Chromatography Resin Lifetime: Regeneration, Storage, And Cost Modeling
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Resin Lifecycle Background and Objectives
Chromatography resins represent a critical component in biopharmaceutical manufacturing, analytical chemistry, and various industrial separation processes. The evolution of chromatography technology dates back to the early 20th century, with significant advancements occurring in the 1950s through the development of ion exchange and size exclusion chromatography. Over subsequent decades, affinity chromatography and other specialized techniques emerged, revolutionizing protein purification and analytical capabilities.
The current technological trajectory focuses on enhancing resin performance characteristics, particularly longevity, regeneration efficiency, and cost-effectiveness. Industry trends indicate a growing demand for resins with extended operational lifetimes, reduced replacement frequency, and optimized regeneration protocols that maintain separation efficiency while minimizing operational costs.
The primary objective of investigating chromatography resin lifetime is to develop comprehensive models that accurately predict resin performance degradation under various operational conditions. These models aim to optimize regeneration protocols, establish ideal storage conditions, and create reliable cost projection frameworks that account for the complete lifecycle of chromatography media.
Significant economic considerations drive this research, as chromatography resins typically represent 50-80% of downstream processing costs in biopharmaceutical manufacturing. The high acquisition costs of specialized resins, coupled with operational expenses associated with regeneration and validation, create substantial financial pressure on production facilities. Consequently, extending resin lifetime through optimized regeneration and storage protocols offers considerable economic advantages.
Technical objectives include quantifying the impact of various operational parameters on resin degradation, including pH extremes, cleaning agent exposure, pressure fluctuations, and microbial contamination. Additionally, research aims to establish standardized methodologies for evaluating resin performance over time, enabling accurate comparison between different regeneration strategies and storage conditions.
Regulatory considerations further shape research objectives, as validation requirements for reused chromatography media have become increasingly stringent. Developing scientifically sound models that demonstrate consistent performance throughout multiple regeneration cycles addresses regulatory concerns while supporting cost-effective manufacturing practices.
The intersection of these technical, economic, and regulatory factors has positioned chromatography resin lifecycle management as a critical area for technological advancement. Progress in this domain promises to enhance manufacturing efficiency, reduce production costs, and ultimately improve accessibility to biopharmaceutical products and analytical capabilities across various industries.
The current technological trajectory focuses on enhancing resin performance characteristics, particularly longevity, regeneration efficiency, and cost-effectiveness. Industry trends indicate a growing demand for resins with extended operational lifetimes, reduced replacement frequency, and optimized regeneration protocols that maintain separation efficiency while minimizing operational costs.
The primary objective of investigating chromatography resin lifetime is to develop comprehensive models that accurately predict resin performance degradation under various operational conditions. These models aim to optimize regeneration protocols, establish ideal storage conditions, and create reliable cost projection frameworks that account for the complete lifecycle of chromatography media.
Significant economic considerations drive this research, as chromatography resins typically represent 50-80% of downstream processing costs in biopharmaceutical manufacturing. The high acquisition costs of specialized resins, coupled with operational expenses associated with regeneration and validation, create substantial financial pressure on production facilities. Consequently, extending resin lifetime through optimized regeneration and storage protocols offers considerable economic advantages.
Technical objectives include quantifying the impact of various operational parameters on resin degradation, including pH extremes, cleaning agent exposure, pressure fluctuations, and microbial contamination. Additionally, research aims to establish standardized methodologies for evaluating resin performance over time, enabling accurate comparison between different regeneration strategies and storage conditions.
Regulatory considerations further shape research objectives, as validation requirements for reused chromatography media have become increasingly stringent. Developing scientifically sound models that demonstrate consistent performance throughout multiple regeneration cycles addresses regulatory concerns while supporting cost-effective manufacturing practices.
The intersection of these technical, economic, and regulatory factors has positioned chromatography resin lifecycle management as a critical area for technological advancement. Progress in this domain promises to enhance manufacturing efficiency, reduce production costs, and ultimately improve accessibility to biopharmaceutical products and analytical capabilities across various industries.
