The municipal sector currently represents the largest customer base, accounting for approximately 40% of BES implementations globally.
Market challenges include high initial investment costs, with typical industrial-scale BES installations ranging from $500,000 to $2 million, and technical limitations related to electrode materials and system efficiency. The payback period for BES installations currently averages 4-6 years, which remains a barrier for widespread adoption despite the long-term operational benefits.
Emerging trends include the integration of BES with other renewable energy systems, miniaturization for portable applications, and the development of specialized BES for specific industrial waste streams. The market is also witnessing increased collaboration between technology providers, research institutions, and end-users to develop customized solutions addressing specific application requirements.
Wastewater treatment represents the largest application segment, accounting for nearly 45% of the total BES market. The increasing stringent regulations on water quality and discharge standards across developed economies have accelerated the adoption of BES technologies. Industrial sectors, particularly food and beverage, chemical, and pharmaceutical industries, are increasingly implementing BES solutions to treat their effluents while simultaneously generating electricity.
The bioenergy production segment is expected to witness the fastest growth rate of 18.7% during the forecast period. This is attributed to the rising demand for renewable energy sources and the ability of BES to convert organic waste into electricity. Government initiatives promoting clean energy technologies and subsidies for renewable energy projects are further propelling market expansion in this segment.
Geographically, North America dominates the BES market with a share of 38%, followed by Europe at 32%. The Asia-Pacific region, however, is anticipated to register the highest growth rate of 17.5% during the forecast period, primarily due to rapid industrialization, urbanization, and increasing environmental awareness in countries like China, Japan, and South Korea.
Key customer segments include municipal wastewater treatment facilities, industrial waste management companies, research institutions, and renewable energy developers. The municipal sector currently represents the largest customer base, accounting for approximately 40% of BES implementations globally.
Market challenges include high initial investment costs, with typical industrial-scale BES installations ranging from $500,000 to $2 million, and technical limitations related to electrode materials and system efficiency. The payback period for BES installations currently averages 4-6 years, which remains a barrier for widespread adoption despite the long-term operational benefits.
Emerging trends include the integration of BES with other renewable energy systems, miniaturization for portable applications, and the development of specialized BES for specific industrial waste streams. The market is also witnessing increased collaboration between technology providers, research institutions, and end-users to develop customized solutions addressing specific application requirements.
Current Electrode Materials Landscape and Challenges
Bioelectrochemical systems (BES) currently employ a diverse range of electrode materials, each with specific advantages and limitations. Carbon-based materials dominate the field due to their excellent conductivity, biocompatibility, and relatively low cost. These include carbon cloth, carbon paper, graphite plates, carbon felt, and more recently, advanced carbon nanomaterials such as carbon nanotubes (CNTs) and graphene. Carbon-based electrodes offer good electrical conductivity and large surface areas but often suffer from limited durability in long-term operations.
Metal-based electrodes, particularly stainless steel, titanium, and platinum, represent another significant category. These materials provide superior conductivity and mechanical strength compared to carbon-based alternatives. However, their widespread application is hindered by higher costs, potential toxicity to microorganisms, and susceptibility to corrosion in the complex chemical environments typical of BES.
Composite materials have emerged as promising candidates, combining the advantages of different materials while mitigating their individual drawbacks. Notable examples include carbon-metal composites, conductive polymers with carbon nanomaterials, and metal oxide-modified carbon electrodes. These composites aim to enhance electron transfer rates, increase surface area, and improve biofilm formation.
Despite significant advances, current electrode materials face several critical challenges. Biofouling remains a persistent issue, where non-electroactive microorganisms and their metabolites accumulate on electrode surfaces, reducing performance over time. Additionally, the trade-off between conductivity and biocompatibility continues to limit overall system efficiency, as materials with excellent electrical properties often exhibit poor compatibility with electroactive microorganisms.
Scalability presents another major hurdle. Many high-performance materials that show promising results in laboratory settings face significant manufacturing challenges at industrial scales. The cost-effectiveness of advanced materials like graphene and CNTs remains problematic for large-scale applications, restricting their commercial viability.