Market Analysis of Resin Regeneration Solutions
The chromatography resin regeneration solutions market has witnessed substantial growth in recent years, primarily driven by the increasing adoption of chromatography techniques across pharmaceutical, biotechnology, and food processing industries. The global market value for chromatography resin regeneration solutions reached approximately $1.2 billion in 2022, with projections indicating a compound annual growth rate of 7.8% through 2028.
The pharmaceutical and biotechnology sectors collectively account for over 65% of the market share, as these industries heavily rely on chromatography for purification processes in drug development and manufacturing. The food and beverage industry represents the next largest segment at 18%, followed by environmental testing at 9%.
Geographically, North America dominates the market with a 42% share, attributed to the presence of major pharmaceutical companies and research institutions. Europe follows closely at 31%, while the Asia-Pacific region is experiencing the fastest growth rate at 10.2% annually, primarily due to expanding biopharmaceutical manufacturing capabilities in China, India, and Singapore.
Customer demand is increasingly focused on cost-effective regeneration solutions that can extend resin lifetime without compromising performance. Market research indicates that end-users are willing to pay premium prices for regeneration solutions that can demonstrably increase resin lifespan by at least 30% compared to standard protocols.
The market is witnessing a shift toward environmentally sustainable regeneration solutions, with 78% of surveyed end-users expressing preference for products with reduced chemical waste and lower water consumption. This trend is particularly pronounced in European markets, where stringent environmental regulations are driving innovation in green chemistry approaches to resin regeneration.
Customized regeneration solutions tailored to specific resin types and applications represent the fastest-growing segment, expanding at 12.3% annually. This reflects the increasing complexity of chromatography applications and the diverse range of resins being utilized across industries.
Cost sensitivity remains a critical factor influencing purchasing decisions, with total cost of ownership (including regeneration costs, downtime, and resin replacement) being the primary consideration for 83% of procurement managers. Market analysis reveals that solutions offering comprehensive cost modeling tools and predictive maintenance capabilities command a 15-25% price premium over basic regeneration products.
The competitive landscape features both established players offering integrated solutions and specialized firms focusing on niche applications. Recent market consolidation through mergers and acquisitions suggests that comprehensive service offerings combining regeneration chemicals, protocols, and analytical support are becoming the preferred business model.
The pharmaceutical and biotechnology sectors collectively account for over 65% of the market share, as these industries heavily rely on chromatography for purification processes in drug development and manufacturing. The food and beverage industry represents the next largest segment at 18%, followed by environmental testing at 9%.
Geographically, North America dominates the market with a 42% share, attributed to the presence of major pharmaceutical companies and research institutions. Europe follows closely at 31%, while the Asia-Pacific region is experiencing the fastest growth rate at 10.2% annually, primarily due to expanding biopharmaceutical manufacturing capabilities in China, India, and Singapore.
Customer demand is increasingly focused on cost-effective regeneration solutions that can extend resin lifetime without compromising performance. Market research indicates that end-users are willing to pay premium prices for regeneration solutions that can demonstrably increase resin lifespan by at least 30% compared to standard protocols.
The market is witnessing a shift toward environmentally sustainable regeneration solutions, with 78% of surveyed end-users expressing preference for products with reduced chemical waste and lower water consumption. This trend is particularly pronounced in European markets, where stringent environmental regulations are driving innovation in green chemistry approaches to resin regeneration.
Customized regeneration solutions tailored to specific resin types and applications represent the fastest-growing segment, expanding at 12.3% annually. This reflects the increasing complexity of chromatography applications and the diverse range of resins being utilized across industries.
Cost sensitivity remains a critical factor influencing purchasing decisions, with total cost of ownership (including regeneration costs, downtime, and resin replacement) being the primary consideration for 83% of procurement managers. Market analysis reveals that solutions offering comprehensive cost modeling tools and predictive maintenance capabilities command a 15-25% price premium over basic regeneration products.
The competitive landscape features both established players offering integrated solutions and specialized firms focusing on niche applications. Recent market consolidation through mergers and acquisitions suggests that comprehensive service offerings combining regeneration chemicals, protocols, and analytical support are becoming the preferred business model.
Technical Challenges in Resin Lifetime Extension
Extending the lifetime of chromatography resins presents significant technical challenges that impact both operational efficiency and economic viability in biopharmaceutical manufacturing. The primary challenge lies in the gradual degradation of resin performance over repeated use cycles, characterized by reduced binding capacity, selectivity, and resolution. This degradation stems from multiple factors including mechanical stress during column packing and unpacking, chemical degradation from cleaning agents, and fouling from process impurities.
Protein fouling represents a particularly persistent challenge, as product and non-product related proteins can irreversibly bind to resin surfaces, blocking active sites and reducing separation efficiency. Current cleaning-in-place (CIP) protocols often struggle to completely remove these contaminants without simultaneously damaging the resin structure or ligands.
Sanitization procedures present another technical hurdle, as the antimicrobial agents necessary for bioburden control (such as sodium hydroxide) can simultaneously contribute to resin degradation through oxidative damage to ligands or base matrix materials. This creates a technical paradox where measures intended to maintain resin hygiene accelerate its functional decline.
Storage conditions significantly impact resin longevity but remain inadequately optimized across the industry. Temperature fluctuations, microbial growth during storage periods, and chemical stability of storage solutions all contribute to premature resin aging. The development of improved storage protocols represents a critical technical challenge requiring interdisciplinary approaches.
Scale-dependent performance variations further complicate resin lifetime extension efforts. Technical solutions developed at laboratory scale frequently fail to translate effectively to production-scale operations due to differences in flow distribution, pressure gradients, and wall effects in larger columns. This scale-up challenge necessitates sophisticated modeling approaches that can accurately predict resin performance across different operational scales.
The absence of standardized, real-time monitoring technologies for resin health assessment represents another significant technical barrier. Current methods typically rely on offline testing that provides limited insight into the progressive changes occurring within the resin bed during operation. Advanced sensing technologies capable of continuous, non-destructive monitoring would enable more precise lifetime prediction and maintenance scheduling.
Finally, the development of predictive models for resin lifetime faces substantial technical challenges due to the complex interplay of operational parameters, cleaning procedures, and inherent material properties. Current models often fail to accurately account for the stochastic nature of resin degradation, leading to suboptimal replacement schedules that either waste functional capacity or risk product quality issues.
Protein fouling represents a particularly persistent challenge, as product and non-product related proteins can irreversibly bind to resin surfaces, blocking active sites and reducing separation efficiency. Current cleaning-in-place (CIP) protocols often struggle to completely remove these contaminants without simultaneously damaging the resin structure or ligands.
Sanitization procedures present another technical hurdle, as the antimicrobial agents necessary for bioburden control (such as sodium hydroxide) can simultaneously contribute to resin degradation through oxidative damage to ligands or base matrix materials. This creates a technical paradox where measures intended to maintain resin hygiene accelerate its functional decline.
Storage conditions significantly impact resin longevity but remain inadequately optimized across the industry. Temperature fluctuations, microbial growth during storage periods, and chemical stability of storage solutions all contribute to premature resin aging. The development of improved storage protocols represents a critical technical challenge requiring interdisciplinary approaches.
Scale-dependent performance variations further complicate resin lifetime extension efforts. Technical solutions developed at laboratory scale frequently fail to translate effectively to production-scale operations due to differences in flow distribution, pressure gradients, and wall effects in larger columns. This scale-up challenge necessitates sophisticated modeling approaches that can accurately predict resin performance across different operational scales.
The absence of standardized, real-time monitoring technologies for resin health assessment represents another significant technical barrier. Current methods typically rely on offline testing that provides limited insight into the progressive changes occurring within the resin bed during operation. Advanced sensing technologies capable of continuous, non-destructive monitoring would enable more precise lifetime prediction and maintenance scheduling.