Stability in diverse operational conditions represents a significant technical barrier. Electrode materials must maintain performance across varying pH levels, temperatures, and chemical compositions typical in real-world applications. Current materials often demonstrate performance degradation under fluctuating conditions, limiting their practical utility.
Geographically, research and development in electrode materials show concentration in North America, Europe, and East Asia, particularly in China, the United States, and Germany. This distribution reflects both academic research clusters and industrial investment patterns in renewable energy and biotechnology sectors, creating potential gaps in technology transfer to other regions where BES applications could provide significant benefits.
Metal-based electrodes, particularly stainless steel, titanium, and platinum, represent another significant category. These materials provide superior conductivity and mechanical strength compared to carbon-based alternatives. However, their widespread application is hindered by higher costs, potential toxicity to microorganisms, and susceptibility to corrosion in the complex chemical environments typical of BES.
Composite materials have emerged as promising candidates, combining the advantages of different materials while mitigating their individual drawbacks. Notable examples include carbon-metal composites, conductive polymers with carbon nanomaterials, and metal oxide-modified carbon electrodes. These composites aim to enhance electron transfer rates, increase surface area, and improve biofilm formation.
Despite significant advances, current electrode materials face several critical challenges. Biofouling remains a persistent issue, where non-electroactive microorganisms and their metabolites accumulate on electrode surfaces, reducing performance over time. Additionally, the trade-off between conductivity and biocompatibility continues to limit overall system efficiency, as materials with excellent electrical properties often exhibit poor compatibility with electroactive microorganisms.
Scalability presents another major hurdle. Many high-performance materials that show promising results in laboratory settings face significant manufacturing challenges at industrial scales. The cost-effectiveness of advanced materials like graphene and CNTs remains problematic for large-scale applications, restricting their commercial viability.
Stability in diverse operational conditions represents a significant technical barrier. Electrode materials must maintain performance across varying pH levels, temperatures, and chemical compositions typical in real-world applications. Current materials often demonstrate performance degradation under fluctuating conditions, limiting their practical utility.
Geographically, research and development in electrode materials show concentration in North America, Europe, and East Asia, particularly in China, the United States, and Germany. This distribution reflects both academic research clusters and industrial investment patterns in renewable energy and biotechnology sectors, creating potential gaps in technology transfer to other regions where BES applications could provide significant benefits.
Current Electrode Design Solutions
01 Carbon-based electrode materials
Carbon-based materials are widely used in electrode applications due to their excellent electrical conductivity, chemical stability, and cost-effectiveness. These materials include graphene, carbon nanotubes, and activated carbon, which can be modified or functionalized to enhance their performance characteristics. Carbon-based electrodes offer advantages such as high surface area, good mechanical strength, and compatibility with various electrolytes, making them suitable for energy storage devices like batteries and supercapacitors.- Carbon-based electrode materials: Carbon-based materials are widely used in electrode applications due to their excellent electrical conductivity, stability, and versatility. These materials include graphene, carbon nanotubes, and activated carbon, which can be modified or functionalized to enhance specific properties such as capacity, cycling stability, and rate capability. Carbon-based electrodes are particularly valuable in energy storage applications like batteries and supercapacitors where high surface area and conductivity are critical for performance.
- Metal oxide electrode materials: Metal oxide materials serve as important electrode components in various electrochemical devices. These materials offer high theoretical capacities, diverse redox mechanisms, and tunable properties through composition control. Common metal oxides used include lithium cobalt oxide, manganese dioxide, and titanium dioxide. Research focuses on improving their conductivity, stability during cycling, and rate performance through doping, nanostructuring, and composite formation with conductive additives.
- Silicon-based electrode materials: Silicon-based materials are promising electrode candidates, particularly for lithium-ion batteries, due to their exceptionally high theoretical capacity. However, these materials face challenges related to volume expansion during charge-discharge cycles, which can lead to structural degradation and capacity fading. Various approaches to address these issues include nanostructuring, creating silicon-carbon composites, and developing specialized binders and electrolyte additives that accommodate volume changes while maintaining electrical contact.
- Composite and hybrid electrode materials: Composite and hybrid electrode materials combine different components to leverage synergistic effects and overcome limitations of single-component materials. These composites often pair high-capacity materials with conductive additives or structural stabilizers to achieve balanced performance. Examples include metal oxide-carbon composites, silicon-graphene hybrids, and polymer-inorganic composites. The design of these materials focuses on optimizing interfaces between components, controlling morphology, and ensuring effective electron and ion transport pathways.
- Electrode material manufacturing and processing techniques: Manufacturing and processing techniques significantly impact electrode material performance. Advanced methods include solution-based synthesis, hydrothermal/solvothermal processing, electrospinning, and various coating technologies. These techniques enable precise control over particle size, morphology, porosity, and surface properties. Post-synthesis treatments such as annealing, surface modification, and activation processes can further enhance electrochemical performance by optimizing crystallinity, removing defects, and creating favorable surface chemistry for specific applications.
02 Metal oxide electrode materials
Metal oxide materials serve as important electrode components due to their diverse electrochemical properties and stability. These materials, including transition metal oxides like manganese dioxide, nickel oxide, and iron oxide, offer high theoretical capacities and redox activity. The performance of metal oxide electrodes can be enhanced through nanostructuring, doping, or creating composite materials to improve conductivity, cycling stability, and rate capability for applications in batteries, supercapacitors, and electrocatalysis.Expand Specific Solutions03 Silicon-based electrode materials
Silicon-based materials are promising electrode candidates, particularly for lithium-ion batteries, due to their exceptionally high theoretical capacity. These materials can store significantly more charge than traditional graphite anodes. However, they face challenges related to volume expansion during charging/discharging cycles, which can lead to structural degradation. Various strategies have been developed to address these issues, including silicon-carbon composites, nanostructured silicon, and silicon alloys that can accommodate volume changes while maintaining electrical contact.Expand Specific Solutions04 Polymer and composite electrode materials
Polymer and composite electrode materials combine the advantages of different material classes to achieve enhanced performance. Conductive polymers like polyaniline and polypyrrole offer flexibility and processability, while polymer-inorganic composites can provide improved mechanical stability and electrochemical properties. These materials often feature synergistic effects between components, resulting in better conductivity, ion transport, and structural integrity during operation. Applications include flexible electronics, wearable devices, and next-generation energy storage systems.Expand Specific Solutions05 Novel electrode material fabrication techniques
Advanced fabrication techniques play a crucial role in developing high-performance electrode materials with controlled structures and properties. These methods include hydrothermal synthesis, electrospinning, atomic layer deposition, and various templating approaches. Novel processing techniques enable the creation of hierarchical structures, core-shell architectures, and precisely engineered interfaces that can significantly enhance electrochemical performance. These fabrication innovations help address challenges related to electron transport, ion diffusion, and structural stability in electrode materials.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The bioelectrochemical systems (BES) market for high-performance electrode materials is in a growth phase, with increasing commercial applications transitioning from research to industrial implementation. The global market is expanding rapidly, driven by sustainable energy and wastewater treatment demands. Technologically, the field shows varying maturity levels across companies. Research institutions like Nanyang Technological University, University of Wollongong, and CNRS lead fundamental innovations, while commercial players demonstrate different specialization levels. Cambrian Innovation has developed scaled BES solutions for resource recovery, while materials manufacturers like Nippon Chemi-Con, SGL Carbon, and Sakai Chemical are advancing electrode technologies. Form Energy's long-duration storage expertise and Festo's automation capabilities represent complementary technological approaches enhancing BES applications.
Cambrian Innovation, Inc.
Technical Solution: Cambrian Innovation has developed EcoVolt, a bioelectrochemical system that combines microbial fuel cell technology with anaerobic digestion for wastewater treatment. Their electrode materials incorporate specialized carbon-based anodes with high surface area and conductivity, optimized for biofilm formation and electron transfer. The company utilizes a proprietary exoelectrogenic biofilm cultivation process that enhances electrode performance through selective microbial communities. Their electrodes feature hierarchical porous structures with nanoscale modifications that maximize microbial attachment while facilitating efficient electron transfer. Cambrian's technology employs a dual-chamber design with specialized proton exchange membranes and cathodes containing platinum-free catalysts based on transition metal compounds, significantly reducing costs while maintaining performance comparable to traditional noble metal catalysts.