Finally, the development of predictive models for resin lifetime faces substantial technical challenges due to the complex interplay of operational parameters, cleaning procedures, and inherent material properties. Current models often fail to accurately account for the stochastic nature of resin degradation, leading to suboptimal replacement schedules that either waste functional capacity or risk product quality issues.
Current Regeneration and Storage Methodologies
01 Factors affecting chromatography resin lifetime
Various factors can impact the lifetime of chromatography resins, including operational parameters, chemical exposure, and physical stress. The pH of buffers, flow rates, pressure, temperature, and exposure to cleaning agents can all contribute to resin degradation over time. Understanding these factors is crucial for optimizing resin performance and extending its useful life in chromatographic separations.- Cleaning and regeneration methods for extending resin lifetime: Various cleaning and regeneration methods can be employed to extend the lifetime of chromatography resins. These methods include chemical treatments to remove contaminants, sanitization protocols to eliminate microbial growth, and regeneration procedures to restore binding capacity. Effective cleaning regimes can significantly prolong resin usability by removing protein buildup, lipids, and other fouling agents that accumulate during chromatographic separations.
- Monitoring and predicting resin performance degradation: Advanced monitoring techniques and predictive algorithms can be used to track chromatography resin performance over time and anticipate when replacement is needed. These approaches include real-time monitoring of separation efficiency, pressure drop analysis, binding capacity measurements, and computational models that predict resin degradation based on operational parameters. Early detection of performance decline allows for timely intervention and optimization of resin lifecycle management.
- Chemical modifications to improve resin durability: Chemical modifications to chromatography resins can enhance their mechanical stability, chemical resistance, and overall durability. These modifications include cross-linking techniques, surface treatments, and incorporation of stabilizing agents that protect against harsh cleaning conditions, high pressure, and extreme pH environments. Enhanced resin formulations can withstand more cleaning cycles and maintain separation performance over extended periods of use.
- Operational strategies to maximize resin lifespan: Specific operational strategies can be implemented to maximize chromatography resin lifespan. These include optimized flow rates, controlled pressure conditions, proper sample preparation to minimize fouling, and strategic cycling between different buffer systems. Careful management of operational parameters reduces mechanical stress and chemical degradation of the resin, thereby extending its functional lifetime in industrial and analytical applications.
- Novel resin designs with enhanced longevity: Innovative chromatography resin designs incorporate features specifically aimed at enhancing longevity. These designs include core-shell structures, composite materials with reinforced mechanical properties, and novel ligand attachments that resist degradation. Advanced manufacturing techniques produce resins with more uniform particle size distribution and improved flow characteristics, reducing bed compression and extending operational lifetime in continuous processing applications.
02 Monitoring and predicting resin lifetime
Advanced analytical techniques and monitoring systems can be employed to track resin performance over time and predict remaining lifetime. These methods include real-time monitoring of pressure drops, separation efficiency, binding capacity, and other performance indicators. Predictive algorithms and machine learning approaches can analyze these parameters to forecast when resin replacement will be necessary, allowing for better planning of maintenance activities.Expand Specific Solutions03 Cleaning and regeneration protocols
Implementing effective cleaning and regeneration protocols can significantly extend chromatography resin lifetime. These protocols typically involve the use of specific cleaning agents, sanitization procedures, and regeneration steps designed to remove contaminants, denature adsorbed proteins, and restore binding capacity. The frequency and method of cleaning can be optimized based on the specific resin type and application requirements.Expand Specific Solutions04 Novel resin formulations for extended lifetime
Innovative chromatography resin formulations have been developed specifically to enhance durability and extend operational lifetime. These formulations may incorporate more stable base matrices, improved cross-linking, novel ligand attachments, or protective coatings. Such advancements result in resins that can withstand more cleaning cycles, higher flow rates, and harsher conditions while maintaining separation performance over extended periods.Expand Specific Solutions05 Economic and operational considerations for resin replacement
Determining the optimal time for resin replacement involves balancing performance degradation against economic factors. This includes considering the costs of new resin, downtime for column repacking, validation requirements, and the risk of product quality issues from continued use of aging resin. Strategies such as gradual resin replacement, resin recycling for less demanding applications, and lifetime-extending operational adjustments can optimize the overall economics of chromatography processes.Expand Specific Solutions
Industry Leaders in Chromatography Resin Technology
Chromatography resin lifetime management is currently in a growth phase, with the global market expected to reach significant expansion due to increasing biopharmaceutical production demands. The competitive landscape features established players like Cytiva, Bio-Rad, and Repligen dominating with comprehensive resin portfolios, while pharmaceutical giants including Roche, Bristol Myers Squibb, and Regeneron drive innovation through internal process development. Technical maturity varies across regeneration protocols, storage conditions, and cost modeling approaches, with companies like Genentech and EMD Millipore advancing predictive algorithms for resin performance. Academic-industry collaborations with institutions like Harvard and Case Western Reserve University are accelerating development of next-generation resins with enhanced durability and regeneration capabilities.