Strengths: Integrated wastewater treatment and energy generation capabilities; scalable modular design for industrial applications; reduced operational costs compared to conventional treatment. Weaknesses: Requires specific wastewater compositions for optimal performance; system complexity necessitates specialized maintenance; electrode longevity under continuous operation remains a challenge.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has pioneered advanced electrode materials for bioelectrochemical systems through their innovative carbon-based nanocomposites. Their research focuses on hierarchically structured electrodes combining carbon nanotubes and graphene with conductive polymers like polyaniline and polypyrrole, creating high surface area materials with enhanced conductivity and biocompatibility. CNRS has developed a unique sol-gel synthesis method for producing mesoporous carbon materials with controlled pore architecture, optimized for bacterial colonization and extracellular electron transfer. Their electrodes incorporate nitrogen-doped carbon structures that significantly improve the electrode-microbe interface by creating favorable surface functional groups. Additionally, CNRS researchers have engineered novel cathode materials using earth-abundant transition metal oxides (particularly manganese and iron-based compounds) that demonstrate oxygen reduction reaction performance approaching that of platinum while maintaining long-term stability in biological environments.
Strengths: Exceptional scientific expertise in materials chemistry and electrochemistry; innovative approaches to electrode functionalization; strong focus on sustainable, platinum-free catalysts. Weaknesses: Some materials remain at laboratory scale with challenges in mass production; higher production costs for specialized nanostructured materials; technology transfer to industrial applications requires further development.
Key Patents and Innovations in BES Electrode Materials
Electrodes for cost-effective bio-electrochemical systems
PatentActiveUS12122689B2
Innovation
- The development of novel electrodes with a planar structure, incorporating a conductive frame and current collectors, along with a non-conductive polymer material and rubber padding, and methods for electrochemical coating to protect against corrosion and enhance bio-compatibility, such as using carbon-based coatings on substrates like aluminum or stainless steel.
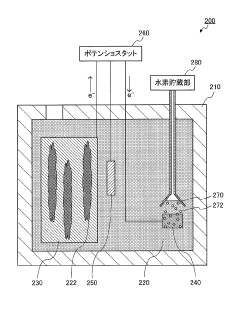
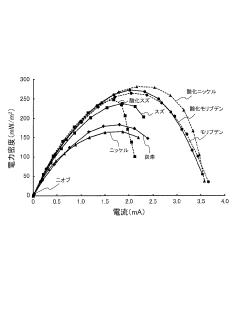
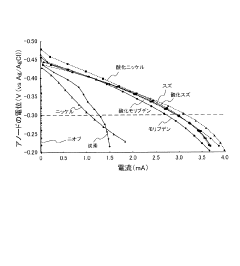
Bioelectrochemistry system, and electrode for bioelectrochemistry system
PatentInactiveJP2017157517A
Innovation
- The use of alternative electrode materials (molybdenum, molybdenum oxide, tin, tin oxide, and nickel oxide) as anode materials in bioelectrochemical systems, replacing conventional carbon electrodes to improve processing speed.
- Precise control of electrode surface composition with the electrode materials covering 0.001-100% of the anode surface area, allowing for optimization of the electrode-microorganism interface.
- System design incorporating a cation-permeable diaphragm between the anode and cathode compartments, enabling selective ion transport while maintaining separation of electrode reactions.
Sustainability and Life Cycle Assessment
Sustainability and life cycle assessment of electrode materials for bioelectrochemical systems (BES) has become increasingly critical as these technologies move toward commercial applications. The environmental impact of electrode materials spans their entire lifecycle, from raw material extraction to manufacturing, operation, and end-of-life disposal or recycling.