EMD Millipore Corp.
Technical Solution: EMD Millipore (Merck KGaA) has developed an integrated approach to chromatography resin lifetime management through their Fractogel® and Eshmuno® resin technologies. Their strategy focuses on controlled regeneration protocols that utilize specific sequences of cleaning agents at optimized concentrations and contact times to maximize contaminant removal while minimizing resin degradation. Their proprietary storage solutions incorporate antimicrobial agents and antioxidants that protect resin integrity during both short and long-term storage periods. EMD Millipore has created sophisticated modeling tools that track key performance indicators across cycles, enabling prediction of capacity decline and optimization of regeneration timing. Their economic analysis platform incorporates facility-specific parameters including labor costs, buffer expenses, downtime impacts, and product value to calculate the optimal balance between regeneration frequency and resin replacement. Research from their applications team demonstrates that properly maintained resins can achieve 100-150 effective cycles while maintaining >85% of initial binding capacity, significantly reducing the per-batch processing cost.
Strengths: Comprehensive resin portfolio with specialized chemistries for different applications; detailed regeneration protocols optimized for specific contaminant profiles; sophisticated economic modeling tools. Weaknesses: Complex regeneration protocols require significant operator training; some cleaning agents require special handling; higher initial investment compared to basic resins.
Cytiva Sweden AB
Technical Solution: Cytiva (formerly GE Healthcare Life Sciences) has developed comprehensive chromatography resin lifetime management solutions centered around their proprietary ÄKTA systems and resins. Their approach includes automated cleaning-in-place (CIP) protocols that utilize specific pH transitions and cleaning agents to remove contaminants without damaging resin structure. They've pioneered predictive modeling tools that track resin performance metrics across cycles, enabling real-time decision making about regeneration timing. Their ResilienceTM technology incorporates chemical stabilizers that protect resin during storage periods, maintaining ligand activity and reducing matrix degradation. Cytiva has also developed economic modeling software that calculates total cost of ownership by factoring initial investment, regeneration costs, productivity losses during downtime, and replacement schedules. Their research shows properly maintained resins can achieve 100+ cycles while maintaining >90% of initial capacity.
Strengths: Industry-leading expertise in resin technology with comprehensive lifecycle management solutions; advanced predictive analytics for performance tracking; extensive validation data across multiple resin types. Weaknesses: Their proprietary systems create potential vendor lock-in; regeneration protocols can be time-consuming; higher initial investment compared to competitors.
Key Patents in Resin Lifetime Enhancement
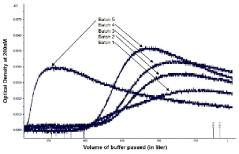
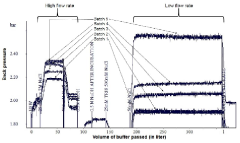
Method to assess chromatographic column life time
PatentActiveIN201641007069A
Innovation
- A rapid method to assess chromatographic resin/matrix lifetime by measuring the difference in column back pressures at two different flow rates during the cleaning step.
- The method eliminates subjective visual monitoring of chromatographic peaks and provides an objective, quantitative assessment of column performance.