Carbon-based electrodes, while offering excellent performance characteristics, present varying sustainability profiles. Conventional carbon materials like graphite and carbon felt demonstrate relatively low environmental footprints compared to more advanced carbon nanomaterials. Carbon nanotubes and graphene, despite their superior electrochemical properties, often involve energy-intensive production processes that generate significant greenhouse gas emissions and require hazardous chemicals during synthesis.
Metal-based electrodes, particularly those utilizing precious metals like platinum or gold, face substantial sustainability challenges due to resource scarcity and environmentally damaging mining practices. Recent research has focused on reducing or eliminating precious metal content through the development of composite materials or non-noble metal alternatives that maintain performance while improving sustainability metrics.
Life cycle assessment (LCA) studies reveal that the operational phase of bioelectrochemical systems typically dominates the environmental impact profile when considering long-term applications. However, the manufacturing phase becomes increasingly significant for advanced nanomaterials and composite electrodes. This highlights the importance of balancing initial environmental investment against operational lifetime and performance efficiency.
Recycling and circular economy approaches are emerging as crucial strategies for improving the sustainability of BES electrode materials. Recovery of precious metals from spent electrodes can significantly reduce the net environmental impact, while research into biodegradable electrode components offers promising pathways for minimizing end-of-life waste management issues.
Energy payback time (EPBT) analysis indicates that most bioelectrochemical systems require 1-3 years of operation to offset the embodied energy in their electrode materials, with advanced nanomaterial electrodes typically falling at the higher end of this range. This metric provides valuable insight for technology developers seeking to optimize sustainability alongside performance characteristics.
Water consumption and toxicity impacts vary significantly across electrode material types, with nanomaterial production processes often requiring substantial water resources and potentially releasing harmful substances if not properly managed. These factors must be carefully considered in sustainability assessments, particularly for applications in water-stressed regions or sensitive environmental contexts.
Carbon-based electrodes, while offering excellent performance characteristics, present varying sustainability profiles. Conventional carbon materials like graphite and carbon felt demonstrate relatively low environmental footprints compared to more advanced carbon nanomaterials. Carbon nanotubes and graphene, despite their superior electrochemical properties, often involve energy-intensive production processes that generate significant greenhouse gas emissions and require hazardous chemicals during synthesis.
Metal-based electrodes, particularly those utilizing precious metals like platinum or gold, face substantial sustainability challenges due to resource scarcity and environmentally damaging mining practices. Recent research has focused on reducing or eliminating precious metal content through the development of composite materials or non-noble metal alternatives that maintain performance while improving sustainability metrics.
Life cycle assessment (LCA) studies reveal that the operational phase of bioelectrochemical systems typically dominates the environmental impact profile when considering long-term applications. However, the manufacturing phase becomes increasingly significant for advanced nanomaterials and composite electrodes. This highlights the importance of balancing initial environmental investment against operational lifetime and performance efficiency.
Recycling and circular economy approaches are emerging as crucial strategies for improving the sustainability of BES electrode materials. Recovery of precious metals from spent electrodes can significantly reduce the net environmental impact, while research into biodegradable electrode components offers promising pathways for minimizing end-of-life waste management issues.
Energy payback time (EPBT) analysis indicates that most bioelectrochemical systems require 1-3 years of operation to offset the embodied energy in their electrode materials, with advanced nanomaterial electrodes typically falling at the higher end of this range. This metric provides valuable insight for technology developers seeking to optimize sustainability alongside performance characteristics.
Water consumption and toxicity impacts vary significantly across electrode material types, with nanomaterial production processes often requiring substantial water resources and potentially releasing harmful substances if not properly managed. These factors must be carefully considered in sustainability assessments, particularly for applications in water-stressed regions or sensitive environmental contexts.
Scalability and Cost-Effectiveness Analysis
The scalability and cost-effectiveness of electrode materials represent critical factors determining the commercial viability of bioelectrochemical systems (BES). Current laboratory-scale demonstrations often utilize expensive materials like platinum, gold, or specialized carbon-based electrodes that become economically prohibitive when scaled to industrial applications.