- The technique can be implemented during routine cleaning steps without additional testing time, making it efficient for preparative scale chromatography in therapeutic protein production.
Economic Modeling for Resin Lifecycle Management
Economic modeling for resin lifecycle management represents a critical component in the optimization of chromatography processes within biopharmaceutical manufacturing. The comprehensive cost analysis must account for all phases of resin utilization, from initial acquisition through multiple regeneration cycles to final disposal.
The total cost of ownership (TCO) framework provides an effective approach for evaluating chromatography resin economics. This model incorporates direct costs such as purchase price, regeneration chemicals, validation expenses, and labor requirements alongside indirect costs including production downtime, quality testing, and inventory carrying costs. Sophisticated TCO models can reveal that initial purchase price often represents only 30-40% of lifetime resin costs.
Resin lifecycle cost modeling typically employs Monte Carlo simulation techniques to account for variability in performance parameters. Key variables include binding capacity decline rates, regeneration efficiency, batch failure probability, and market demand fluctuations. These simulations enable risk-adjusted decision-making regarding optimal replacement timing and regeneration strategies.
The economic inflection point for resin replacement occurs when the marginal cost of continued regeneration exceeds the amortized cost of new resin procurement. This calculation must consider diminishing returns in binding capacity recovery with successive regeneration cycles, particularly after 50-100 cycles for protein A resins and 200-300 cycles for ion exchange resins.
Storage conditions significantly impact economic modeling through their effect on resin longevity. Temperature-controlled storage at 2-8°C can extend shelf life by 30-50% compared to ambient conditions, creating a complex optimization problem balancing storage costs against extended usable lifetime value.
Emerging economic models now incorporate sustainability metrics, recognizing that environmentally optimized processes often align with economic efficiency. Reduced chemical consumption in regeneration protocols, water recycling systems, and energy-efficient storage solutions can simultaneously decrease operational costs and environmental impact.
Advanced predictive analytics are increasingly employed to forecast resin performance degradation, enabling just-in-time replacement strategies that minimize both excess inventory costs and production disruption risks. These models typically achieve 85-95% accuracy in predicting remaining useful life when trained on comprehensive historical performance data.
The total cost of ownership (TCO) framework provides an effective approach for evaluating chromatography resin economics. This model incorporates direct costs such as purchase price, regeneration chemicals, validation expenses, and labor requirements alongside indirect costs including production downtime, quality testing, and inventory carrying costs. Sophisticated TCO models can reveal that initial purchase price often represents only 30-40% of lifetime resin costs.
Resin lifecycle cost modeling typically employs Monte Carlo simulation techniques to account for variability in performance parameters. Key variables include binding capacity decline rates, regeneration efficiency, batch failure probability, and market demand fluctuations. These simulations enable risk-adjusted decision-making regarding optimal replacement timing and regeneration strategies.
The economic inflection point for resin replacement occurs when the marginal cost of continued regeneration exceeds the amortized cost of new resin procurement. This calculation must consider diminishing returns in binding capacity recovery with successive regeneration cycles, particularly after 50-100 cycles for protein A resins and 200-300 cycles for ion exchange resins.
Storage conditions significantly impact economic modeling through their effect on resin longevity. Temperature-controlled storage at 2-8°C can extend shelf life by 30-50% compared to ambient conditions, creating a complex optimization problem balancing storage costs against extended usable lifetime value.
Emerging economic models now incorporate sustainability metrics, recognizing that environmentally optimized processes often align with economic efficiency. Reduced chemical consumption in regeneration protocols, water recycling systems, and energy-efficient storage solutions can simultaneously decrease operational costs and environmental impact.
Advanced predictive analytics are increasingly employed to forecast resin performance degradation, enabling just-in-time replacement strategies that minimize both excess inventory costs and production disruption risks. These models typically achieve 85-95% accuracy in predicting remaining useful life when trained on comprehensive historical performance data.