Manufacturing scalability presents significant challenges for advanced electrode materials. While carbon-based materials such as graphite, carbon cloth, and carbon paper offer reasonable scalability pathways, newer nanomaterials including carbon nanotubes and graphene face substantial production limitations. The synthesis of these advanced materials typically involves energy-intensive processes, specialized equipment, and often hazardous chemicals, creating bottlenecks for mass production.
Cost analysis reveals substantial variations across electrode material categories. Conventional carbon-based electrodes range from $10-100/m², while noble metal-coated electrodes can exceed $1,000/m². Modified electrodes incorporating nanomaterials typically fall between $200-800/m², depending on modification complexity. These cost structures significantly impact the economic feasibility of large-scale BES implementation, particularly for wastewater treatment applications where profit margins remain tight.
Life-cycle cost assessment further complicates the economic picture. While initial material costs represent a significant investment, operational longevity and performance stability over time ultimately determine true cost-effectiveness. Materials exhibiting rapid performance degradation necessitate frequent replacement, negating any initial cost advantages. Current research indicates that many high-performance nanomaterials suffer from stability issues in real-world operating conditions, limiting their practical application despite impressive laboratory performance.
Production scaling pathways differ substantially between material types. Traditional carbon-based electrodes benefit from established manufacturing infrastructure, while emerging nanomaterials require development of new production methodologies. Recent advances in chemical vapor deposition techniques for graphene production and continuous flow synthesis for carbon nanotubes show promise for reducing production costs, potentially bringing these materials within economically viable ranges for certain high-value applications.
Market adoption barriers extend beyond pure material costs to include integration expenses, system redesign requirements, and operational learning curves. The most promising electrode materials demonstrate not only enhanced performance but also compatibility with existing manufacturing processes and system designs, minimizing transition costs for industry adoption.
Future cost reduction opportunities primarily lie in manufacturing process optimization, material hybridization approaches that minimize expensive components, and development of standardized production methods. Research targeting scalable synthesis of nanomaterials under ambient conditions represents a particularly promising direction for improving cost-effectiveness while maintaining performance advantages.
Manufacturing scalability presents significant challenges for advanced electrode materials. While carbon-based materials such as graphite, carbon cloth, and carbon paper offer reasonable scalability pathways, newer nanomaterials including carbon nanotubes and graphene face substantial production limitations. The synthesis of these advanced materials typically involves energy-intensive processes, specialized equipment, and often hazardous chemicals, creating bottlenecks for mass production.
Cost analysis reveals substantial variations across electrode material categories. Conventional carbon-based electrodes range from $10-100/m², while noble metal-coated electrodes can exceed $1,000/m². Modified electrodes incorporating nanomaterials typically fall between $200-800/m², depending on modification complexity. These cost structures significantly impact the economic feasibility of large-scale BES implementation, particularly for wastewater treatment applications where profit margins remain tight.
Life-cycle cost assessment further complicates the economic picture. While initial material costs represent a significant investment, operational longevity and performance stability over time ultimately determine true cost-effectiveness. Materials exhibiting rapid performance degradation necessitate frequent replacement, negating any initial cost advantages. Current research indicates that many high-performance nanomaterials suffer from stability issues in real-world operating conditions, limiting their practical application despite impressive laboratory performance.
Production scaling pathways differ substantially between material types. Traditional carbon-based electrodes benefit from established manufacturing infrastructure, while emerging nanomaterials require development of new production methodologies. Recent advances in chemical vapor deposition techniques for graphene production and continuous flow synthesis for carbon nanotubes show promise for reducing production costs, potentially bringing these materials within economically viable ranges for certain high-value applications.
Market adoption barriers extend beyond pure material costs to include integration expenses, system redesign requirements, and operational learning curves. The most promising electrode materials demonstrate not only enhanced performance but also compatibility with existing manufacturing processes and system designs, minimizing transition costs for industry adoption.
Future cost reduction opportunities primarily lie in manufacturing process optimization, material hybridization approaches that minimize expensive components, and development of standardized production methods. Research targeting scalable synthesis of nanomaterials under ambient conditions represents a particularly promising direction for improving cost-effectiveness while maintaining performance advantages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!