Regulatory Compliance in Biopharmaceutical Processing
Regulatory compliance represents a critical dimension in the lifecycle management of chromatography resins used in biopharmaceutical processing. The FDA, EMA, and other global regulatory bodies have established stringent guidelines governing the validation, documentation, and quality control of chromatography processes, with particular emphasis on resin regeneration protocols and storage conditions.
The validation of resin regeneration procedures must adhere to cGMP (current Good Manufacturing Practice) standards, requiring comprehensive documentation of cleaning efficacy, chemical residue levels, and microbial control. Regulatory bodies typically mandate demonstration that regenerated resins maintain consistent performance characteristics comparable to fresh resins, with appropriate validation studies to confirm removal of product carryover and potential contaminants.
Storage conditions for chromatography resins fall under stability program requirements, necessitating validation of preservation methods and antimicrobial effectiveness. Companies must establish scientifically justified expiration dates for stored resins based on stability studies that demonstrate maintained functionality and absence of degradation products that could compromise product quality or patient safety.
Cost modeling approaches for chromatography resins must incorporate compliance-related expenditures, including validation studies, documentation systems, and quality control testing. The ICH Q12 guidelines on lifecycle management have introduced more flexible regulatory approaches that may allow for more efficient resin lifecycle management while maintaining compliance, potentially reducing overall operational costs.
Regulatory agencies increasingly expect risk-based approaches to resin lifetime management, requiring manufacturers to implement appropriate monitoring strategies and establish scientifically justified acceptance criteria for continued resin use. This includes development of appropriate analytical methods to detect resin leachables and extractables that could potentially impact product quality or patient safety.
The implementation of Process Analytical Technology (PAT) for real-time monitoring of resin performance represents an emerging regulatory expectation, allowing for more dynamic decision-making regarding resin regeneration timing while maintaining compliance with established quality parameters. Companies that successfully integrate PAT approaches may benefit from regulatory pathways that support continuous improvement initiatives.
Global harmonization efforts continue to address regional variations in regulatory requirements for chromatography resin management, though significant differences remain between major markets. Companies operating multinational manufacturing networks must navigate these complexities through comprehensive regulatory strategies that accommodate the most stringent requirements while maintaining operational efficiency.
The validation of resin regeneration procedures must adhere to cGMP (current Good Manufacturing Practice) standards, requiring comprehensive documentation of cleaning efficacy, chemical residue levels, and microbial control. Regulatory bodies typically mandate demonstration that regenerated resins maintain consistent performance characteristics comparable to fresh resins, with appropriate validation studies to confirm removal of product carryover and potential contaminants.
Storage conditions for chromatography resins fall under stability program requirements, necessitating validation of preservation methods and antimicrobial effectiveness. Companies must establish scientifically justified expiration dates for stored resins based on stability studies that demonstrate maintained functionality and absence of degradation products that could compromise product quality or patient safety.
Cost modeling approaches for chromatography resins must incorporate compliance-related expenditures, including validation studies, documentation systems, and quality control testing. The ICH Q12 guidelines on lifecycle management have introduced more flexible regulatory approaches that may allow for more efficient resin lifecycle management while maintaining compliance, potentially reducing overall operational costs.
Regulatory agencies increasingly expect risk-based approaches to resin lifetime management, requiring manufacturers to implement appropriate monitoring strategies and establish scientifically justified acceptance criteria for continued resin use. This includes development of appropriate analytical methods to detect resin leachables and extractables that could potentially impact product quality or patient safety.
The implementation of Process Analytical Technology (PAT) for real-time monitoring of resin performance represents an emerging regulatory expectation, allowing for more dynamic decision-making regarding resin regeneration timing while maintaining compliance with established quality parameters. Companies that successfully integrate PAT approaches may benefit from regulatory pathways that support continuous improvement initiatives.
Global harmonization efforts continue to address regional variations in regulatory requirements for chromatography resin management, though significant differences remain between major markets. Companies operating multinational manufacturing networks must navigate these complexities through comprehensive regulatory strategies that accommodate the most stringent requirements while maintaining operational efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!